Abstract
Background: Acquired platelet dysfunction with eosinophilia (APDE) is characterized by a temporary impairment in platelet function accompanied by significant eosinophilia. This condition, also known as "non-thrombocytopenic purpura with eosinophilia," primarily affects children in the South-East Asian region, presenting as a bleeding disorder.
Case Presentation: In this case series, we report on three patients who were admitted to our hospital displaying skin bruising, despite being previously healthy, with no history of drug use or recent travel. These patients exhibited classic signs of APDE, and interestingly, some showed spontaneous recovery without the need for medical intervention. Diagnostic evaluations revealed an increased eosinophil count, whereas the basic hemostatic parameters and platelet counts remained within normal limits. However, platelet aggregation studies indicated abnormalities.
Conclusion: It is crucial to identify this benign disorder promptly, as providing reassurance to patients and their families plays a critical role in the management of APDE.
Introduction
Acquired Platelet Dysfunction with Eosinophilia (APDE), as the name suggests, is a transient hematological disorder characterized by eosinophilia and a normal platelet count. This condition was initially described by Prof. Chulee Mitrakul in 1975 and later designated as APDE by Prof. Parttraporn Isarangkura in 1977. It is also referred to as ‘non-thrombocytopenic purpura with eosinophilia’1. APDE is distinguished by temporary bleeding manifestations, particularly spontaneous bruising, in individuals who are otherwise healthy. A hallmark of APDE is spontaneous ecchymosis, which is reported in nearly all patients2. The diagnostic criteria for APDE encompass three essential components: clinical presentation characterized by spontaneous ecchymosis occurring on the trunk or extremities, hemogram results indicating eosinophilia, and laboratory evidence confirming platelet dysfunction.
Case Report: A 9-Year-Old Malay Female with Spontaneous Bruising and Eosinophilia
A previously healthy 9-year-old Malay girl was presented with spontaneous bruising on bilateral lower limbs and her forearm, persisting for two weeks without any preceding trauma. The patient had no personal or family history of bleeding tendencies and was not under any medication at the time of presentation. A physical examination disclosed multiple bruises, both old and new, ranging from 2 to 5 cm in diameter across the reported areas.
Laboratory investigations revealed an elevated white blood cell (WBC) count, mild anemia, slight thrombocytosis, and notably severe eosinophilia at 49%. Coagulation profiles were within normal limits. Peripheral blood smear analysis identified hypochromic microcytic red blood cells, occasional dysplastic eosinophils, and reactive lymphocytes. Stool examinations for ova, cysts, and culture for common enteropathogens including Salmonella, Shigella, Enteropathogenic E. coli, Enterohemorrhagic E. coli, Aeromonas hydrophila, and Campylobacter jejuni yielded negative results. Autoimmune screening, including anti-double stranded DNA antibodies, was also negative. Platelet aggregation tests indicated defective aggregation in response to adenosine diphosphate (ADP) and collagen.
Despite the absence of positive stool culture findings, the patient was treated with anthelmintic therapy (syrup Albendazole). This intervention led to the resolution of bruising, cessation of new bleeding events, and a significant decrease in eosinophil count after a three-month follow-up period. The notable reduction in eosinophilia, coupled with the clinical outcome, suggests a parasitic infestation as the probable cause, which was not identified through stool analysis.
Case 2
A 69-year-old Malay male, with a history of diabetes mellitus, presented with swelling and bruises on his left hand, persisting for three days. These symptoms developed after the patient lifted a heavy object. He had no history of bleeding tendencies, nor a family history of bleeding disorders. The patient reported consuming traditional herbal supplements a month prior to the onset of these symptoms. Physical examination revealed swelling over the left dorsal hand, approximately 3x3 cm in size, accompanied by ecchymoses extending from the third and fourth fingers to the lateral aspect of the wrist and forearm.
Full blood count (FBC) indicated a normal white blood cell count, mild anemia, and a normal platelet count, but with a moderate eosinophilia at 25%. Coagulation screening results were within normal limits. The blood film presented normochromic normocytic red cells with the occasional presence of dysplastic eosinophils and a few large platelets. Platelet aggregation tests disclosed defective aggregation in response to low dose adenosine diphosphate (ADP) and ristocetin.
Radiographic imaging of the hand did not reveal any fractures or signs of osteomyelitis. The patient was not started on any pharmacological treatments but was advised to undergo close monitoring of his FBC and to seek follow-up if his symptoms worsened or recurred. He was also advised to discontinue the use of traditional herbs. In a follow-up consultation two months later, the patient's symptoms of swelling and bruising in the left hand had resolved, and there was a noted decrease in the eosinophil count. The likely cause of the patient's eosinophilia appears to be an adverse reaction to the traditional herbal supplements he had consumed.
Case 3
Case 3 involved a 23-year-old Malay male with no previous medical conditions who presented with multiple cases of bruising across his upper and lower limbs over the course of one month. These symptoms developed subsequent to receiving a COVID-19 vaccination. The patient reported no prior incidence of bruising or bleeding tendencies, and there was no familial history of bleeding disorders. Upon physical examination, the patient exhibited non-tender bruises on the right upper limb measuring 10x5 cm and on the left knee and thigh, each measuring 5x5 cm, along with multiple smaller bruises across his back.
Laboratory analysis, including a full blood count, indicated a normal white blood cell (WBC) count, mild anemia, a normal platelet count, and a mild eosinophilia at 11.5%. Coagulation screening results were within normal limits. Examination of the blood film revealed normochromic normocytic red blood cells with eosinophilia and normal platelet morphology. Platelet aggregation tests identified defective aggregation responses to ADP, collagen, ristocetin, and arachidonic acid. At a follow-up three months later, the patient’s bruising had resolved, accompanied by a decrease in eosinophil count. The eosinophilia in this patient is likely related to the recent vaccination. A summary of investigations for all three cases is provided in Table 1.
| Case 1 | Case 2 | Case 3 | Normal ranges | |
|---|---|---|---|---|
| Demographic data Gender/ age (years) | Girl/9 | Male /69 | Male /23 | |
| Full blood count | ||||
| WBC (x10 9 /L) | 20.2 *(3.4-10.1) | 5.78 | 10.15 | 3.80-9.70 |
| Hb (g/dL) | 9.0 *(11.6-15.1) | 11.2 | 12.9 | 13.5-17.4 |
| Platelet (x 10 9 /L) | 535 *(158-410) | 239 | 194 | 167-376 |
| Eosinophils (x 10 9 /L) Initial Presentation | 9.9 *(0.03-0.28) | 1.50 | 1.17 | 0.08-0.28 |
| Follow Up | 1.45 *(0.03-0.28) | 1.28 | 0.74 | |
| Platelet Aggregation Test | ||||
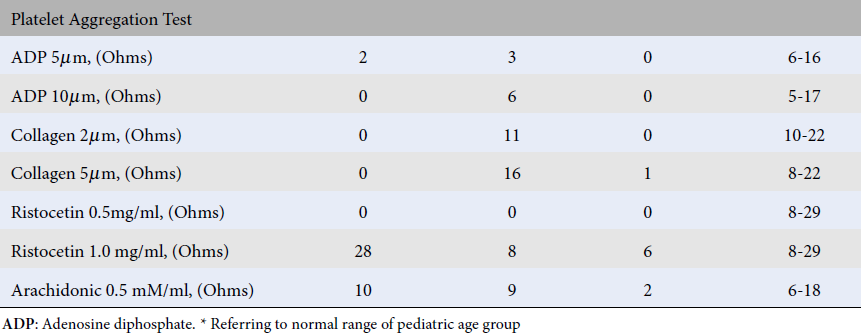
| ADP 5µm, (Ohms) | 2 | 3 | 0 | 6-16 |
| ADP 10µm, (Ohms) | 0 | 6 | 0 | 5-17 |
| Collagen 2µm, (Ohms) | 0 | 11 | 0 | 10-22 |
| Collagen 5µm, (Ohms) | 0 | 16 | 1 | 8-22 |
| Ristocetin 0.5mg/ml, (Ohms) | 0 | 0 | 0 | 8-29 |
| Ristocetin 1.0 mg/ml, (Ohms) | 28 | 8 | 6 | 8-29 |
| Arachidonic 0.5 mM/ml, (Ohms) | 10 | 9 | 2 | 6-18 |
Discussion
Acquired Platelet Dysfunction with Eosinophilia (APDE) has been identified predominantly within the geographic limits of Thailand, Malaysia, and Singapore3, 4. While chiefly affecting the pediatric demographic, its occurrence in adults is not uncommon5. The rarity of this condition outside these endemic regions necessitates the inclusion of travel history in the diagnostic assessment. Notably, instances of APDE have emerged from Western nations such as Canada, the United Kingdom, and Hong Kong, all linked by a common denominator of travel to Southeast Asia5. This report underscores the identification of three Malay patients presenting with APDE in the absence of recent travel history. Although the primary incidence is observed in children aged between 1 to 12 years, adult cases are also documented, as evidenced by Case 2 and Case 3. The distribution of APDE between genders is relatively equitable, albeit with a slight male predominance6, 7.
The diagnostic challenge of APDE lies in its lack of definitive signs or tests, necessitating the exclusion of other conditions. Diagnostic efforts are supported by history-taking, physical examination, and basic laboratory investigations such as full blood counts and coagulation profiles, including platelet aggregation tests3. Eosinophilia presents heterogeneously, ranging from asymptomatic states to severe, complex presentations, and is categorized into primary, or neoplastic eosinophilia, and secondary eosinophilia—the latter often prompted by parasitic infections4, 8, 9. The spectrum of secondary eosinophilia also encompasses allergies, autoimmune and inflammatory disorders, and drug hypersensitivity9, 10.
Persistent eosinophilia with a normal coagulation profile is revealed through Full Blood Count (FBC) analysis, while mild leukocytosis is observed in approximately 80% of cases11. Microscopic examination of Wright-stained blood smears may reveal abnormal eosinophils and platelets, with platelets displaying diminished granulation and constituting 30-80% of total platelet counts11, 12. Stool examinations ascertain the absence of parasitic infestations, as illustrated in Case 1. Even when tests are not performed, as in Cases 2 and 3, the possibility of overlooked parasitic infestations cannot be entirely dismissed given that 10-25% of such infections go undiagnosed13.
Platelet aggregation tests in APDE demonstrate decreased or absent aggregation in response to collagen, ADP, and epinephrine3, 11, 14. Notably, a study by the National Blood Centre found 21.9% of impaired ADP agonist platelet aggregation cases linked to APDE, with eosinophil percentages ranging from 4.4 to 8.3%8. In some instances, an increase in IgE levels is noted, though a consistent correlation between IgE levels and eosinophilia remains elusive3.
The underlying mechanisms connecting eosinophilia to platelet dysfunction are speculative. One theory suggests that elevated IgE levels, in response to parasitic infections, provoke mast cell degranulation and the subsequent release of platelet-activating factor (PAF), impairing platelet function15, 16. However, no direct correlation has been established between serum IgE levels and the severity of bleeding. Other hypotheses include eosinophilia-induced antibody production leading to immune complexes binding to platelets, causing adenine nucleotides release and acquired storage pool deficiency15. It's noteworthy that despite abnormal ADP release, platelet interaction with von Willebrand factor remains unaffected. Eosinophil cationic protein has been implicated as a potential platelet aggregation inhibitor3.
In the context of post-COVID-19 vaccination, the observed mild eosinophilia in the third case may reflect an immunological response to the vaccine or the virus itself, indicating the dual roles of eosinophils in protective immunity and antiviral responses17, 18. Recovery from APDE typically coincides with the normalization of eosinophil counts and platelet function, exhibiting a benign, self-limiting course that spontaneously resolves within a span of 3 to 6 months up to a year. Aside from potential empirical anthelmintic treatment, no specific interventions have proven efficacious7. Recognizing this benign condition is crucial for avoiding misdiagnosis of more severe bleeding disorders in children presenting with ecchymosis. Follow-up observations in all three cases confirmed symptomatic improvement correlating with eosinophil count reduction.
Conclusion
The precise mechanisms underlying Acquired Platelet Dysfunction with Eosinophilia (APDE) are yet to be fully elucidated. Possible explanations for its pathogenesis include the inhibition of platelet aggregation by eosinophil cationic protein and noticeable abnormalities in platelet morphology, as evidenced through light microscopy. Additionally, the presence of gray platelets has been observed in several instances, though not consistently. This variability underscores the need for further research to fully elucidate the mechanisms driving APDE19.
In conclusion, APDE is characterized as a transient and benign coagulopathy, typically persisting between four to six months and resolving spontaneously without therapeutic intervention. Despite its self-limiting nature, patients often undergo extensive testing that yields inconclusive results, posing a diagnostic challenge for clinicians. Although APDE is considered rare in local contexts, recognizing this benign condition is of paramount importance. The mainstay of management involves providing reassurance to both the patient and their families and ensuring diligent follow-up care, which are generally sufficient for patient management.
Abbreviations
ADP - Adenosine Diphosphate, APDE - Acquired Platelet Dysfunction with Eosinophilia, DNA - Deoxyribonucleic Acid, E. coli - Escherichia coli, FBC - Full Blood Count, IgE - Immunoglobulin E, PAF - Platelet-Activating Factor, WBC - White Blood Cel
Acknowledgments
We would like to thank Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia and all the staff at the Universiti Sains Malaysia, Kubang Kerian, Kelantan.
Author’s contributions
Writing—original draft preparation: Nur Ilyia Syazwani Saidin, Fatin Amirah Nik Min and Noor Haslina Mohd Noor
Writing—review and editing: Wan Suriana Wan Ab Rahman, Mohd Nazri Hassan, Salfarina Iberahim and Zefarina Zulkafli
All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Anuar
M. A.,
Talib
N. A.,
Misaridin
N. F. I.,
Acquired Platelet Dysfunction with Eosinophilia (APDE): Contemplating Physical Abuse. IIUM Medical Journal Malaysia.
2019;
18
(2)
:
10-1
.
-
Shih
M.Y.,
Wang
R.C.,
Liang
C.W.,
Wang
J.D.,
Acquired platelet dysfunction with eosinophilia in two patients. Pediatrics and Neonatology.
2020;
61
(3)
:
346-7
.
View Article PubMed Google Scholar -
Laosombat
V.,
Wongchanchailert
M.,
Sattayasevana
B.,
Kietthubthew
S.,
Wiriyasateinkul
A.,
Acquired platelet dysfunction with eosinophilia in children in the south of Thailand. Platelets.
2001;
12
(1)
:
5-14
.
View Article PubMed Google Scholar -
Suvatte
V.,
Mahasandana
C.,
Tanphaichitr
V.,
Tuchinda
S.,
Acquired platelet dysfunction with eosinophilia: study of platelet function in 62 cases. The Southeast Asian Journal of Tropical Medicine and Public Health.
1979;
10
(3)
:
358-67
.
PubMed Google Scholar -
Lee
A.C.,
Unusual hematologic disease affecting Caucasian children traveling to Southeast Asia: acquired platelet dysfunction with eosinophilia. Hematology Reports.
2012;
4
(1)
:
e5
.
View Article PubMed Google Scholar -
Zhou
X.,
S.Y. Ha,
S.K. Ma,
T.L. Lee,
G.C. Chan,
Y.L. Lau,
Case Report Acquired Platelet Dysfunction with Eosinophilia: Report of Two Cases. HK J Paediatr (New Series).
2000;
5
(2)
:
143-5
.
-
Yadav
D.D.,
Nayar
P.S.,
Manchanda
R.V.,
Acquired Platelet Dysfunction with Eosinophilia (APDE) Syndrome: A Case Report. Indian Journal of Hematology & Blood Transfusion : An Official Journal of Indian Society of Hematology and Blood Transfusion.
2016;
32
(S1)
:
235-8
.
View Article PubMed Google Scholar -
Muhsin
A.,
Raha
M.T. Eusni,
Sabariah
M.N.,
Faraizah
A.K.,
Roshida
H.,
Salmiah
M.S.,
Impaired Platelet Aggregation to Adenosine Diphosphate (ADP) Agonist in National Blood Centre, Malaysia. Asian Journal of Epidemiology.
2012;
5
(4)
:
114-22
.
View Article Google Scholar -
Leru
P.M.,
Eosinophilic disorders: evaluation of current classification and diagnostic criteria, proposal of a practical diagnostic algorithm. Clinical and Translational Allergy.
2019;
9
(1)
:
36
.
View Article PubMed Google Scholar -
Klion
A.D.,
How I treat hypereosinophilic syndromes. Blood.
2015;
126
(9)
:
1069-77
.
View Article PubMed Google Scholar -
Brij Mohan Kumar Singh
A.K.,
Barnini Banerjee, Elcid Vijay Kumar, Acquired Platelet Dysfunction with Eosinophilia: A Report of 3 Cases with. Review of Literature. International Journal of Health Sciences and Research.
2014;
4
(8)
:
297-303
.
-
E. Villanueva III,
A.M. Espaldon,
Acquired Platelet Dysfunction with Eosinophilia. Philippine Journal of Pathology.
2020;
5
(1)
:
50-51
.
View Article Google Scholar -
Cartwright
C.P.,
Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. Journal of Clinical Microbiology.
1999;
37
(8)
:
2408-11
.
View Article PubMed Google Scholar -
Shih
M.Y.,
Wang
R.C.,
Liang
C.W.,
Wang
J.D.,
Acquired platelet dysfunction with eosinophilia in two patients. Pediatrics and Neonatology.
2020;
61
(3)
:
346-7
.
View Article PubMed Google Scholar -
Chotsampancharoen
T.,
Sripornsawan
P.,
Duangchu
S.,
McNeil
E.,
Clinical Course
Outcome of Childhood Acquired Platelet Dysfunction with Eosinophilia
Clinical Course and Outcome of Childhood Acquired Platelet Dysfunction with Eosinophilia. Acta Haematologica.
2018;
139
(1)
:
28-32
.
View Article PubMed Google Scholar -
Zaki
M.M.,
Parasite platelet interactions.. Parasitologists United Journal.
2011;
4
:
127-36
.
-
Lucas
G. N.,
Acquired platelet dysfunction with eosinophilia (APDE). Sri Lanka Journal of Child Health.
2002;
31
:
89-90
.
View Article Google Scholar -
Lindsley
A.W.,
Schwartz
J.T.,
Rothenberg
M.E.,
Eosinophil responses during COVID-19 infections and coronavirus vaccination. The Journal of Allergy and Clinical Immunology.
2020;
146
(1)
:
1-7
.
View Article PubMed Google Scholar -
Shih
M.Y.,
Wang
J.D.,
Reply to acquired platelet dysfunction with eosinophilia: A false premise. Pediatrics and Neonatology.
2020;
61
(5)
:
568-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 5 (2024)
Page No.: 6442-6446
Published on: 2024-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3086 times
- PDF downloaded - 968 times
- XML downloaded - 108 times
 Biomedpress
Biomedpress

