Abstract
Introduction: Dual antiplatelet therapy (DAPT), incorporating aspirin and a P2Y12 receptor inhibitor, is advised for patients experiencing acute coronary syndrome and for those with coronary artery stents, aiming to mitigate the risk of cardiac events attributable to thromboses. Nevertheless, the therapeutic challenge lies within the narrow therapeutic window where either excessive or deficient platelet reactivity (PR) during DAPT can predispose to thrombotic events or hemorrhage, respectively. This underscores the criticality of attaining an optimal PR level throughout the course of therapy. Accordingly, this study was conducted to evaluate PR among Vietnamese patients administered with DAPT, examining the implications for coronary artery disease management. This study aimed to evaluate PR in the context of aspirin therapy, utilizing the VerifyNow system, and to evaluate PR in the context of clopidogrel therapy, also utilizing the VerifyNow system.
Methods: A prospective, cross-sectional, descriptive analysis was executed over the period April 2020 to August 2020. In total, 55 patients diagnosed with coronary artery disease and receiving DAPT comprising aspirin and clopidogrel were enrolled from the Interventional Cardiology Department at Cho Ray Hospital. PR was assessed via blood samples using PRU and ARU measurement kits for clopidogrel and aspirin, respectively. Data were compiled and subjected to analysis employing SPSS version 20. The delineation of high PR was set at >550 ARU for aspirin and >208 PRU for clopidogrel.
Results: The cohort's mean age stood at 65.7 years, with a male predominance (male-to-female ratio of 1.6). Mean PR values for clopidogrel and aspirin were 158.966.8 and 462.578, respectively. A significant 25.5% of the population demonstrated high PR to clopidogrel, whereas aspirin-related high PR was observed in 7.1% of the patients.
Conclusion: In the subset of Vietnamese patients undergoing coronary stent implantation, a notable proportion exhibited high PR in response to clopidogrel, aligning with observations in international cohorts. Elevated age emerged as a contributing factor for increased PR, while diabetes was implicated in diminished platelet responsiveness. This investigation enriches the comprehension of PR dynamics in patients subjected to DAPT, suggesting a pressing need for additional studies to corroborate these findings and explore therapeutic adjustments.
Introduction
Dual antiplatelet therapy, which consists of aspirin and a P2Y12 receptor inhibitor, is recommended for patients with acute coronary syndrome and for those undergoing coronary artery stent implantation to prevent cardiac events related to blood clot formation. Clopidogrel is the most frequently used P2Y12 inhibitor. However, unlike ticagrelor, which is immediately active upon administration, both prasugrel and clopidogrel require metabolic activation to inhibit platelet aggregation effectively. This metabolic process is dependent on several hepatic enzymes, including CYP2C19, CYP3A4/5, CYP2B6, CYP2C9, and CYP1A2. Notably, mutations in the CYP2C19 gene can lead to a loss of enzymatic function, thereby reducing the metabolism of clopidogrel. This reduction in metabolism can result in decreased effectiveness of clopidogrel in inhibiting platelet aggregation, which may be associated with adverse cardiovascular outcomes, including stent thrombosis. CYP2C19 is identified as the critical enzyme in the metabolism of clopidogrel1. Furthermore, a high prevalence of mutations in this enzyme has been reported in the Asian population, affecting up to 60% of individuals2. This diminished platelet inhibition manifests as high platelet reactivity (HPR) in platelet function tests. On the contrary, low platelet reactivity (LPR) or an excessive response to antiplatelet therapy increases the risk of bleeding complications. Therefore, optimizing platelet reactivity is crucial within the therapeutic window. To address this, we conducted research to assess platelet reactivity in Vietnamese patients with coronary artery disease who were receiving dual antiplatelet therapy with aspirin and clopidogrel.
Methods
Study Design
This research was conducted as a cross-sectional descriptive analysis.
Study Participants
The inclusion criteria targeted patients receiving treatment within the Interventional Cardiology Department at Cho Ray Hospital for coronary artery disease that required dual antiplatelet therapy. This therapy consisted of Aspirin at a minimum dosage of 162 mg at least 3 hours prior to testing or 81 mg for at least 5 days before testing, coupled with Clopidogrel administered at a minimum dosage of 300 mg at least 3 hours prior to testing or 75 mg for at least 5 days before testing. The exclusion criteria eliminated patients with a history of myocardial infarction (MI) or prior coronary artery interventions, patients who experienced bleeding events or mortality, and those not consenting to participate in the study.
Procedure
Prior to undergoing cardiac catheterization and percutaneous coronary intervention, 4ml of the patient's blood was collected into anticoagulant tubes. These tubes were then incubated at room temperature for 10 minutes before undergoing platelet reactivity testing utilizing an aspirin-specific (ARU test) and a clopidogrel-specific (PRU test) kit. Results from the platelet reactivity tests were documented for each participant.
Diagnostic Criteria
Coronary artery disease
The diagnosis follows the 2019 criteria established by the American College of Cardiology and the European Society of Cardiology for stable angina and acute coronary syndromes, which include unstable angina, non-ST elevation myocardial infarction, and ST elevation myocardial infarction.
Diabetes mellitus (DM)
The condition is diagnosed based on a pre-existing patient history or as newly diagnosed DM, adhering to the criteria defined by the American Diabetes Association in 2013.
Hypertension (HTN)
Diagnosis is founded on pre-existing patient history or as newly identified HTN, in line with the Vietnam Heart Association's 2014 guidelines for prevention, detection, evaluation, and treatment of high blood pressure.
Dyslipidemia (DL)
DL definitions and classifications adhere to the criteria from the NCEP (ATP III) study.
Smoking (SM)
Smoking status is diagnosed using the COMMIT study criteria, differentiating between current smokers (those with a history of smoking 100 or more cigarettes or those who have ceased smoking within the last 5 years) and non-smokers (those who have never smoked or have quit smoking for more than 5 years prior to the onset of disease).
Heart failure with preserved ejection fraction (HFpEF)
Diagnosis aligns with the diagnostic criteria from the American College of Cardiology in 2016.
Platelet reactivity was determined using the VerifyNow test, a point-of-care platelet function test known for its convenience and proven accuracy, showing high congruence with the gold-standard LTA test among available market options. High platelet reactivity (HPR) is identified when results exceed 208 for clopidogrel (PRU > 208) and 550 for aspirin (ARU > 550), whereas low platelet reactivity (LPR) for clopidogrel is marked by results below 95 (PRU < 95). Therapeutic range platelet reactivity for clopidogrel is delineated between 95 and 208, and for aspirin as less than 550 (ARU < 550).
Variables
Considered variables include Age, Gender, BMI, Hypertension (HTN), Smoking status (SM), Diabetes Mellitus (DM), Dyslipidemia (DL), Heart failure, and platelet reactivity to Aspirin (ARU) and Clopidogrel (PRU), with cutoff points established at 550 for high aspirin platelet reactivity and 208 for high clopidogrel platelet reactivity. The cutoff for low clopidogrel platelet reactivity is set at 95.
Data Processing and Analysis
Data analysis was executed using SPSS 20 software, with results presented as mean values (± standard deviation) or median (interquartile range), and as percentages (%).
| Characteristics | Frequency (Percentage) |
|---|---|
| Age (Mean ± SD) | 65.7 (±9.9) |
| Gender Male | 34 (62%) |
| Weight (Mean ± SD) | 60.6 (±1.3) |
| Hypertension | 49 (89%) |
| Diabetes Mellitus | 10 (18%) |
| Smoking | 36 (65%) |
| Dyslipidemia | 53 (96%) |
| Heart Failure | 12 (22%) |
| Clinical Presentation | |
| Unstable Angina | 17 (31%) |
| Non-ST Elevation MI | 3 (5%) |
| ST Elevation MI | 16 (29%) |
| Non-Fatal MI | 19 (35%) |
| Sample | Male | Female | p | |
| (mean ± SD) | ||||
| Age | 65.7 ± 9.91 | 63.5 ± 8.7 | 69.1 ± 11 | 0.039 |
| Sample | Male | Female | p | |
| (mean ± SD) | ||||
| BMI (kg/m 2 ) | 23.2 ± 2.9 | 23.6 ± 3.2 | 22.4 ± 2.2 | 0.13 |
| Index | Mean (± SD) | Min | Max | Median | Interquartile Range |
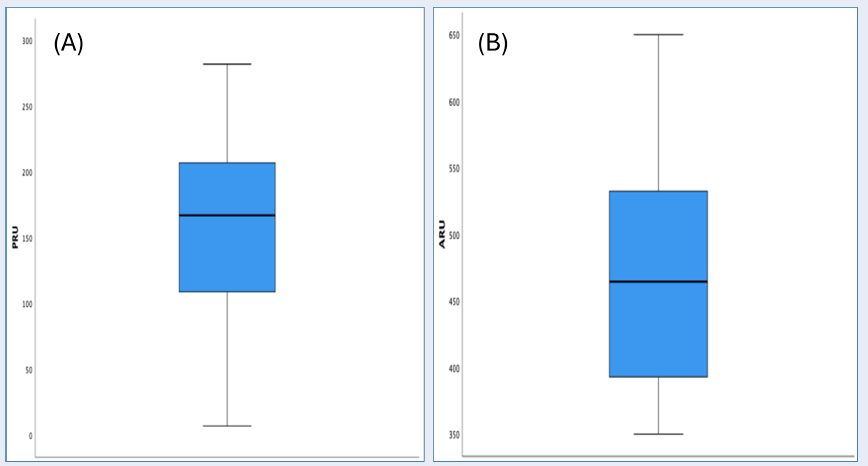
| PRU | 159 (±69) | 7 | 282 | 167 | 101 |
| ARU | 463 (±78) | 350 | 650 | 465 | 145 |
| PRU | ARU | ||||
| Cut-off | N | % | Cut-off | N | % |
| ≥ 208 | 14 | 25.5 | ≥ 550 | 2 | 7.1 |
| 95 - 208 | 32 | 58.2 | < 550 | 26 | 92.9 |
| < 95 | 9 | 16.4 | |||
| Characteristics | Normal/Low Platelet Reactivity (N=41) | High Platelet Reactivity (N=14) | p |
|---|---|---|---|
| Characteristics | 66.5 | 63.2 | <0.001 |
| Age (Mean ± SD) | 28 | 6 | 0.117 |
| Gender Male | 61.7 | 57.4 | 0.12 |
| Weight (Mean ± SD) | 29 | 7 | 0.159 |
| Hypertension | 35 | 14 | 0.129 |
| Diabetes Mellitus | 39 | 14 | 0.399 |
| Smoking | 5 | 5 | 0.048 |
| Dyslipidemia | 7 | 5 | 0.144 |
| Clinical Presentation | |||
| Unstable Angina | 12 | 5 | 0.742 |
| Non-ST Elevation MI | 3 | 0 | 1 |
| ST Elevation MI | 12 | 4 | 1 |
| Non-Fatal MI | 12 | 5 | 0.099 |
Results
Study Population Characteristics
This study comprised 55 patients diagnosed with coronary artery disease undergoing percutaneous coronary intervention. The mean age of the participants was 65.7 years, with a predominant male representation of 62% (Table 1). The average age of male participants was significantly lower than that of their female counterparts, a disparity that was statistically significant (p=0.039, student's t-test) (Table 2). The mean body mass index (BMI) across the cohort was 23, with no significant difference observed between genders (student's t-test) (Table 3).
Clinical characteristics of the study population revealed a high prevalence of hypertension and dyslipidemia, followed by smoking, which affected approximately two-thirds of the participants. Diabetes and heart failure were present in about one-fifth of the cohort. Furthermore, patients were categorized based on specific medical conditions, with the largest subgroup having NMCTSTCL, followed by ĐTNÔĐ and NMCTKSTCL, while the smallest subgroup was identified as ĐTNKÔĐ.
Platelet Reactivity to Clopidogrel and Aspirin
In terms of platelet reactivity, measured by platelet reactivity unit (PRU) and aspirin reaction unit (ARU), the PRU levels varied between 7 and 282, with a median of 167, whereas ARU levels ranged from 350 to 650, with a median of 465 (Table 4). Clopidogrel response categorization using predefined cutoffs showed that 25.5% of the patients exhibited high PRU levels (≥ 208), 58.2% were within the therapeutic range (95-208), and 16.4% had low PRU levels (< 95) (Table 5 ). For aspirin, based on the cutoffs for classifying PRU levels, high (>550) and within therapeutic range (< 550), the distributions were 7.2% and 92.9%, respectively.
An analysis of the clinical characteristics among patients exhibiting high PRU levels on clopidogrel, indicative of suboptimal drug response, revealed a significantly lower mean age (p < 0.001) and an increased prevalence of type 2 diabetes mellitus (p = 0.048) in comparison with patients achieving therapeutic PRU levels (Table 6).
Discussion
Clopidogrel Platelet Reactivity
In our investigation, the mean platelet reactivity in response to clopidogrel administration was documented at 159 ± 69 P2Y12 Reaction Units (PRU), exhibiting a broad range from 7 to 282 PRU. This contrasts with the findings from the study by Fakilahiel et al.3, 4, conducted on a similar cohort of patients with coronary artery disease, which reported an average of 178 ± 88 PRU and a range from 4 to 385 PRU. Our results indicate a relative decrease in platelet reactivity and a narrower distribution in comparison. Several international studies have highlighted that the prevalence of resistance to clopidogrel is notably higher among Asian populations than those in European and American groups, challenging the prevalent assumptions. This discrepancy may stem from differences in racial composition and clinical profiles of the studied populations. A more definitive metric of interest has been the incidence of elevated clopidogrel platelet reactivity, frequently linked to adverse outcomes such as all-cause mortality, non-fatal myocardial infarctions, stent thrombosis, and ischemic strokes5, 6. In our research, a cutoff of 208 PRU was employed to delineate between high and normal clopidogrel platelet reactivity, given its established utility in predicting cardiovascular events across multiple large-scale international studies. The prevalence of patients manifesting high platelet reactivity was 25.5%, aligning with global observations on suboptimal platelet inhibition7, 8, 9. This rate is comparable to that found in the 3T/2R study10 and lower than those reported in TRIGGER-PCI11, ARCTIC12, and GRAVITAS13.
Pertaining to the correlation of clinical factors such as age, weight, and diabetes mellitus with high PRU levels, our analysis and other studies, including the GRAVITAS trial, show statistically significant outcomes that suggest heavier patients tend to have higher PRU levels (p < 0.001). Despite varying BMI associations across different studies, our findings did not replicate this trend, possibly due to the specific characteristics of our study population and the need for more extensive data sets. A detailed examination of individuals with elevated PRU levels demonstrated a significantly younger average age in this subgroup (p < 0.001), consistent with characterized trends from other research and a higher prevalence of diabetes mellitus (p = 0.048), underlining the potential contribution of hyperglycemia, insulin resistance, or deficiency to heightened inflammatory responses and endothelial dysfunction14.
Aspirin Platelet Reactivity
The VerifyNow assay employing arachidonic acid, for assessing the antiplatelet effects of aspirin, proved equivalent to the light transmission aggregometry (LTA), the accepted standard for platelet function assessment15. Our data indicated an average aspirin reactivity unit (ARU) of 463 ± 78, spanning from 350 to 650 ARU, with a high platelet reactivity occurrence of 7.1%. This rate is in line with findings from the ADAPT-DES study16, modestly surpasses the 2.1% observed in Lee's Korean cohort17, and is significantly lower than the 13.5% reported in the 3R/2T study10. The term "aspirin resistance" frequently misused to describe patients with elevated platelet reactivity under aspirin therapy scarcely reflects the true resistance mechanism, which involves the failure of aspirin to acetylate COX-1, a phenomenon established as exceedingly rare by laboratory analyses. In effect, high platelet reactivity during aspirin therapy may largely attribute to suboptimal drug absorption or might improve with increased aspirin dosages18, 19, 20, without necessitating prior assessment of platelet responses to aspirin.
Limitations and Conclusion
The study’s limitations include a potentially non-generalizable finding due to the sample size or selection criteria, the cross-sectional design precluding causal relationships, and the lack of longitudinal data to evaluate the temporal evolution of platelet reactivity. Future research should focus on elucidating mechanisms underpinning high platelet reactivity in specific patient groups, evaluating alternative antiplatelet strategies, and exploring long-term outcomes associated with heightened platelet reactivity. Approximately a quarter of patients with coronary artery disease undergoing angiography or intervention in this study presented with elevated clopidogrel platelet reactivity, corresponding with global reports. Notably, advanced age and diabetes mellitus emerged as risk factors for increased platelet reactivity. Platelet reactivity testing in patients experiencing cardiac events or suspected of clopidogrel resistance could inform tailored therapeutic approaches.
Abbreviations
ARU: Aspirin Reaction Unit, COMMIT: ClOpidogrel and Metoprolol in Myocardial Infarction Trial, CYP1A2: Designations for specific cytochrome P450 enzyme involved in drug metabolism, CYP2B6: Designations for specific cytochrome P450 enzyme involved in drug metabolism, CYP2C19: Designations for specific cytochrome P450 enzyme involved in drug metabolism, CYP2C9: Designations for specific cytochrome P450 enzyme involved in drug metabolism, CYP3A4/5: Designations for specific cytochrome P450 enzymes involved in drug metabolism, DAPT: Dual Antiplatelet Therapy, DL: Dyslipidemia, DM: Diabetes Mellitus, HFpEF: Heart Failure with Preserved Ejection Fraction, HTN: Hypertension, LTA: Light Transmission Aggregometry, MI: Myocardial Infarction, NCEP (ATP III): National Cholesterol Education Program (Adult Treatment Panel III), PR: Platelet Reactivity, PRU: P2Y12 Reaction Unit, SM: Smoking, SPSS: Statistical Package for the Social Sciences
Acknowledgments
Phan Thi Bach Tuyet, RN (Cho Ray hospital) was involved in performing the tests.
Author’s contributions
Nghia T N, MD: Conceptualization; Writing - original draft; Critical Review. My H T N, MD: Formal analysis; Writing - original draft; Manuscrift editing; Critical Review. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Following information must be provided in order for this form to be processed accurately. Patients have the right to refuse to sign this consent form; refusal to sign this form will not affect the care in any way. ”I hereby give my consent for images or other clinical information relating to my case to be reported in a medical publication. I understand that my name and initials will not be published and that efforts will be made to conceal my identity, but that anonymity cannot be guaranteed. I understand that the material may be published in a journal, website or other form of publication. At a result, I understand that the material may be seen by the general public. I understand that the material may be included in medical books.”
Competing interests
The authors declare that they have no competing interests.
References
-
Cavallari
L.H.,
Jeong
H.,
Bress
A.,
Role of cytochrome P450 genotype in the steps toward personalized drug therapy. Pharmacogenomics and Personalized Medicine.
2011;
4
:
123-36
.
View Article PubMed Google Scholar -
Myrand
S.P.,
Sekiguchi
K.,
Man
M.Z.,
Lin
X.,
Tzeng
R.Y.,
Teng
C.H.,
Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clinical Pharmacology and Therapeutics.
2008;
84
(3)
:
347-61
.
View Article PubMed Google Scholar -
Nakahara
H.,
Sarker
T.,
Dean
C.L.,
Skukalek
S.L.,
Sniecinski
R.M.,
Cawley
C.M.,
A Sticky Situation: Variable Agreement Between Platelet Function Tests Used to Assess Anti-platelet Therapy Response. Frontiers in Cardiovascular Medicine.
2022;
9
:
899594
.
View Article PubMed Google Scholar -
Mshelbwala
F.S.,
Hugenberg
D.W.,
Kreutz
R.P.,
Impact of Routine Platelet Reactivity Testing with VerifyNow Assay on Antiplatelet Choice After Percutaneous Coronary Intervention. Clinical Pharmacology.
2020;
12
:
35-41
.
View Article PubMed Google Scholar -
Breet
N.J.,
van Werkum
J.W.,
Bouman
H.J.,
Kelder
J.C.,
Ruven
H.J.,
Bal
E.T.,
Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. Journal of the American Medical Association.
2010;
303
(8)
:
754-62
.
View Article PubMed Google Scholar -
Lee
S.J.,
Cha
J.J.,
Jeong
Y.H.,
Hong
S.J.,
Ahn
C.M.,
Kim
J.S.,
Investigators
PTRG,
Platelet Reactivity and Clinical Outcomes After Drug-Eluting Stent Implantation: Results From the PTRG-DES Consortium. JACC: Cardiovascular Interventions.
2022;
15
(22)
:
2253-65
.
View Article PubMed Google Scholar -
Bonello
L.,
Tantry
U.S.,
Marcucci
R.,
Blindt
R.,
Angiolillo
D.J.,
Becker
R.,
Working Group on High On-Treatment Platelet Reactivity
Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. Journal of the American College of Cardiology.
2010;
56
(12)
:
919-33
.
View Article PubMed Google Scholar -
Brar
S.S.,
ten Berg
J.,
Marcucci
R.,
Price
M.J.,
Valgimigli
M.,
Kim
H.S.,
Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. Journal of the American College of Cardiology.
2011;
58
(19)
:
1945-54
.
View Article PubMed Google Scholar -
Combescure
C.,
Fontana
P.,
Mallouk
N.,
Berdague
P.,
Labruyere
C.,
Barazer
I.,
CLOpidogrel
Vascular ISchemic Events Meta-analysis Study Group
Clinical implications of clopidogrel non-response in cardiovascular patients: a systematic review and meta-analysis. Journal of Thrombosis and Haemostasis.
2010;
8
(5)
:
923-33
.
View Article PubMed Google Scholar -
Valgimigli
M.,
Campo
G.,
de Cesare
N.,
Meliga
E.,
Vranckx
P.,
Furgieri
A.,
Tailoring Treatment With Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel (3T/2R) Investigators
Intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double-blind, prospective, randomized Tailoring Treatment with Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel study. Circulation.
2009;
119
(25)
:
3215-22
.
View Article PubMed Google Scholar -
Trenk
D.,
Stone
G.W.,
Gawaz
M.,
Kastrati
A.,
Angiolillo
D.J.,
Müller
U.,
A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. Journal of the American College of Cardiology.
2012;
59
(24)
:
2159-64
.
View Article PubMed Google Scholar -
Collet
J.P.,
Cuisset
T.,
Rangé
G.,
Cayla
G.,
Elhadad
S.,
Pouillot
C.,
Investigators
ARCTIC,
Bedside monitoring to adjust antiplatelet therapy for coronary stenting. The New England Journal of Medicine.
2012;
367
(22)
:
2100-9
.
View Article PubMed Google Scholar -
Price
M.J.,
Berger
P.B.,
Teirstein
P.S.,
Tanguay
J.F.,
Angiolillo
D.J.,
Spriggs
D.,
Investigators
GRAVITAS,
Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. Journal of the American Medical Association.
2011;
305
(11)
:
1097-105
.
View Article PubMed Google Scholar -
Schneider
D.J.,
Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care.
2009;
32
(4)
:
525-7
.
View Article PubMed Google Scholar -
Cattaneo
M.,
Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. Journal of Thrombosis and Haemostasis.
2007;
5
:
230-7
.
View Article PubMed Google Scholar -
Stone
G.W.,
Witzenbichler
B.,
Weisz
G.,
Rinaldi
M.J.,
Neumann
F.J.,
Metzger
D.C.,
Investigators
ADAPT-DES,
Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet.
2013;
382
(9892)
:
614-23
.
View Article PubMed Google Scholar -
Lee
D.H.,
Arat
A.,
Morsi
H.,
Shaltoni
H.,
Harris
J.R.,
Mawad
M.E.,
Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test: a single-center experience. AJNR. American Journal of Neuroradiology.
2008;
29
(7)
:
1389-94
.
View Article PubMed Google Scholar -
Gross
L.,
Aradi
D.,
Sibbing
D.,
Platelet Function Testing in Patients on Antiplatelet Medications. Seminars in Thrombosis and Hemostasis.
2016;
42
(3)
:
306-20
.
View Article PubMed Google Scholar -
Pettersen
A.A.,
Seljeflot
I.,
Abdelnoor
M.,
Arnesen
H.,
High on-aspirin platelet reactivity and clinical outcome in patients with stable coronary artery disease: results from ASCET (Aspirin Nonrespon- siveness and Clopidogrel Endpoint Trial). Journal of the American Heart Association.
2012;
1
(3)
:
e000703
.
View Article PubMed Google Scholar -
Grosser
T.,
Fries
S.,
Lawson
J.A.,
Kapoor
S.C.,
Grant
G.R.,
FitzGerald
G.A.,
Drug resistance and pseudoresistance: an unintended consequence of enteric coating aspirin. Circulation.
2013;
127
(3)
:
377-85
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 5 (2024)
Page No.: 6426-6433
Published on: 2024-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2871 times
- PDF downloaded - 907 times
- XML downloaded - 139 times
 Biomedpress
Biomedpress




