Abstract
Background: Oral cancer is a prevalent global health issue, with chemotherapy and radiation therapy commonly used in its treatment.
Methods: This study aimed to investigate the correlation between oral cytological changes and exposure to chemotherapy and/or radiotherapy. A total of 111 participants were included, consisting of 75 cancer patients undergoing radiotherapy and/or chemotherapy (case group) and 36 healthy individuals (control group).
Results: Participants' ages ranged from 15 to 70 years. Pap stain analysis revealed that 15.3% of samples exhibited acute inflammation, with 18.7% in the treatment group and 8.3% in healthy individuals. Among chemotherapy dose groups, varying degrees of atypia were observed, with a higher incidence of atypia associated with increased chemotherapy dose. Similarly, radiation therapy doses showed an increase in atypical cellular changes with higher doses.
Conclusions: Chemotherapy and radiation therapy were found to influence atypical cellular changes and inflammatory infiltration in the oral cavity. The incidence of atypia was observed to increase with higher doses of chemotherapy and radiation therapy.
Introduction
Oral cancer poses a significant global health challenge, impacting diverse populations worldwide1. Treatment modalities such as chemotherapy and radiation therapy are commonly utilized in managing this condition. Prior research has documented the adverse effects of these treatments, including oral mucositis and disruptions to the normal cell cycle, leading to the development of carcinogenic changes and alterations in epithelial cell regeneration2, 3.
This study employs cytological techniques to investigate the impact of short and long durations of cancer treatment on the development of cellular changes in the oral cavity. Specifically, we aim to detect oral cytological changes associated with exposure to chemotherapy and/or radiotherapy. By examining the cellular alterations in oral tissues, we seek to enhance our understanding of the effects of chemotherapy and radiation therapy on the oral mucosa and potentially identify markers of treatment-related changes4.
The research is set against the backdrop of the widespread incidence of oral cancer and the importance of elucidating the mechanisms underlying treatment-induced cellular changes5, 6, 7. By elucidating the relationship between chemotherapy, radiotherapy, and oral cytological alterations, this study aims to contribute to the existing body of knowledge on oral disorders among cancer patients, addressing a critical gap in current research.
Methods
This descriptive analytical cross-sectional study was conducted at the Radiation and Isotope Centre in Khartoum from November 2021 to April 2022. The study aimed to assess the effects of radiotherapy and/or chemotherapy on the oral mucosa using cytological techniques.
The study cohort comprised 111 individuals, with 75 patients undergoing radiotherapy and/or chemotherapy considered as the case group, and 36 apparently healthy individuals serving as the control group. Data collection involved interviews using a pre-tested questionnaire to gather information related to the disease and behavior of the participants.
Cytological techniques were employed to analyze oral cells through the Papanicolaou (PAP) stain method. Smears were fixed with 95% ethanol, stained with Harris Hematoxylin and Eosin Azure, and mounted with DPX for examination. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software, version 23.0, and STATA 11, including frequency, mean, and chi-square tests.
Ethical approval was obtained from the Khartoum State Ministry of Health Innovation, Development, and Scientific Research Committee in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants, and confidentiality of patient information was strictly maintained throughout the study. Samples were collected with proper protective measures in place to adhere to safety protocols, including COVID-19 precautions. Ethical clearance code: KMOH-RES/06-01-2022.
| Variables | Cytological diagnosis | Fisher exact test P value | |||
| Benign | Inflammation | Atypia | |||
| Chemotherapy number of doses | One | 1 | 0 | 0 | 0.664* |
| 100.00% | 0.00% | 0.00% | |||
| Two | 3 | 0 | 0 | ||
| 100.00% | 0.00% | 0.00% | |||
| Three | 14 | 6 | 3 | ||
| 60.90% | 26.10% | 13.00% | |||
| Four | 7 | 2 | 3 | ||
| 58.30% | 16.70% | 25.00% | |||
| Five | 0 | 1 | 0 | ||
| 0.00% | 100.00% | 0.00% | |||
| Radiotherapy number of doses | One | 1 | 0 | 0 | 0.120* |
| 100.00% | 0.00% | 0.00% | |||
| Two | 2 | 0 | 1 | ||
| 66.70% | 0.00% | 33.30% | |||
| Three | 17 | 3 | 0 | ||
| 85.00% | 15.00% | 0.00% | |||
| Four | 6 | 2 | 3 | ||
| 54.50% | 18.20% | 27.30% | |||
| Variables | Cytological Atypia | Fisher exact test P value | ||
| No | Yes | |||
| Groups | Under treatment | 65 | 10 | 0.029** |
| 86.70% | 13.30% | |||
| Healthy individuals | 36 | 0 | ||
| 100.00% | 0.00% | |||
| Gender | Male | 25 | 5 | 0.130* |
| 83.30% | 16.70% | |||
| Female | 76 | 5 | ||
| 93.80% | 6.20% | |||
| Age groups | 15-20 years | 8 | 0 | 0.142* |
| 100.00% | 0.00% | |||
| 21-30 years | 21 | 0 | ||
| 100.00% | 0.00% | |||
| 31-40 years | 25 | 2 | ||
| 92.60% | 7.40% | |||
| 41-50 years | 27 | 3 | ||
| 90.00% | 10.00% | |||
| 51-60 years | 15 | 5 | ||
| 75.00% | 25.00% | |||
| 61-70 years | 5 | 0 | ||
| 100.00% | 0.00% | |||
| Variables | Cytological Atypia | Fisher exact test P value | ||
| No | Yes | |||
| Under treatment gender | Male | 25 | 5 | 0.508* |
| 83.30% | 16.70% | |||
| Female | 40 | 5 | ||
| 88.90% | 11.10% | |||
| Under treatment age groups | 15-20 years | 5 | 0 | 0.389* |
| 100.00% | 0.00% | |||
| 21-30 years | 7 | 0 | ||
| 100.00% | 0.00% | |||
| 31-40 years | 18 | 2 | ||
| 90.00% | 10.00% | |||
| 41-50 years | 21 | 3 | ||
| 87.50% | 12.50% | |||
| 51-60 years | 11 | 5 | ||
| 68.80% | 31.30% | |||
| 61-70 years | 3 | 0 | ||
| 100.00% | 0.00% | |||
| Type of treatment | Chemotherapy | 34 | 6 | 0.742* |
| 85.00% | 15.00% | |||
| Radiotherapy | 31 | 4 | ||
| 88.60% | 11.40% | |||
| Total number of dose | One | 2 | 0 | 0.201* |
| 100.00% | 0.00% | |||
| Two | 5 | 1 | ||
| 83.30% | 16.70% | |||
| Three | 40 | 3 | ||
| 93.00% | 7.00% | |||
| Four | 17 | 6 | ||
| 73.90% | 26.10% | |||
| Five | 1 | 0 | ||
| 100.00% | 0.00% | |||
| Variables | Cytological Atypia | Fisher exact test P value | ||
| No | Yes | |||
| Chemotherapy number of doses | One | 1 | 0 | 0.743* |
| 100.00% | 0.00% | |||
| Two | 3 | 0 | ||
| 100.00% | 0.00% | |||
| Three | 20 | 3 | ||
| 87.00% | 13.00% | |||
| Four | 9 | 3 | ||
| 75.00% | 25.00% | |||
| Five | 1 | 0 | ||
| 100.00% | 0.00% | |||
| Radiotherapy number of doses | One | 1 | 0 | 0.028** |
| 100.00% | 0.00% | |||
| Two | 2 | 1 | ||
| 66.70% | 33.30% | |||
| Three | 20 | 0 | ||
| 100.00% | 0.00% | |||
| Four | 8 | 3 | ||
| 72.70% | 27.30% | |||
Results
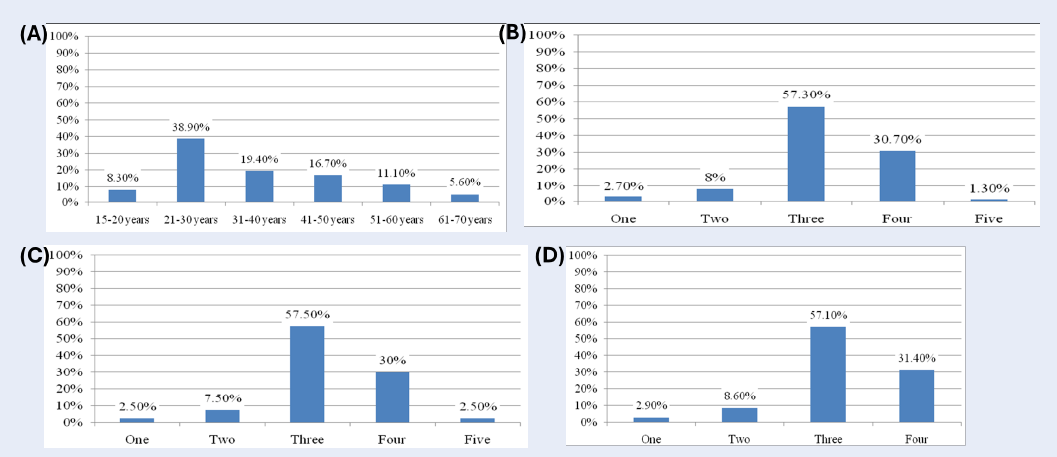
A total of 111 buccal smear samples were included in the study, with participants' ages ranging from 15 to 70 years. Of these samples, 75 were from patients undergoing radiotherapy and/or chemotherapy, while 36 were from healthy individuals. Pap stain analysis revealed that 15.3% of the samples exhibited acute inflammation, with 18.7% found in the treatment group and 8.3% in healthy individuals (Figure 1).
Analysis of cytological findings based on chemotherapy dose showed varying degrees of atypia, with 3 cases of mild atypia observed in the 3-dose group, 3 cases in the 4-dose group (one patient with moderate atypia and two patients with severe atypia). In the radiation therapy group, no cytological changes were seen after the first dose, while one case of moderate atypia was noted after the second dose and three cases of atypia (one severe, two mild) after the fourth dose.
The distribution of study participants by gender showed 27% male and 73% female. Age-wise distribution included 7.2% in the 15-20 age group, 18.9% in the 21-30 age group, 24.3% in the 31-40 age group, 27% in the 41-50 age group, 18% in the 51-60 age group, and 4.5% in the 61-70 age group (Figure 1).
Furthermore, correlation analysis between cytological diagnosis and treatment doses revealed significant findings. For instance, chemotherapy dose showed a statistically insignificant correlation with cytological diagnosis (p > 0.05), while radiation therapy dose exhibited a statistically significant correlation (p < 0.05) with atypical cellular changes (Table 1, Table 2, Table 3, Table 4).
Overall, the results indicate a relationship between chemotherapy, radiation therapy, and oral cytological changes, with a higher incidence of atypia associated with increased treatment doses. Additionally, findings suggest variations in cytological diagnosis between the treatment group and healthy individuals.
Discussion
The current study examined 111 samples from patients undergoing chemotherapy and/or radiation therapy in Khartoum state, aged between 15 and 70 years. Of the 111 samples, 75 were from patients undergoing chemotherapy and/or radiation therapy, while 36 were from healthy individuals. The results indicated a mean of 43 with a standard deviation of 12 in cases, and a mean of 35 with a standard deviation of 14 in healthy individuals, yielding a p-value of 0.011.
Our study is supported by several previous reports. For instance, Ahmed and Elemirri's study identified infections and cellular infiltration post-cancer treatment8. Minhas et al. reported various nuclear atypia in normal buccal mucosa in patients receiving chemoradiotherapy9, while Naidu et al. in 2004 observed oral mucositis complications associated with radiation therapy and chemotherapy9.
In Pap smears, analysis revealed that out of 111 samples, 17 samples (15.3%) from both cases and healthy individuals exhibited acute inflammation, 14 samples (18.7%) were under treatment, and 3 samples (8.3%) were from healthy individuals. Our study findings are in line with those of Ahmed and Elemirri8. Notably, Pap stain serves as an effective method for detecting cellular changes in the buccal mucosa10.
Conclusions
Analysis of Papanicolaou (Pap) stain indicates that chemotherapy and radiation therapy have varying effects on atypical cellular changes and inflammatory infiltration in the oral cavity. Specifically, research shows that the incidence of atypia increases with higher doses of chemotherapy and radiation therapy.
Abbreviations
COVID-19: Coronavirus Disease 2019, DPX: Dibutylphthalate Polystyrene Xylene, KMOH-RES: Khartoum State Ministry of Health Research Ethics Section, PAP: Papanicolaou, SPSS: Statistical Package for Social Sciences
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Khartoum State Ministry of Health Innovation, Development and Scientific Research Committee in accordance with the Declaration of Helsinki Principles, and the agreement was taken from all hospital administrations before taking sample and collecting data. The patient’s information were highly secured and not used for other purposes than scientific inquiry. Each participant was asked to sign a written ethical consent form during the interview, before the specimen was taken. The informed ethical consent form was designed and approved by the ethical committee of the Khartoum State Ministry of Health. Ethical clearance code number: KMOH-RES/06-01-2022 Date: 11/12/2022.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Mummudi
N.,
Agarwal
J.P.,
Chatterjee
S.,
Mallick
I.,
Ghosh-Laskar
S.,
Oral Cavity Cancer in the Indian Subcontinent - Challenges and Opportunities. Clinical Oncology (Royal College of Radiologists (Great Britain)).
2019;
31
(8)
:
520-8
.
View Article PubMed Google Scholar -
National Institute of Dental and Craniofacial Research (NIDCR). .https://www.nih.gov/about-nih/what-we-do/nih-almanac/national-institute-dental-craniofacial-research-nidcr.
.
-
Ibrahim
E.M.,
al-Mulhim
F.A.,
Effect of granulocyte-macrophage colony-stimulating factor on chemotherapy-induced oral mucositis in non-neutropenic cancer patients. Medical Oncology (Northwood, London, England).
1997;
14
(1)
:
47-51
.
View Article PubMed Google Scholar -
Johnson
N.W.,
Ranasinghe
A.W.,
Warnakulasuriya
K.A.,
Potentially malignant lesions and conditions of the mouth and oropharynx: natural history cellular and molecular markers of risk. European Journal of Cancer Prevention.
1993;
2
:
31-51
.
View Article PubMed Google Scholar -
Mehrotra
R.,
Goel
N.,
Singh
M.,
Kumar
D.,
Radiation-related cytological changes in oral malignant cells. Indian Journal of Pathology & Microbiology.
2004;
47
(3)
:
343-7
.
PubMed Google Scholar -
Mehrotra
R.,
Gupta
A.,
Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Molecular cancer.
2006;
5
(11)
.
View Article Google Scholar -
Seiwert
T.Y.,
Salama
J.K.,
Vokes
E.E.,
The chemoradiation paradigm in head and neck cancer. Nature Clinical Practice. Oncology.
2007;
4
(3)
:
156-71
.
View Article PubMed Google Scholar -
Ahmed
H.G.,
Elemirri
D.A.,
Assessment of oral cytological changes associated with exposure to chemotherapy and/or radiotherapy. CytoJournal.
2009;
6
:
8
.
View Article PubMed Google Scholar -
Minhas
S.,
Kashif
M.,
Altaf
W.,
Afzal
N.,
Nagi
A.H.,
Concomitant-chemoradiotherapy-associated oral lesions in patients with oral squamous-cell carcinoma. Cancer Biology & Medicine.
2017;
14
(2)
:
176-82
.
View Article PubMed Google Scholar -
Ahmad
H.G.,
Ebnoof
S.G.,
Hussin
M.O.,
Gbreel
A.Y.,
Oral epithelial atypical changes in apparently healthy oral mucosa exposed oral mucosa to smoking, alcohl, peppers and hot meals, using the AgNOR and papanicolaou staining techniques. Diagnostic Cytopathology.
2009;
38
(7)
:
489-95
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 5 (2024)
Page No.: 6421-6425
Published on: 2024-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2321 times
- PDF downloaded - 853 times
- XML downloaded - 155 times
 Biomedpress
Biomedpress



