Abstract
Introduction: Liver and kidney disorders are of substantial concern in global health, posing significant challenges due to the unwanted side effects often associated with conventional treatment drugs. The exploration of natural antioxidants for their management has garnered attention due to the potential for fewer side effects. This study focuses on the protective effects of Amaranthus cruentus hydroethanolic leaf extract (HE) against lead-induced hepatorenal toxicity in rats, aiming to provide a safer alternative in managing these conditions.
Methods: The study embarked on a comprehensive assessment involving phytochemistry, heavy metal analysis, in vitro antioxidant, and anti-inflammatory activities of the hydroethanolic leaf extract. Lead-induced hepatorenal toxicity was established in rats through intraperitoneal injection at 25 mg/kg body weight. Following this, oral treatments were administered at varied dosages of 100 mg/kg, 250 mg/kg, and 500 mg/kg body weight respectively. Evaluations were made using hematological, biochemical, inflammatory indices, and histological assessments to determine the extract's protective efficacy.
Results: The phytochemical analysis revealed a rich presence of phenols, flavonoids, saponins, tannins, coumarins, cardiac glycosides, and steroids. Also detected were heavy metals including Fe, Cd, Pb, and Ni. In terms of antioxidant capacity, the DPPH percentage inhibition was noted at 72.4 ± 0.002. The total phenol and flavonoid contents were quantified at 1832.88 ± 11.96 mg GAE/100g and 196.47 ± 1.23 mg QE/g, respectively. The HRBC membrane stabilization exhibited a range between 64.4 - 74.7%, compared to the standard drug, diclofenac sodium, which ranged between 63.9 - 84.02%. Significant restoration was observed in the levels of ALT, AST, ALP, bilirubin, albumin, globulin, urea, and creatinine. Furthermore, the NLR and PLR levels were significantly reduced. Histopathological examinations also disclosed significant alleviation in liver and kidney damage.
Conclusion: The investigation highlights the considerable potential of using natural antioxidants from food crops like Amaranthus cruentus in managing liver and kidney disorders. The study demonstrated that the hydroethanolic leaf extract could significantly mitigate lead-induced hepatorenal toxicity in rats, showcasing an effective restoration of biochemical, hematological, and histopathological parameters. This suggests that the extract offers a promising alternative with minimal to no side effects, meriting further exploration for clinical application in liver and kidney disease management.
Introduction
Liver and kidney diseases pose significant global health challenges, exacerbating the burden of chronic conditions and financial pressures on healthcare systems, particularly in resource-limited settings such as Ghana. Traditional treatments for these disorders are available; however, their utility is often compromised by undesirable side effects and the prohibitively high costs associated with liver therapy and haemodialysis, which are unaffordable for the average Ghanaian1. The pathogenesis of liver and kidney maladies is complex, with oxidative stress—stemming from an imbalance between antioxidant systems and reactive oxygen species (ROS)—playing a crucial role. ROS can damage cellular components, including DNA, proteins, and lipids, thus compromising the integrity of cell membranes2, 3. Medicinal plants, known for their rich antioxidant content, have been shown to effectively counteract oxidative damage with minimal adverse effects.
Amaranthus cruentus, cultivated primarily for its nutritious grains, was an essential dietary element in pre-Columbian American societies. Contemporary research has underscored its wide array of pharmacological benefits, encompassing antidiabetic, anticancer, antihypertensive, anti-hypercholesterolemia, and cardioprotective effects4. Despite these known benefits, the hepatorenal protective capacities of Amaranthus cruentus remain largely uninvestigated. This study, therefore, seeks to address this gap by evaluating the hepatorenal protective effects of Amaranthus cruentus leaf extract against lead-induced toxicity in rat models.
Methods
Reagents
The study utilized reagents of analytical grade, including gallic acid, quercetin, Folin-Ciocalteu's phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ethanol, aluminum chloride, nitric acid, perchloric acid, formaldehyde, Silymarin (Legalon 70 mg, Bukwang Pharm, Seoul, Korea), Diclofenac sodium 75 mg (Entrance Pharmaceutical Company, Ghana), and sodium carbonate. All additional solvents and reagents used in this study were of analytical grade.
Plant Collection, Identification, and Authentication
Fresh leaves were collected between 7:00 and 9:00 am from the Tamale Metropolis, Northern Ghana. The Department of Pharmacognosy at Kwame Nkrumah University of Science and Technology (KNUST) authenticated the plant species. A voucher specimen (KNUST/HMI/2022/L016) was deposited at the Department’s herbarium for future reference.
Preparation of Plant Extract
Approximately 450 g of pulverized air-shade dried plant materials were macerated in 50% ethanol and allowed to stand overnight. The liquid fraction was decanted, and the remaining sediments were compressed and filtered through a clean cloth and sterile cotton wool. The filtrate was then lyophilized using a benchtop freeze dryer (LYO60B-1P) at the Central Lab-KNUST. The yield percentage of the extract was calculated and stored in a zip-locked bag at refrigeration.
Phytochemical Screening
A qualitative analysis was conducted on the extract to determine the presence of flavonoids, phenolics, tannins, coumarins, terpenoids, cardiac glycosides, saponins, alkaloids, and steroids, following methods described in the literature5, 6.
In Vitro Antioxidant Assay and Polyphenolic Content
The hydroethanolic extract’s antioxidant capacity was evaluated using the DPPH scavenging activity assay, along with total phenolic and total flavonoid content determinations.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
A 20 mg sample of the extract was dissolved in 20 ml of distilled water. Subsequently, 1 ml of this solution was combined with 1 ml of DPPH working solution in a test tube. The mixture was incubated in darkness for 30 minutes, after which the absorbance was measured at 517 nm utilizing a UV-Vis Spectrophotometer (Mettler Toledo UV 5). A reference control, containing 2 ml of the DPPH radical solution, was utilized for comparison purposes. The percentage of DPPH radical scavenging activity (AA%) was calculated using the formula:
Estimation of Total Flavonoid Content
To assess the total flavonoid content, a measured 20 mg sample of the extract was dissolved in 50 ml of 80% ethanol. Subsequently, 1 ml of this solution was combined with 1 ml of 2% AlCl3 ethanol solution and left to stand for 1 hour. The development of a golden yellow coloration, indicative of flavonoid presence, was quantified at 420 nm with a UV-Visible Spectrophotometer (Mettler Toledo UV 5). The flavonoid concentration was deduced using a Quercetin calibration curve and expressed in terms of mg Quercetin Equivalent (QE) per g of extract.
Determination of Total Phenolic Content
For the total phenolic content, a 20 mg sample of the extract was prepared in 50 ml of distilled water, and 1 ml of this preparation was mixed with 1 ml of Folin-Ciocalteu reagent. The mixture was vortexed and, after a 3-minute interval, 1 ml of a 20% sodium carbonate solution was incorporated and incubated for 1 hour. Absorbance of the resultant color was measured at 760 nm using a UV-Visible Spectrophotometer (Mettler Toledo UV 5). The total phenolic content was calculated from a Gallic Acid standard curve and reported as mg Gallic Acid Equivalent (GAE) per 100 g of extract.
Heavy Metal Screening Protocol
For heavy metal analysis, 1.00 g of the extract was combined with 2 ml of double-distilled water, followed by the addition of 8 ml of a 1:1 nitric acid-perchloric acid mixture and 5 ml of concentrated H2SO4. The mixture was heated to 200°C for 30 minutes until a clear solution emanating white fumes was achieved. After cooling, the volume was adjusted to 50 ml with double-distilled water and transferred into a pre-washed PET bottle for subsequent metal analysis.
In Vitro Anti-inflammatory Activity Assessment
The in vitro anti-inflammatory efficacy of the hydroethanolic extract was evaluated using a human red blood cells (HRBC) membrane stabilization assay. Initially, HRBCs from healthy donors were combined with an equivalent volume of Alsever’s solution and centrifuged at 3000 rpm for 10 minutes to remove the supernatant. The packed cells were repetitively washed with an isotonic saline solution and a 10% v/v cell suspension was prepared in isotonic saline. The assay involved preparing reaction mixtures containing 1 ml of phosphate buffer (0.15 M, pH 7.4), 2 ml of a hyposaline solution (0.36%), 0.5 ml of the HRBC suspension, and 1 ml of the extract at various concentrations (0.125, 0.250, and 0.500 mg/ml). Similarly, mixtures containing the standard drug, diclofenac sodium, and control samples using distilled water instead of the extract were prepared. Post incubation at 37°C for 30 minutes and subsequent centrifugation at 3000 rpm for 10 minutes, the absorbance of liberated hemoglobin was measured at 560 nm spectrophotometrically. The percentage stabilization of HRBC by the extract and the standard drug was determined by the expression:
| Group | Number of rats | Group name | Treatment |
|---|---|---|---|
| I | N = 4 | Normal | Animals were given free access to normal water and standard feed. |
| II | N = 4 | Silymarin+Pb | Animals received 1 mL of Silymarin orally once daily via gavage @120mg/kg body weight dissolved in distilled water for 10 days and 1 mL of lead acetate intraperitoneally (IP) @25mg/kg body weight for 5 days starting from day 6 to day 10. |
| III | N = 4 | Pb only | Animals received 1mL of lead acetate IP once daily @25mg/kg body weight for 5 days starting from day 6 to day 10. |
| IV | N = 4 | 100HE only | Animals receive 1 mL of crude drug orally once daily via gavage @ 100mg/kg body weight dissolved in distilled water for 10 days. |
| V | N = 4 | 250HE only | Animals receive 1 mL of crude drug orally once daily via gavage @ 250mg/kg body weight dissolved in distilled water for 10 days. |
| VI | N = 4 | 500HE only | Animals receive 1 mL of crude drug orally once daily via gavage @ 500mg/kg body weight dissolved in distilled water for 10 days. |
| VII | N = 4 | 100HE +Pb | Animals received 1 mL of crude drug once daily via gavage @100mg/kg body weight dissolved in distilled water for 10 days and 1mL of lead acetate IP @25mg/kg body weight for 5 days starting from day 6 to day 10. |
| VIII | N = 4 | 250HE +Pb | Animals received 1 mL of crude drug orally once daily via gavage @250mg/kg body weight dissolved in distilled water for 10 days and 1 mL of lead acetate IP @25mg/kg body weight for 5 days starting from day 6 to day 10. |
| IX | N = 4 | 500HE +Pb | Animals received 1 mL of crude drug orally once daily via gavage @500mg/kg body weight dissolved in distilled water for 10 days and 1 mL of lead acetate IP @25mg/kg body weight for 5 days starting from day 6 to day 10. |
Hepatorenal Study Design
Experimental Approach
The induction of liver and kidney injury in this study was performed on female Wistar rats, following the methodology outlined by7. The study incorporated a total of 36 rats, systematically divided into nine groups, each comprising four subjects.
Haematological and Biochemical Parameters Assessment
The experimental subjects were euthanized via cervical dislocation after fasting overnight, specifically on the 10th day following the initiation of the study. Immediate post-mortem neck incisions facilitated the collection of blood samples. These samples were then segregated into two categories: those infused with EDTA for haematological evaluations and those collected into gel-activated tubes for subsequent biochemical assays.
The haematological analysis encompassed a comprehensive panel of indicators, including white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), platelets (PLT), lymphocytes (LYM), neutrophils (NEUT), hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), red cell distribution width (RDW), platecrit, platelet distribution width (PDW), and platelet-large cell ratio (P-LCR). These assessments were precisely conducted using the Sysmex haematological analyzer.
Biochemical analyses were directed at evaluating liver and kidney function markers from the serum extracted from gel-activated tubes. These markers included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBil), direct bilirubin (DBil), total protein (TP), albumin (Alb), globulins (Glo), urea, and creatinine levels.
Percentage protection from different extract concentrations was assessed based on liver and kidney protection indicators using the expression:
Determination of inflammation using inflammatory indicators: NLR AND PLR
Complete blood count indices were used. Briefly, the absolute number of neutrophils were divided by the absolute number of lymphocytes to obtain the NLR. Also, the absolute number of platelets was divided by the absolute number of lymphocytes to obtain the PLR. The following expressions were used:
Histopathological examination
The liver and kidney tissues of animals were surgically removed and immediately washed with normal saline (0.9% NaCl) and blotted dry. The organs were separately weighed to obtain the absolute liver weight (ALW) and absolute kidney weight (AKW). The relative organ weight were obtained from the expression:
Cut-out sections from the liver and kidney were first preserved in 10% formalin, then embedded in paraffin, and sliced into 5 µm sections from each block. These paraffin-embedded liver sections underwent hematoxylin-eosin staining for histopathological analysis using a light microscopy (Olympus Manual System Microscope BX43).
Statistical analysis
Statistical analysis of data was done using GraphPad Prism for Windows version 9.0 (GraphPad Software, San Diego, CA, USA). A two-way Analysis of Variance (ANOVA) test was done and the data were presented as mean±SEM. Multiple comparisons between groups were performed using Tukey Multiple comparison test and statistical significance between groups were considered at p < 0.05.
| Phytochemical | Results |
|---|---|
| Flavonoids | + |
| Phenols | + |
| Tannins | + |
| Coumarins | + |
| Terpenoids | + |
| Cardiac glycosides | + |
| Saponins | + |
| Alkaloids | - |
| Steroids | + |
| Heavy metal | Fe (mg/kg) | Cd (mg/kg) | Pb (mg/kg) | Ni (mg/kg) |
|---|---|---|---|---|
| ACR | 0.1317±0.0028 | 0.0064±0.001 | 0.0055±0.0001 | 0.0021±0.00002 |
| HE | 27.899±0.321 | 0.095±0.003 | 0.007±0.0030 | 0.031±0.0002 |
| Extract | Total phenol (mgGAE/100g) | Total flavonoids (mgQE/g) | DPPH (%AA) |
|---|---|---|---|
| HE | 1832.887 ±0.011.96 | 196.47±1.23 | 72.4±0.002 |
| Treatment | D2 | D4 | D6 | D8 | D10 |
|---|---|---|---|---|---|
| Normal | 2.99±0.81 | 5.34±0.66 | 7.58±1.85 | 5.47±0.58 | 10.01±1.87 |
| Silymarin+Pb | 1.15±0.33 | 2.87±0.52 | 5.76±1.08 | 3.01±1.22 | 2.00±1.24 |
| Pb only | 1.97±0.55 | 3.13±0.81 | 4.84±0.62 | 3.01±0.48 | 1.82±0.86 |
| 100HE only | 1.76±0.25 | 3.25±0.62 | 5.02±0.81 | 6.94±2.03 | 9.24±1.58 |
| 250HE only | 1.69±0.32 | 2.17±0.33 | 4.32±0.27 | 4.20±1.01 | 6.38±0.70 |
| 500HE only | 2.52±0.48 | 2.61±0.79 | 4.00±0.58 | 3.82±0.84 | 8.37±0.86 |
| 100HE+Pb | 1.66±0.43 | 1.54±±0.37 | 3.71±0.57 | 1.66±0.53 | 1.16±0.49 |
| 250HE+Pb | 1.70±0.12 | 2.97±0.59 | 2.72±0.89 | 1.8±0.42 | 1.19±0.67 |
| 500HE+Pb | 2.190.70 | 2.73±0.51 | 4.61±1.25 | 3.80±1.51 | 1.92±0.39 |
| Treatment | Normal | Siymarin + Pb | 100mg HE only | 250mg HE 0nly | 500mg HE only | Pb only | 100HE + Pb | 250HE + Pb | 500HE + Pb |
|---|---|---|---|---|---|---|---|---|---|
| WBC | 13.33 ± 10.33 | 13.18 ± 1.18 | 12.83 ± 1.12 | 12.10 ± 1.29 | 12.70 ± 0.49 | 14.90 ± 1.53 | 11.45 ± 1.74 | 14.30 ± 0.64 | 12.53 ± 1.00 |
| RBC | 7.43 ± 0.27 | 6.54 ± 0.26 | 7.38 ± 0.36 | 7.67 ± 0.11 | 7.71 ± 0.13 | 7.01 ± 0.06 | 6.98 ± 0.34 | 7.48 ± 0.26 | 7.65 ± 0.15 |
| HGB | 13.53 ± 0.38 | 11.53 ± 0.31 | 13.28 ± 0.77 | 13.68 ± 0.15 | 13.38 ± 0.42 | 12.58 ± 0.34 | 12.33 ± 0.49 | 13.13 ± 0.30 | 13.20 ± 0.47 |
| HCT | 54.28 ± 1.62 | 46.73 ± 1.29 | 52.05 ± 2.26 | 54.40 ± 1.16 | 54.30 ± 1.55 | 50.38 ± 1.15 | 49.85 ± 2.34 | 55.33 ± 2.28 | 52.80 ± 1.58 |
| PLT | 1050.50 ± 52.95 | 1381.75 ± 184.88 b | 1219.00 ± 214.78 | 991.00 ± 106.93 | 1187.00 ± 54.14 | 1669.25 ± 39.26 a | 1178.25 ± 163.64 | 1531.25 ± 81.67 b | 1350.00 ± 63.46 b |
| LYM# | 10.23 ± 0.89 | 5.13 ± 1.82 | 8.98 ± 1.32 | 5.08 ± 0.68 | 6.90 ± 0.43 | 4.88 ± 1.89 | 6.10 ± 0.43 | 6.63 ± 0.20 | 6.40 ± 0.47 |
| NEUT# | 2.30 ± 0.56 | 6.60 ± 2.05 | 3.20 ± 1.05 | 5.45 ± 0.93 | 4.65 ± 0.37 | 5.80 ± 1.12 | 4.25 ± 1.08 | 6.30 ± 0.70 | 3.93 ± 0.26 |
| P-LCR | 20.03 ± 0.66 | 20.38 ± 0.62 | 14.75 ± 1.11 | 16.83 ± 0.71 | 16.23 ± 1.45 | 17.20 ± 1.53 | 18.95 ± 0.95 | 17.13 ± 1.71 | 19.00 ± 0.80 |
| PCT | 0.97 ± 0.05 | 1.28 ± 0.17 | 1.01 ± 0.15 | 0.86 ± 0.08 | 1.02 ± 0.05 | 1.47 ± 0.02 | 1.05 ± 0.14 | 1.33 ± 0.04 | 1.40 ± 0.20 |
Results
The Percent Yield of Extract
The 450g of powdered material yielded 78g of crude extract, resulting in a percent yield of 17.3%.
Phytochemical Screening
Qualitative analysis was conducted to ascertain the phytoconstituents present in the hydroethanolic extract (HE) of A. cruentus, with the results presented in Table 2. The extract was found to have a rich phytochemical content, including flavonoids, phenols, tannins, coumarins, terpenoids, cardiac glycosides, saponins, and steroids. The pharmacological properties observed in the study may be attributed to these bioactive compounds.
In-vitro Antioxidant Assay and Polyphenolic Content
To determine the HE’s ability to neutralize free radicals, assays for total phenolic content, total flavonoid content, and DPPH scavenging activity were conducted. Gallic acid and quercetin standard curves were used to extrapolate the total phenolic and flavonoid content of the extract. The total phenolic content was found to be 1832.887 ± 0.011.96, the total flavonoid content was 196.47 ± 1.23, and the DPPH scavenging activity was 72.4 ± 0.002, as represented in Table 3. These results suggest that the HE possesses antioxidant properties. Figure 1 and Figure 2 present the standard calibration curves used to derive the total phenolic and total flavonoid contents of the hydroethanolic extract.
Heavy Metal Analysis
To ascertain the safety of the HE and the raw plant material, analyses for the presence of heavy metals, including Pb, Cd, Fe, and Ni, were conducted. The raw plant material showed concentrations of Fe, Cd, Pb, and Ni at 0.1317 ± 0.0028, 0.0064 ± 0.001, 0.0055 ± 0.0001, and 0.0021 ± 0.00002, respectively. The HE revealed the presence of Fe, Cd, Pb, and Ni at concentrations of 27.899 ± 0.321, 0.095 ± 0.003, 0.007 ± 0.003, and 0.031 ± 0.0002, respectively, as shown in Table 3.
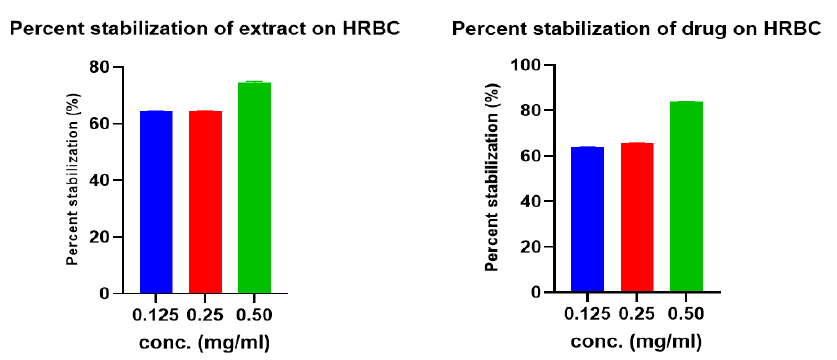
In-vitro Anti-Inflammatory Activity
Figure 3 illustrates the results for the in-vitro anti-inflammatory activity of various concentrations of the hydroethanolic extract and the standard drug (diclofenac sodium) at 0.125, 0.250, 0.500 mg/ml. The standard drug showed the maximum percentage stabilization (84.02 ± 0.0006) compared to 74.7 ± 0.001 from the extract.
In Vivo Evaluation of Hepatorenal Protective Activity
Effect of Treatment on Body Variations
Table 5 demonstrates the variations in body weight of experimental subjects from Day 2 (D2) to Day 10 (D10). The normal group, 100mg/kg HE only, 250mg/kg HE only, and 500mg/kg body weight HE only groups increased in body weight from D2 until the end of the study (D10). The Pb-treated groups (Pb only, Silymarin+Pb, 100mg/kg HE+Pb, 250mg/kg HE+Pb, and 500mg/kg body weight HE+Pb) exhibited a decrease in body weight after Day 6 (D6) when the toxicant was introduced, an observation likely attributable to the toxic effects of Pb affecting their eating patterns.
Effect of Treatment on Relative Organ Weight
Figure 4 shows the effect of the hydroethanolic extract on the relative liver and kidney weights. There was an increase in relative liver and kidney weights in the Pb-only group compared to the normal, though the increase was not statistically significant. The obtained values for relative liver weight (RLW) and relative kidney weight (RKW) under various treatments are delineated, indicating that co-administration of the extract, especially at 500mg/kg body weight, reduced the RLW and RKW.
Effect of Treatment on Hematological Parameters
The impacts of the treatment on hematological parameters are represented as mean±SEM. Key hematological parameters like WBC, RBC, HGB, HCT, LYM, NEUT, PCT, and PL-LCR showed no significant difference, except for a significant difference observed in the PLT count between the normal and Pb-only groups, and between the Pb-only group and extract plus lead groups, showcasing the extract's potential benefits.
Effect of Treatment on Key Liver Enzymes
Figure 5 details the effects of the extract on key liver enzymes, indicating no significant increase in ALT, AST, and ALP levels in the normal and extract only groups. However, a significant increase was observed for the Pb only group, which was mitigated by treatment with the extract in a dose-dependent manner, suggesting the extract's protective efficacy.
Effect of Treatment on Some Liver Biochemical Parameters
Figure 6 elaborates on how treatment affected several liver biochemical parameters, demonstrating a significant amelioration in total proteins, albumin, and globulin levels, as well as a reduction in bilirubin levels, particularly notable in the 500mg/kg body weight group when compared to the Pb-only group, highlighting the extract's beneficial effects.
Effect of Treatment on Some Kidney Biochemical Parameters
Figure 7 details the extract’s impact on key kidney biochemical parameters, showing a significant reduction in creatinine and urea levels, especially in the 500mg/kg HE+Pb group compared to the Pb-only group, indicating the extract’s potential protective effect on kidney function.
Effect of Treatment on Some Inflammatory Indices
Figure 8 illustrates the effect of the extract on some inflammatory indices like NLR and PLR, with a significant decrease observed in these values upon treatment with the extract in a dose-dependent manner, particularly in the 500mg/kg body weight group compared to the Pb-only group, suggesting the extract's anti-inflammatory properties.
Histological Examination
The extract only groups showed normal liver and kidney architecture. The Pb only group showed severe damage to the liver and kidney tissues. Meanwhile, the co-administration with the extract reduced the insult of the Pb on the liver and kidney tissues which are indicative of the protective properties of the extract especially in the 500 mg HE+Pb group.
Discussion
Lead (Pb) has a non-biodegradable nature, contributing to elevated levels in water, food, and biological systems. Pb’s toxicity is well-documented, with oxidative stress as a known mechanism through the depletion of antioxidant systems. Pb triggers ROS production that attacks crucial components of cells, including DNA, proteins, and lipids, leading to DNA damage, changes in protein structure and functions, and lipid peroxidation8.
Food crops contain bioactive compounds with numerous therapeutic properties, including antioxidants, antidiabetic, anticancer, anti-inflammatory, hepatoprotective, and nephroprotective activities. These bioactive compounds elicit their functions by acting as scavengers and chelators of ROS9. Additionally, polyphenols regulate inflammation by acting on numerous cell signaling pathways implicated in inflammation, such as nuclear factor-kappa β, mitogen-activated protein kinases, Wnt/β-catenin, as well as phosphatidylinositol 3-kinase and protein kinase B pathways10.
The phytochemical analysis of the hydroethanolic extract indicated the presence of flavonoids, phenolics, tannins, coumarins, cardiac glycosides, saponins, and steroids. The hepatoprotective and nephroprotective properties of the extract revealed in the study may be attributed to the presence of these bioactive compounds. The results corroborate with other reports11, 12. Differences in phytoconstituents may be due to solvent differences. Heavy metals, including Pb, Cd, Cr, and Cu, have been reported to be hepato- and nephrotoxic even at low concentrations. Research shows that exposure to these metals increases renal toxicity leading to tubular dysfunction and chronic kidney disease (CKD)13. In the current studies, there were traces of heavy metals (Table 4) in the extract. However, these were within the acceptable limits proposed by WHO, and hence the extract is safe14.
Polyphenols react with Folin-Ciocalteu's reagent by transferring an electron to molybdenum, and this aids in the measurement of reducing capacity, which is then reported as the total phenolic compounds in the sample. Phenols can remove free radicals from biological systems, serve as metal chelators, activate antioxidant enzymes, and inhibit oxidases15. This study recorded a total phenolic content of 1827.9 ± 11.96 mg QE/100g. This result corroborates other reports16, 17. The extract's total flavonoid content was 196.47 ± 1.23 mg GAE. Results are consistent with other reports17.
The extract's free radical scavenging potential was measured using the DPPH assay. This is because antioxidants react with DPPH, leading to its conversion to 1,1-diphenyl-1-2-picrylhydrazine. The percentage antioxidant activity (%AA) was 72.4 ± 0.002. This property may be due to the polyphenols' ability to transfer hydrogen atoms or donate an electron to the DPPH radical, hence neutralizing the free radical. The result of the DPPH assay suggests the extract is a potential antioxidant. Other studies reported values ranging from 78.33 ± 0.18 to 85.67 ± 0.5918, which are consistent with what was revealed in the current study.
The human red blood cell (HRBC) acts just like the lysosomal cell membrane and is therefore used to assess the anti-inflammatory activity of extracts19. The in-vitro anti-inflammatory activity of the extract on HRBC revealed a percentage stabilization of 64.4 – 74.7% compared to 63.9 – 84.02% in the standard drug (diclofenac sodium).
The effect of the extract on the relative kidney and liver weight was estimated using the absolute organ weight. The study revealed an increase in the relative organ weight of the rats treated with Pb only compared to the control group. Nevertheless, treatment with the extract seems to have reversed the effect of the toxicant in the extract plus Pb-treated groups. The increase in relative organ weight could be due to the infiltration of inflammatory cells that add to the tissue weight20.
There was an increase in the weight of rats treated with the extract on days 2, 4, and 6 before the toxicant introduction. The extract-only Group, as well as the normal group, continued to increase in weight until the end of the experiment. Meanwhile, rats treated with toxicants decreased in weight after day 6 to the end of the experiment. The increase in the extract-only groups may signify the non-toxicity of the extract. It also suggests that the extract did not affect the eating patterns and appetite of the rats, thus promoting their growth. The decrease in the weight of the Pb-treated groups (toxicant group) suggests that the toxicant affected the eating pattern and appetite of the rats, which influenced their eating habit. Additionally, since the rats may not be feeding properly, their bodies may be forced to utilize fat and protein stores to synthesize glucose, potentially reducing their body mass. These findings corroborate the observations made by21, 22.
Pb has been associated with changes in the cytoskeleton that further deteriorate cell membranes, leading to the release of cellular contents into the blood. This increases the concentration of certain biomarkers that serve as clues to liver and kidney damage23. There was an increase in the ALT, AST, ALP, and bilirubin levels in the lead-only group compared to the normal, which signifies some damage to the hepatocytes. However, co-administration with the extract restored their levels significantly. There was also a decline in total protein, albumin, and globulin in the lead-treated groups compared to the normal and extract-only groups. The decrease in these parameters may be attributed to the destructive effect of Pb on the endoplasmic reticulum via the impairment of Ca2+ homeostasis, affecting protein biosynthesis24. This could account for the decrease in proteins, albumin, and globulin across the Pb-treated groups. The slight increase in levels of the biomarkers in the Pb plus extract-treated groups is an indication of the hepatorenal protective properties of the extract, as this is in line with other reports25, 26.
Increased protein degradation leads to elevated levels of ammonia in serum and a further increase in urea levels27. Furthermore, free radical-induced disruption of brush border epithelial cells makes them impermeable to urea and creatinine by renal tubules. This further increases these biomarkers, serving as indicators of renal damage28, 29. It was revealed that the creatinine and urea levels in the lead-only group were elevated. However, co-administration with the extract brought about a reduction in the creatinine and urea levels in the lead and extract groups, attributable to the antioxidant capacity of the extract to lessen the impact of the toxicant on the kidney cells, which is in line with other reports25, 30.
Plants contain bioactive compounds that regulate the composition of gut microbiota and reduce the production of inflammatory mediators such as ROS and pro-inflammatory cytokines. Additionally, plant extracts can inhibit the activity of enzymes including COX and lipoxygenase, which have been implicated in the production of inflammatory mediators. While stabilizing cell membranes, plant extracts elicit anti-inflammatory properties by inhibiting lysis and subsequent release of cytoplasmic components31. This prevents further damage to cells and tissues and inflammatory responses.
Systemic inflammatory biomarkers, such as PLR (platelet-to-lymphocyte ratio) and NLR (neutrophil-to-lymphocyte ratio), are indicators associated with the immune response. These biomarkers have been studied extensively in various cancers to assess the prognosis of aggressive tumors. Additionally, numerous studies have demonstrated their relevance in evaluating the progression and prognosis of conditions like cardiovascular diseases, sudden deafness, vestibular neuritis, and diseases linked to thrombosis32, 33, 34.
The neutrophil-to-lymphocyte (NLR) ratio increased significantly in the toxicant-only group, predicting possible systemic inflammation. However, the values were reduced in the toxicant plus extract groups, indicative of the amelioration of the insult caused by the toxicant on the liver and kidney tissues. This was further confirmed by the platelet-to-lymphocyte ratio (PLR), which yielded a higher value in the toxicant-only group compared to the toxicant-plus extract groups. The findings are consistent with other reports35, who reported a significant increase in the NLR in autoimmune patients. However, Liu et al. (2017) reported a decrease in WBC, neutrophils, and lymphocytes but a significant increase in NLR values36. The high values obtained for the PLR corroborate the report of Rodríguez-Yoldi (2021) and Asemota et al. (2019)31, 32, who found higher values of PLR as indicative of an inflammatory response in coronary atherosclerosis. Higher values of NLR and PLR have been implicated in Psoriasis vulgaris patients as indicative of inflammation37, 38, 39. Also, increased PLR has been associated with inflammation, atherosclerosis, and thrombosis40, 41.
Pb toxicity manifests in the kidney as renal tubular injury, vascular engorgement, and expansion of Bowman's capsule42. Other indicators of Pb-induced hepatorenal damage include the invasion of lymphocytes and macrophages, localized cell death, and deterioration of hepatocytes and kidney cells43. As evidenced by the study, the histology of the Pb-only group showed severe degeneration of hepatocytes and portal fibrous strands. There was infiltration of inflammatory cells and mild degeneration and sinusoidal dilation in the 100 mg/kg body weight and 250 mg/kg body weight groups. However, co-administration of the extract partly prevented the effect of the toxicant on the hepatocytes, especially in the 500 mg/kg body weight group. A study by Genfi et al. (2020)1 reported the ability of Ocimum extract to ameliorate para-induced hepatotoxicity, while Sarfo-Antwi et al. (2019)44 reported the ameliorative properties of Ageratum conyzoides extract against CCl4–induced hepatotoxicity.
There was obvious central necrosis and destruction of the Bowman’s capsule and glomerular apparatus in the Pb-only group compared with the Pb and extract groups. This could be due to the preservative influence of the extract on the liver and kidney cells. The finding corroborates the reports of Kandemir et al. (2019)26. Regeneration in the liver and kidneys, even though it involves complex processes, is very crucial and possible after sustained injury. The liver, for example, has a high regenerative capacity if the cause of damage can be removed. The kidney employs mechanisms such as polyploidization in its recovery processes45. In instances where there is liver failure, kidney dysfunction cannot be overlooked, and it is well-established that liver cirrhosis parallels kidney damage46. An in-depth knowledge of the recovery and regeneration of liver and kidney structure and function is thus needed in developing therapeutic options for treating liver and kidney disorders.
Regarding the percentage of protection, the main indicators used for hepatoprotection were ALT, AST, ALP, Bil, ALB, and TP levels, while Crea and Urea were used for percentage nephroprotection. The study saw a dose-dependent effect, as supported by the photomicrographs. The 100 mg/kg, 250 mg/kg, and 500 mg/kg body weight protected the liver against damage up to 35.2%, 53.8%, and 72.1%, respectively. For nephroprotection, the 100 mg/kg, 250 mg/kg, and 500 mg/kg body weight doses showed protection up to 36%, 42%, and 54.1%, respectively, while the standard drug, Silymarin, recorded 83.2% hepatoprotection and 70% nephroprotection. These findings suggest that the protective effect of the extract was more pronounced in the 500 mg/kg body weight compared to the lower dosages. These findings suggest that Amaranthus cruentus HE offers a promising natural alternative with fewer side effects for managing hepatorenal disorders.
Even though the extract exhibited significant hepatorenal protective properties, the exact compounds present in the extract that exhibited those pharmacological properties were not determined, and it is therefore recommended to be investigated with a larger sample size. Again, the study could not determine the mechanism of action of the extract and is, therefore, recommended to be further investigated.
Conclusions
The research examined the hepatorenal properties of the hydroethanolic extract of Amaranthus cruentus in rat models. Hematological, biochemical, and histological indices were used as indicators of liver and kidney protection. The phytoconstituents identified were flavonoids, phenolics, tannins, coumarins, steroids, cardiac glycosides, and saponins. Traces of Fe, Cd, Pb, and Ni were found in the extract and raw plant material. Significant antioxidant activity against DPPH radicals was recorded. The extract contained significant amounts of flavonoids and phenolic compounds. There was a notable percentage stabilization of the extract on HRBC. Significant increases in liver and kidney biomarkers in the toxicant-treated groups were reversed by co-administration with the extract. Microarchitectural changes in the liver and kidneys were also reversed following co-administration with the extract. These findings regarding Amaranthus cruentus have implications for managing liver and kidney conditions using natural antioxidants from food crops, which have little to no side effects on biological systems.
Abbreviations
AKW - Absolute Kidney Weight, ALP - Alkaline Phosphatase, ALW - Absolute Liver Weight, Alb - Albumin, ANOVA - Analysis of Variance, AST - Aspartate Aminotransferase, ALT - Alanine Aminotransferase, Cd - Cadmium, CKD - Chronic Kidney Disease, DBil - Direct Bilirubin, DPPH - 2,2-diphenyl-1-picrylhydrazyl, EDTA - EthyleneDiamineTetraacetic Acid, Fe - Iron, Glo - Globulin, Hb - Hemoglobin, HE - Hydroethanolic Extract, HRBC - Human Red Blood Cell, KNUST - Kwame Nkrumah University of Science and Technology, LYM - Lymphocyte, MCH - Mean Corpuscular Hemoglobin, MCV - Mean Corpuscular Volume, NEUT - Neutrophil, Ni - Nickel, NLR - Neutrophil-to-Lymphocyte Ratio, Pb - Lead, PET - Polyethylene Terephthalate, PLR - Platelet-to-Lymphocyte Ratio, PLT - Platelet, RDW - Red Cell Distribution Width, RBC - Red Blood Cell, ROS - Reactive Oxygen Species, ROW - Relative Organ Weight, SEM - Standard Error of Mean, TBil - Total Bilirubin, TP - Total Protein, UV - Ultraviolet, WBC - White Blood Cell, WHO - World Health Organization, mgGAE/100g - Milligrams of Gallic Acid Equivalent per 100 grams, mgQE/g - Milligrams of Quercetin Equivalent per gram
Acknowledgments
None.
Author’s contributions
James Otabil was the principal investigator throughout the study. All other authors contributed intellectually to the design of the study and interpretation of analyses. Ossei Paul Poku Sampene contributed to the histological examination and interpretation of photomicrographs. All authors read and approved for the final version of manuscript for publishing.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The animals were identified with tail marks made with permanent markers. All animal studies were conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA, New Delhi, India) and the guide for the care and use of laboratory animals [National Research Council. 2011. Guide for care and use of laboratory animal (8th ed) National Academic Press Washington]. All animals were humanely handled during the experiment according to the approved protocol by a veterinarian on the research team.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Genfi
A.K.,
Larbie
C.,
Emikpe
B.O.,
Oyagbemi
A.A.,
Firempong
C.K.,
Adjei
C.O.,
Modulation of Oxidative Stress and Inflammatory Cytokines as Therapeutic Mechanisms of Ocimum americanum L Extract in Carbon Tetrachloride and Acetaminophen-Induced Toxicity in Rats. Journal of Evidence-based Integrative Medicine.
2020;
25
.
View Article PubMed Google Scholar -
Eaton
D.L.,
Gallagher
E.P.,
Vandivort
T.C.,
General Overview of Toxicology. Comprehensive Toxicology.
2018;
1
:
1-38
.
View Article Google Scholar -
Unsal
V.,
Cicek
M.,
Sabancilar
İ.,
Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Reviews on environmental health.
2021;
36
(2)
:
279-95
.
View Article Google Scholar -
Ma
X.,
Vaistij
F.E.,
Li
Y.,
Jansen van Rensburg
W.S.,
Harvey
S.,
Bairu
M.W.,
A chromosome-level Amaranthus cruentus genome assembly highlights gene family evolution and biosynthetic gene clusters that may underpin the nutritional value of this traditional crop. The Plant Journal.
2021;
107
(2)
:
613-28
.
View Article PubMed Google Scholar -
T. Thilagavathi,
R. Arvindganth,
D. Vidhya,
R. Dhivya,
Preliminary phytochemical screening of different solvent mediated medicinal plant extracts evaluated. International Research Journal of Pharmacy.
2015;
6
(4)
:
246-8
.
View Article Google Scholar -
Ismail
M.,
Ali
S.,
Ali
I.,
Shaheen
R.,
Murtaza
G.,
Qualitative analysis for phytochemicals of selected medicinal plants from Gilgit-Baltistan, Pakistan. Asian J Chem.
;
29
(9)
:
1929-32
.
-
Jalali
S.M.,
Najafzadeh
H.,
Bahmei
S.,
Protective role of silymarin and D-penicillamine against lead-induced liver toxicity and oxidative stress. Toxicology and Industrial Health.
2017;
33
(6)
:
512-8
.
View Article PubMed Google Scholar -
Ni
Z.,
Hou
S.,
Barton
C.H.,
Vaziri
N.D.,
Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells. Kidney International.
2004;
66
(6)
:
2329-36
.
View Article PubMed Google Scholar -
Sorrenti
V.,
Burò
I.,
Consoli
V.,
Vanella
L.,
Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. International Journal of Molecular Sciences.
2023;
24
(3)
:
2019
.
View Article Google Scholar -
Jantan
I.,
Haque
M.A.,
Arshad
L.,
Harikrishnan
H.,
Septama
A.W.,
Mohamed-Hussein
Z.A.,
Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. The Journal of nutritional biochemistry.
2021;
93
:
108634
.
View Article Google Scholar -
Nana
F.W.,
Hilou
A.,
Millogo
J.F.,
Nacoulma
O.G.,
Phytochemical composition, antioxidant and xanthine oxidase inhibitory activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts. Pharmaceuticals (Basel, Switzerland).
2012;
5
(6)
:
613-28
.
View Article PubMed Google Scholar -
Karanje
P.S.,
Doijad
R.C.,
Bhosale
R.R.,
Formulation and evaluation of herbal lipstick containing amaranthus cruentus linn. International Journal of Research and Analytical Reviews.
2020;
7
(1)
:
246-255
.
-
Yuan
T.H.,
Jhuang
M.J.,
Yeh
Y.P.,
Chen
Y.H.,
Lu
S.,
Chan
C.C.,
Relationship between renal function and metal exposure of residents living near the No. 6 Naphtha Cracking Complex: A cross-sectional study. Journal of the Formosan Medical Association.
2021;
120
(10)
:
1845-54
.
View Article PubMed Google Scholar -
FAO-WHO-maximum-permissible-values-of-heavy-metals-in-vegetables.
.
-
Foti
M.C.,
Antioxidant properties of phenols. The Journal of Pharmacy and Pharmacology.
2007;
59
(12)
:
1673-85
.
View Article PubMed Google Scholar -
Torane
R.,
Gaikwad
S.,
Khatiwora
E.,
Adsul
V.,
Comparative evaluation of Phenol and flavonoid content of Medicinaly important plant-Amaranthus curentus Comparative Estimation of Phenol and Flavonoid Content of Medicinally Important Plant-Amaranthus curentus. International Journal of ChemTech Research.
2019;
10
(4)
:
306-10
.
-
Oboh
G.,
Short Communication Prevention of Garlic-Induced Hemolytic Anemia Using Some Tropical Green Leafy Vegetables. Journal of medicinal food..
2004;
7
(4)
:
498-501
.
View Article Google Scholar -
Igbinosa
E.O.,
Uzunuigbe
E.O.,
Igbinosa
I.H.,
Odjadjare
E.E.,
Igiehon
N.O.,
Emuedo
O.A.,
. In vitro assessment of antioxidant, phytochemical and nutritional properties of extracts from the leaves of Ocimum gratissimum (Linn). African journal of traditional, complementary, and alternative medicines.
2013;
10
(5)
:
292-8
.
-
Yesmin
S.,
Paul
A.,
Naz
T.,
Rahman
A.B.M.A.,
Akhter
S.F.,
Wahed
M.I.I.,
Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clinical Phytoscience.
2020;
6
:
59
.
View Article Google Scholar -
Guzik
T.J.,
Skiba
D.S.,
Touyz
R.M.,
Harrison
D.G.,
The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovascular research.
2017;
113
(9)
:
1009-23
.
View Article Google Scholar -
Annabi Berrahal
A.,
Nehdi
A.,
Hajjaji
N.,
Gharbi
N.,
El-Fazâa
S.,
Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. Comptes Rendus Biologies.
2007;
330
(8)
:
581-8
.
View Article PubMed Google Scholar -
Allouche
L.,
Hamadouche
M.,
Touabti
A.,
Khennouf
S.,
Effect of Long-term Exposure to Low or Moderate Lead Concentrations on Growth, Lipid Profile and Liver Function in Albino Rats. Advances in Biological Research (Faisalabad).
2011;
5
(6)
:
339-47
.
-
Abdel-Moneim
A.M.,
El-Toweissy
M.Y.,
Ali
A.M.,
Awad Allah
A.A.,
Darwish
H.S.,
Sadek
I.A.,
Curcumin Ameliorates Lead (Pb(2+))-Induced Hemato-Biochemical Alterations and Renal Oxidative Damage in a Rat Model. Biological Trace Element Research.
2015;
168
(1)
:
206-20
.
View Article PubMed Google Scholar -
Mekahli
D.,
Bultynck
G.,
Parys
J.B.,
De Smedt
H.,
Missiaen
L.,
Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harbor Perspectives in Biology.
2011;
3
(6)
:
1-30
.
View Article PubMed Google Scholar -
Amin
I.,
Hussain
I.,
Rehman
M.U.,
Mir
B.A.,
Ganaie
S.A.,
Ahmad
S.B.,
Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. Journal of Food Biochemistry.
2021;
45
(3)
:
e13241
.
View Article PubMed Google Scholar -
Kandemir
F.M.,
Yildirim
S.,
Caglayan
C.,
Kucukler
S.,
Eser
G.,
Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environmental Science and Pollution Research International.
2019;
26
(22)
:
22562-74
.
View Article PubMed Google Scholar -
Ali
R.,
Nagalli
S.,
Hyperammonemia. InStatPearls [Internet] 2023 Apr 7. StatPearls Publishing..
.
-
Yuan
G.,
Dai
S.,
Yin
Z.,
Lu
H.,
Jia
R.,
Xu
J.,
Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats . International journal of clinical and experimental pathology.
2014;
7
(6)
:
2905-2914
.
PubMed Google Scholar -
Renugadevi
J.,
Prabu
S.M.,
Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Experimental and Toxicologic Pathology.
2010;
62
(2)
:
171-81
.
View Article PubMed Google Scholar -
Adeyemi
O.,
Ajayi
J.O.,
Olajuyin
A.M.,
Oloyede
O.B.,
Oladiji
A.T.,
Oluba
O.M.,
Toxicological evaluation of the effect of water contaminated with lead, phenol and benzene on liver, kidney and colon of Albino rats. Food and Chemical Toxicology.
2009;
47
(4)
:
885-7
.
View Article PubMed Google Scholar -
Rodríguez-Yoldi
M.J.,
Anti-inflammatory and antioxidant properties of plant extracts. Antioxidants.
2021;
10Volume 10
(6)
:
921
.
View Article Google Scholar -
Asemota
K.E.,
Ekene
E.N.,
Ehebha
S.E.,
Olowe
G.T.,
Leucocyte Profile of Adult Nigerians as Indicator of Severity Level of Acute Musculoskeletal Trauma. International blood research & reviews.
2019;
9
(1)
:
1-9
.
View Article Google Scholar -
Arigami
T.,
Uenosono
Y.,
Matsushita
D.,
Yanagita
S.,
Uchikado
Y.,
Kita
Y.,
Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncology Letters.
2016;
11
(2)
:
1537-44
.
View Article PubMed Google Scholar -
Walsh
S.R.,
Cook
E.J.,
Goulder
F.,
Justin
T.A.,
Keeling
N.J.,
Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of Surgical Oncology.
2005;
91
(3)
:
181-4
.
View Article PubMed Google Scholar -
Xie
Y.,
Yang
W.,
Tang
F.,
Chen
X.,
Ren
L.,
Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Current Medicinal Chemistry.
2015;
22
(1)
:
132-49
.
View Article PubMed Google Scholar -
Liu
L.,
Zhang
L.,
Liu
G.J.,
Fu
P.,
Peritoneal dialysis for acute kidney injury. Cochrane Database of Systematic Reviews.
2017;
12
:
CD011457
.
View Article Google Scholar -
Akboga
M.K.,
Canpolat
U.,
Yayla
C.,
Ozcan
F.,
Ozeke
O.,
Topaloglu
S.,
Association of Platelet to Lymphocyte Ratio With Inflammation and Severity of Coronary Atherosclerosis in Patients With Stable Coronary Artery Disease. Angiology.
2016;
67
(1)
:
89-95
.
View Article PubMed Google Scholar -
Zhen
Y.,
Chang
Z.,
Liu
Z.,
Zheng
J.,
Platelet to lymphocyte ratio predicting 6-month primary patency of drug-coated balloon for femoropopliteal disease. BMC Cardiovascular Disorders.
2020;
20
(1)
:
9
.
View Article PubMed Google Scholar -
Kim
D.S.,
Shin
D.,
Lee
M.S.,
Kim
H.J.,
Kim
D.Y.,
Kim
S.M.,
Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. The Journal of Dermatology.
2016;
43
(3)
:
305-10
.
View Article PubMed Google Scholar -
Gasparyan
A.Y.,
Stavropoulos-Kalinoglou
A.,
Mikhailidis
D.P.,
Douglas
K.M.,
Kitas
G.D.,
Platelet function in rheumatoid arthritis: arthritic and cardiovascular implications. Rheumatology International.
2011;
31
(2)
:
153-64
.
View Article PubMed Google Scholar -
Scherlinger
M.,
Guillotin
V.,
Truchetet
M.E.,
Contin-Bordes
C.,
Sisirak
V.,
Duffau
P.,
Systemic lupus erythematosus and systemic sclerosis: all roads lead to platelets. Autoimmunity reviews.
2018;
17
(6)
:
625-35
.
-
Ekong
E.B.,
Jaar
B.G.,
Weaver
V.M.,
Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney International.
2006;
70
(12)
:
2074-84
.
View Article PubMed Google Scholar -
Offor
S.J.,
Mbagwu
H.O.,
Orisakwe
O.E.,
Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Frontiers in Pharmacology.
2017;
8
(MAR)
:
107
.
View Article PubMed Google Scholar -
Sarfo-Antwi
F.,
Larbie
C.,
Babatunde
D.,
Extracts of Ageratum Conyzoides L. Protects against Carbon Tetrachloride \textendash Induced Toxicity in Rats through Inhibiting Oxidative Stress. Journal of Advances in Medical and Pharmaceutical Sciences.
2019;
19
(2)
:
1-14
.
View Article Google Scholar -
Kellum
J.A.,
Romagnani
P.,
Ashuntantang
G.,
Ronco
C.,
Zarbock
A.,
Anders
H.J.,
Acute kidney injury. Nature reviews Disease primers.
2021;
7
(1)
:
52
.
View Article Google Scholar -
Kanduri
S.R.,
Velez
J.C.Q.,
Kidney Dysfunction in the Setting of Liver Failure: Core Curriculum 2024. American Journal of Kidney Diseases.
2024;
83
(3)
:
386-401
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 5 (2024)
Page No.: 6457-6473
Published on: 2024-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2794 times
- PDF downloaded - 894 times
- XML downloaded - 83 times
 Biomedpress
Biomedpress












