Abstract
Introduction: Circulating apoptotic endothelial cell-derived micro particles (EMPs) are a marker of endothelial dysfunction and cardiovascular (CV) risk in type 2 diabetes mellitus patients. There is evidence regarding association between apoptotic EMP number and CV disease in obese individuals. The aim of the study to investigate whether increased number of circulating apoptotic EMPs may predict transformation of Met-HO into Met-UHO.
Methods: The study was retrospectively evolved 89 patients with established abdominal obesity (47 patients with Met-UHO determined as MetS and 42 subjects with Met-HO) from the large cohort of abdominal obesity patients (n=268). Thirty five healthy volunteers matched for age and sex were involved in the study as a control cohort. Obesity-related biomarker (adiponectin, leptin, vistafin) and EMPs were measured at baseline. Flow cytometry was used to determine EMPs with immune phenotype CD31+/annexin V+ and CD144+/annexin V+.
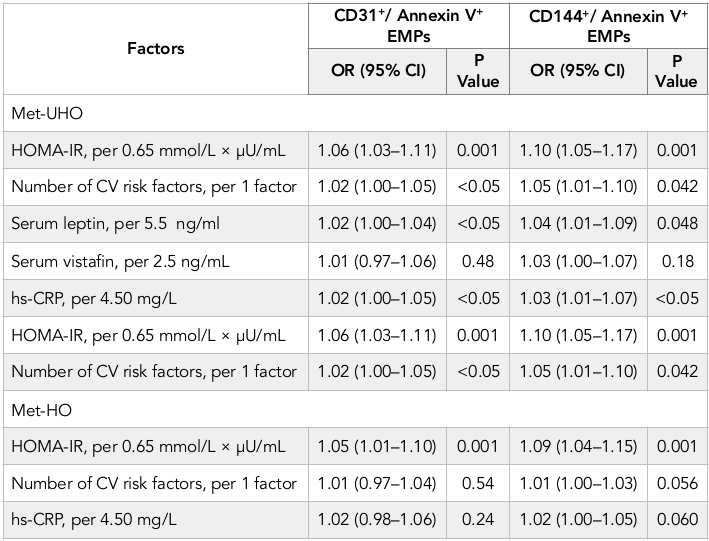
Results: There was not found a significant difference between numbers of EMPs labeled CD31+/ Annexin V+ in Met-UHO and Met-HO patients, while Met-UHO patients had a significantly increased level of circulating CD144+/ Annexin V+ compared with Met-HO individuals. Multivariate logistic regression analysis has revealed the HOMA-IR, number of CV risk factors, serum leptin and hs-CRP independently predicted numbers of circulating CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs in Met-UHO. In Met-HO patients HOMA-IR remained an independent predictor of increased numbers of circulating CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs.
Conclusion: in the investigation we found that the increased number of CD31+/Annexin V+ and CD144+/ Annexin V+ EMPs added to the based predictive model (HOMA-IR) may predict transformation of Met-HO into Met-UHO.
Introduction
The prevalence of abdominal obesity and (T2DM) has been raised worldwide Basterra-Gortari et al., 2017Flegal et al., 2010. Obesity associates with substantially increased all cause and cardiovascular (CV) morbidity and mortality, as well as relates to highest risk of type 2 diabetes mellitus (T2DM) Rey-López et al., 2014Vanuzzo et al., 2008. Recent epidemiological investigations, clinical studies and some registers have shown higher prevalence of CV risk factors in obese individuals especially with morbid obesity (body mass index [BMI] more than 40 kg/m+) Antwi et al., 2012Finucane et al., 2011Sturm, 2007Valdés et al., 2014. Because progressive increases in yearly pre-diabetes / T2DM prevalence has observed for all classes of BMI irrespective age and sex Sterling et al., 2016, there is reason to expect that abdominal obesity mediates CV risk and risk of T2DM through underlying co-morbidities independently BMI Grundy et al., 2005Grundy et al., 2004.
In this context, obese individuals with similar BMI may be protected or opposite predisposed to obesity-related complications (i.e. T2DM, insulin resistance (IR), dyslipidaemia, hypertension) and CV disease Cuthbertson et al., 2017. The heterogeneity of obesity leads to understanding of being of emerging metabolic phenotypes e.g. metabolically unhealthy obesity (Met-UHO) and metabolically healthy obesity (Met-HO) distinguished from each other for CV risk Kim et al., 2015. Moreover, it is suggesting that Met-HO is a transient state in the pathway to cardiometabolic disease, i.e. Met-UHO and T2DM Mongraw-Chaffin et al., 2016.
Based on the Adult Treatment Panel-III criteria subjects with established obesity and co-existing other metabolic abnormalities including dyslipidemia, insulin resistance (IR), increased fasting glucose and impaired glucose tolerance, are referred Met-UHO, whereas obese individuals without these abnormalities might be defined as Met-HO Grundy et al., 2005Ryden et al., 2013. The mechanisms underlying the change in phenotype from metabolically healthy to metabolically unhealthy obesity are still unclear Ryden et al., 2013.
Micro particles (MPs) are defined a heterogeneous sub-population of extracellular vesicles with diameter average from 100 to1000 nm originated from plasma membranes of mother’ cells Berezin et al., 2015a. As a derivate of cellular membrane MPs are discussed powerful paracrine regulators of target cell structure and functions. MP released by apoptotic endothelial cells posse a wide spectrum of biological effects on intercellular communication by transferring different active molecules (proteins, peptides, hormones, growth factors, microRNAs) exhibiting coagulation activity, mediating cell growth and tissue differentiation Alexandru et al., 2016. Additionally, apoptotic endothelial cell-derived MPs (EMPs) may directly worse endothelial integrity and vascular function playing a pivotal role in development of microvascular inflammation and IR Alexandru et al., 2016Berezin et al., 2016b.
Recent clinical studies have shown that the circulating levels of apoptotic EMPs were significantly increased in T2DM patients as compared with healthy volunteers Berezin et al., 2016a and they mediated CV risk in patients with established metabolic syndrome (MetS) and T2DM Agouni et al., 2014Berezin et al., 2015bBerezin et al., 2016c. Whether apoptotic EMPs are involved in the transformation of Met-HO into Met-UHO determining the risk of T2DM and CV disease is not fully clear. The aim of the study: to investigate whether increased number of circulating apoptotic EMPs may predict transformation of Met-HO into Met-UHO.
Materials-Methods
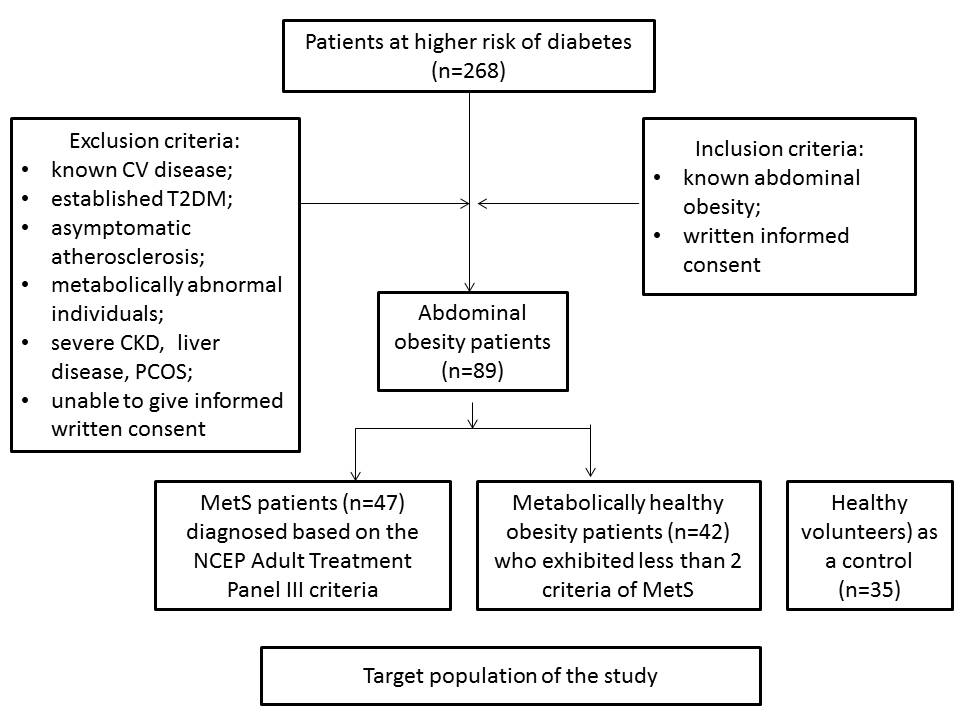
The study was retrospectively evolved 89 patients with established abdominal obesity (47 patients with Met-UHO determined as MetS and 42 subjects with Met-HO) from the large cohort of abdominal obesity patients (n=268) who were examined between February 2012 and July 2016. We have enrolled obese subjects (body mass index was more 30 kg/m+) without known CV disease including angina pectoris, previous myocardial infarction / stroke, heart failure, and asymptomatic atherosclerosis (defined by negative result of the contrast-enhanced multi-spiral tomography angiography). Thirty five healthy volunteers matched for age and sex were involved in the study as a control cohort. All patients have given their informed written consent for a participation in the study.
MetS was diagnosed based on the National Cholesterol Education Program Adult Treatment Panel III criteria Williams, 2002. Patients were enrolled in the MetS cohort when at least three of the following components were defined: waist circumference ≥90 cm or ≥80 cm in men and women respectively; high density lipoprotein (HDL) cholesterol <1.03 mmol/L or <1.3 mmol/L in men and women respectively; triglycerides ≥1.7 mmol/L; blood pressure ≥130/85 mmHg or current exposure of antihypertensive drugs; fasting plasma glucose ≥5.6 mmol/L. Participants who had less than 2 criteria of MetS were classified as Met-HO patients. Those who had 2 or more criteria of MetS were classified as metabolically abnormal Williams, 2002 and were not considered candidates for this study. Participants with abdominal obesity who had less than 3 criteria of MetS were classified those who had Met-HO. Therefore, individuals with non-alcoholic fatty liver disease, polycystic ovary syndrome, and those who had higher levels of HBV / HCV antibodies, were not enrolled in the study. The flow chart with inclusion / exclusion criteria is reported in Figure 1 .
Smoking status
Current smoking was defined as consumption of one cigarette daily for three months Lindson-Hawley et al., 2013.
Anthropometric measurements
Anthropometric measurements (weight, height, body mass, body mass index, waist circumference, and waist-to-hip ratio) were made using standard procedures Ashwell et al., 2012Consultation, 2008. Height and weight were measured by professional health staff with the participants standing without shoes and heavy outer garments with a wall-mounted stadiometer (OMRON, Japan). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured at the level midway between the lower rib margin and the iliac crest with participants in a standing position without heavy outer garments and with emptied pockets, breathing out gently.
Hip circumference was recorded as the maximum circumference over the buttocks.
Calculation of glomerular filtration rate
Glomerular filtration rate (GFR) was calculated with CKD-EPI formula Levey et al., 2009.
Measurement of circulating biomarkers
To determine circulating biomarkers, blood samples were collected at baseline in the morning (at 7-8 a.m.) after at least 10 h fasting into cooled silicone test tubes wherein 2 mL of 5% Trilon B solution were added. Then they were centrifuged upon permanent cooling at 6,000 rpm for 3 minutes. Plasma was collected and refrigerated immediately to be stored at a temperature -70оС. The levels of high-sensitive C-reactive protein (hs-CRP), adiponectin, leptin, vistafin were measured by commercially available standard kit (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). The intra-assay and inter-assay coefficients of variation were <5% for all cases. Fasting insulin level was measured by a double-antibody sandwich immunoassay (Elecsys 1010 analyzer, F. Hoffmann-La Roche Diagnostics, Mannheim, Germany).
The intra-assay and inter-assay coefficients of variation were <5%. The lower detection limit of insulin level was 1.39 pmol/L. Insulin resistance was assessed by the homeostasis model assessment for insulin resistance (HOMA-IR) Matthews et al., 1985 using the following formula:
HOMA-IR (mmol/L × µU/mL) = fasting glucose (mmol/L) × fasting insulin (µU/mL)/ 22.5
IR was arbitrarily defined as a homeostasis model assessment-IR index (HOMA-IR) value above the 75th percentile of normal glucose tolerance equal 2.45 mmol/L × µU/mL.
Hemoglobin A1c (HbA1c) were determined by high-pressure liquid chromatography method. Concentrations of total cholesterol (TC), cholesterol of high-density lipoproteins (HDL-C), triglycerides (TG), and low-density lipoproteins (LDL-C) were measured by direct enzymatic method (Roche P800 analyzer, Basel, Switzerland).
Quality control was assessed daily for all determinations.
Assay of circulating endothelial-derived microparticles
Circulating MPs were isolated from 5 ml of venous citrated blood drawn from the fistula-free arm. No hemolysis in the samples was found. All samples were not frozen before analysis. To prevent contamination of samples platelet-free plasma (PFP) was separated from whole blood. PFP was centrifugated at 70,476 × g for 70 min. MP pellets were washed with DMEM (supplemented with 10 μg/mL polymyxin B, 100 UI of streptomycin, and 100 U/ml penicillin) and centrifuged again (70,476 × g for 90 min) Cvjetkovic et al., 2014. The obtained supernatant was extracted, and MP pellets were re-suspended into the remaining 200 μL of supernatant. PFP, MPs, and supernatant were diluted five-, 10-, and five-fold in PBS, respectively. Only 100 μL of supernatant was prepared for further analysis through incubation with different fluorochrome-labeled antibodies or their respective isotypic immunoglobulins (Beckman Coulter).
MPs were labeled and characterized by flow cytometry by phycoerythrin (PE)-conjugated monoclonal antibody against CD31 (platelet endothelial cell adhesion molecule [PECAM]-1), CD144 (vascular endothelial (VE)-cadherin), CD62E (E-selectin), and Annexin V (BD Biosciences, USA) followed by incubation with fluorescein isothiocyanate (FITC)-conjugated Annexin V (BD Biosciences, USA) per HD-FACS (High-Definition Fluorescence Activated Cell Sorter) methodology independently after supernatant diluted without freeze Orozco and Lewis, 2010.
The samples were incubated in the dark for 15 min at room temperature according to the manufacturer’s instructions. It was performed the analysis of area, height, and width forward scatter (FSC) and side scatter (SSC) parameters as well as side scatter width (SSC-W). Particle sizing by dynamic light scattering revealed a characteristic size of the MPs (Sigma, St Louis, MO, USA). A MPs’ gate was established on the FACS Aria instrument by preliminary standardization experiments using a blend of size-calibrated fluorescent beads, with sizes ranging from 0.1 to 1.0 µm. Two size gates were defined based on forward angle light scattering from polystyrene microsphere (0.5-0.9 µm) accordingly standard protocol. The upper and the outer limit of the MP gate was established just above the size distribution of the 0.9-µm beads in a FSC-A and SSC-A setting (log scale) using the “auto-gate” function. Accordingly, MPs’ gate was defined less than a 0.4 µm polystyrene microsphere extending down to the noise threshold level that is equivalent to cell-derived MPs < 1 µm diameter. The lower limit was the noise threshold of the instrument, and an absolute minimum threshold of 200 was set at the SSC-A parameter (instead of FSC-A) to avoid exclusion of the smallest events. In order to separate true events from background noise, we defined MPs as particles that were less than 1.0 µm in diameter, and expressed cell specific markers.
For each sample, 500 thousand events have been analyzed. Compensation tubes were used with similar reagents as were used in the sample tubes.
Calculation of the number of MPs per liter plasma was based upon the particle count per unit time, the flow rate of the flow cytometer, and the net dilution during sample preparation of the analyzed MP suspension. MP-exposed antigen concentrations were calculated in each sample by multiplying the total concentration of positive MPs by the mean fluorescence intensity of the antigen exposure of the total positive MP population. The reproducibility of EPCs using standard protocol was 4.5%.
Determination of endothelial cell-derived MP populations
CD31 antigen was determined as common marker for endothelial cells, mononuclears and platelets. CD144+ antigen is essential for endothelial cells and used to identify a pure population of endothelial cells. To characterize entire population of MPs originated from endothelial cells we used both antigens’ determination. CD31+/Annexin V+ and CD144+/Annexin V+ MPs were defined as apoptotic endothelial cell-derived MPs Lacroix et al., 2013.
Statistical Analysis
Statistical analysis of the results obtained was performed in SPSS system for Windows, Version 22 (SPSS Inc, Chicago, IL, USA). The data were presented as mean (М) and standard deviation (±SD) or 95% confidence interval (CI); as well as median (Ме) and 25%-75% interquartile range (IQR). To compare the main parameters of patient cohorts, two-tailed Student t-test or Shapiro–Wilk U-test were used. To compare categorical variables between groups, Chi2 test (χ2) and Fisher F exact test were used. Univariant and multivariant linear regression models were used to determine a relation between circulating number of microprticles and other biomarkers. C-statistics, integrated discrimination indices (IDI) and net-reclassification improvement (NRI) were utilized for prediction performance analyses. A two-tailed probability value of <0.05 was considered as significant.
Results
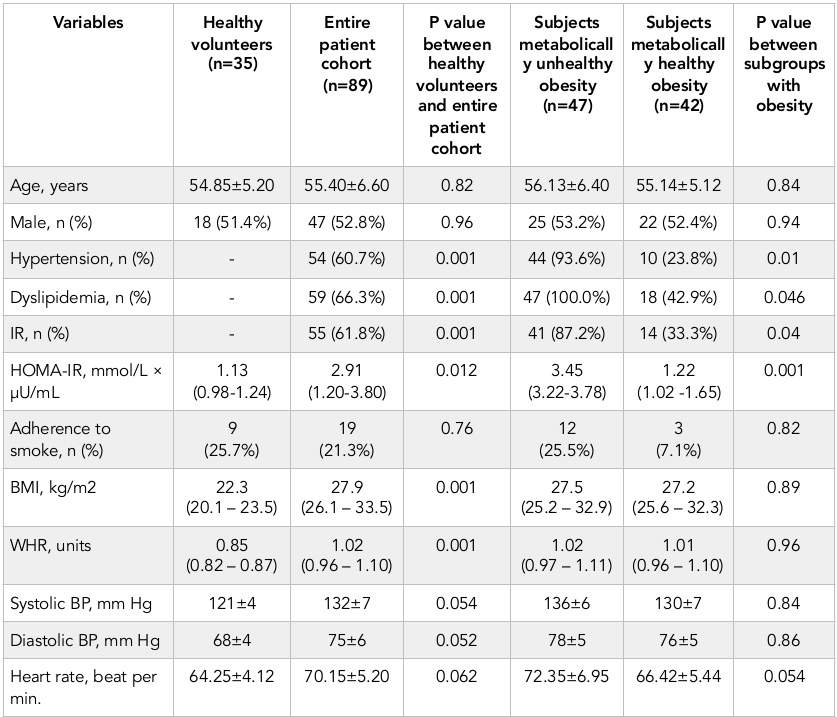
The demographic and anthropometric characteristics, the prevalence of CV risk factors are reported in Table 1 . There was not a significant difference between healthy volunteers and entire cohort, as well as between subjects with metabolically unhealthy obesity (Met-UHO) and metabolically healthy obesity (Met-HO) in age, sex, adherence to smoke and haemodynamic performances. Abdominal obesity subjects exhibited higher BMI, WHR, HOMA-IR, as well as they had increased frequency of LVH, hypertension and IR presentation. Met-UHO patients had higher HOMA-IR, than those with Met-HO, while BMI, systolic and diastolic blood pressure, heart rate were similar in both cohorts. Additionally, hypertension, IR and dyslipidemia presentation were found frequently in Met-UHO patients compared to Met-HO individuals.
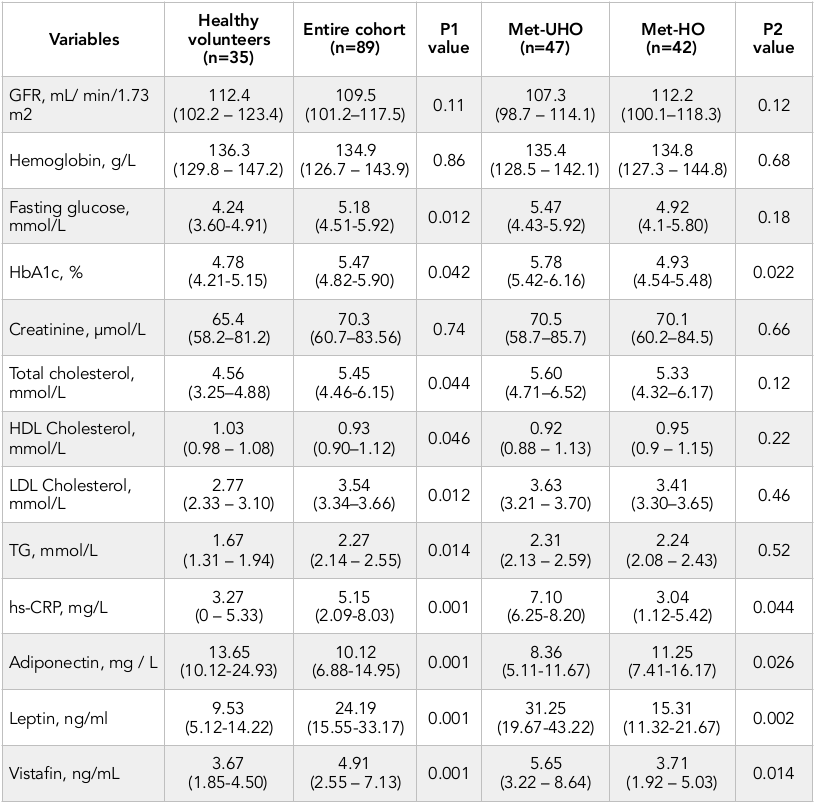
Healthy volunteers and abdominal obesity individuals from entire cohort had similar level of GFR, hemoglobin, and creatinine. Consequently, abdominal obesity individuals had higher level of fasting glucose, HbA1c, total cholesterol, LDL cholesterol, triglycerides, hs-CRP, vistafin, leptin and lower adiponectin ( Table 2 ). No difference was seen in GFR, hemoglobin, fasting glucose, creatinine, and lipids’ level between Met-UHO and Met-HO patients. However, Met-UHO patients had higher HbA1c, vistafin, leptin and lower hs-CRP than those with Met-HO.
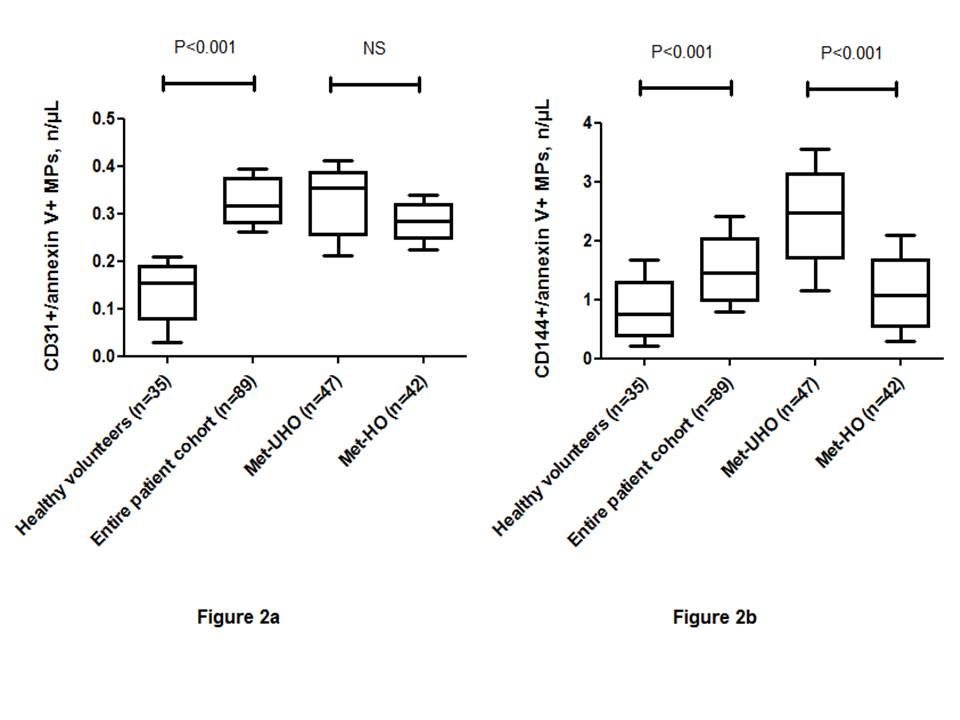
Healthy volunteers had significantly decreased level of EMPs labeled CD31+/Annexin V+ and CD144+/ Annexin V+ than those with abdominal obesity ( Figure 2 ). However, there was not found a significant difference between numbers of EMPs labeled CD31+/Annexin V+ in Met-UHO and Met-HO patients. In contrast, Met-UHO patients had a significantly increased level of circulating CD144+/ Annexin V+ compared with Met-HO individuals.
The univariate linear regression analysis between numerous of EPMs with immune phenotypes determined CD31+/ Annexin V+ and CD144+/ Annexin V+, CV risk factors, hemodynamic performances, and other biomarkers was performed. In Met-UHO patients the number of CD31+/ Annexin V+ EMPs received from peripheral blood positively related to HOMA-IR (r =0.35, P = 0.003), hs-CRP (r = 0.33, P = 0.001), number of CV risk factors (r = 0.32, P = 0.001), BMI (r = 0.31, P = 0.001), serum leptin (r = 0.31, P < 0.001), fasting glucose (r = 0.30, P < 0.001), serum vistafin (r = 0.29, P < 0.001), LDL cholesterol (r = 0.27, P = 0.003), but inversely associated with serum adiponectin (r = -0.31, P < 0.001). In contrast, in Met-HO individuals HOMA-IR (r =0.32, P = 0.001) and number of CV risk factors (r =0.31, P = 0.001) significantly related to number of CD31+/ Annexin V+ EMPs. There were not sufficient relations between number of CD31+/ Annexin V+ EMPs and biomarkers of obesity, i.e. leptin and vistafin, whereas between adiponectin and number of CD31+/ Annexin V+ EMPs an inversely weak association was found (r = -0.25, P = 0.01). Table 2 . The biomarkers of the patients enrolled in the study. Therefore, the number of CD144+/ Annexin V+ EMPs inversely related to a level of serum adiponectin (r = -0.28, P = 0.001) and positively associated with HOMA-IR (r =0.36, P = 0.001), hs-CRP (r = 0.31, P = 0.001), serum vistafin (r = 0.33, P = 0.001), number of CV risk factors (r = 0.32, P = 0.001), serum leptin (r = 0.32, P = 0.001), fasting glucose (r = 0.29, P = 0.012) in Met-UHO patients. In contrast, there was not a significant association between number of CD144+/Annexin V+ EMPs and metabolic biomarkers of obesity, such as adiponectin, leptin and vistafin in Met-HO individuals, whereas they were related to HOMA-IR (r =0.36, P = 0.001), BMI (r = 0.32, P = 0.001) and hs-CRP (r = 0.30, P = 0.001).
Multivariate unadjusted linear regression analysis has shown that in Met-UHO patients the numbers of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs related to HOMA-IR (r = 0.32, P = 0.001 and r =0.33, P = 0.001), hs-CRP (r = 0.30, P = 0.001 and r =0.31, P = 0.001), number of CV risk factors (r =0.29, P = 0.001 and r =0.30, P = 0.001), serum leptin (r = 0.30, P = 0.001 and r = 0.31, P = 0.001), serum vistafin (r = 0.28, P = 0.001 and r = 0.30, P = 0.001), serum adiponectin (r = -0.26, P = 0.001 and r = -0.29, P = 0.001) and LDL cholesterol (r = 0.27, P = 0.001 and r =0.28, P = 0.003) respectively. In Met-HO individuals the numbers of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs related significantly to HOMA-IR (r =0.32, P = 0.001 and r =0.36; P<0.001), BMI (r = 0.27, P = 0.001 and r =0.31; P<0.001) and hs-CRP (r = 0.26, P = 0.001 and r = 0.30, P = 0.001) respectively.
After adjustment for BMI HOMA-IR remained the most profound factor related to numbers of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs (r = 0.33, P = 0.001 and r =0.36, P = 0.001) in Met-UHO. We also determined a relation between numbers of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs and number of CV risk factors (r =0.27, P = 0.001 and r =0.30, P = 0.001), serum leptin (r = 0.27, P = 0.001 and r = 0.29, P = 0.001), serum vistafin (r = 0.28, P = 0.001 and r = 0.30, P = 0.001). Therefore, mild association of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs with HOMA-IR (r = 0.34, P = 0.001 and r =0.36, P = 0.001) was found in Met-HO patients. However, the multivariate adjusted for BMI linear regression analysis has shown that in Met-UHO numbers of CD31+/Annexin V+ and CD144+/ Annexin V+ EMPs associated with serum hs-CRP (r = 0.27, P = 0.001 and r = 0.31, P = 0.001) respectively. In multivariate logistic regression analysis we found that HOMA-IR, number of CV risk factors, serum leptin and hs-CRP were independent predictors for increased numbers of circulating CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs in Met-UHO ( Table 3 ). In Met-HO patients HOMA-IR remained an independent predictor of increased numbers of circulating CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs.
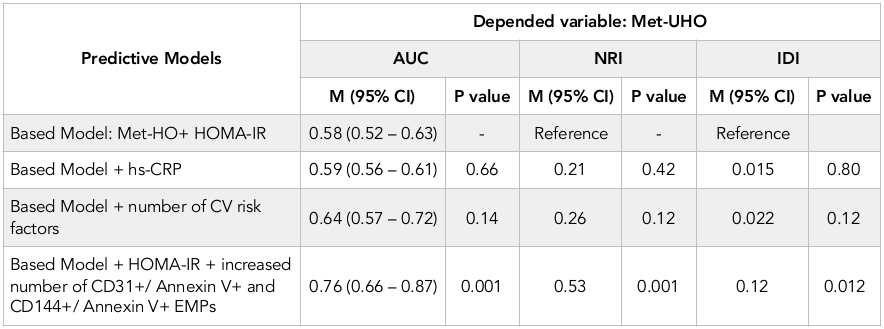
Statistics for model fit for the prediction of Met-UHO development is reported in Table 4 . One can see increased number of CD31+/ Annexin V+ and CD144+/Annexin V+ EMPs added to the based predictive model (Met-HO + HOMA-IR) may sufficiently improve prognostication of based model regarding development of Met-UHO.
Discussion
In this study we reported that increased number of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs much more pretty accurate predict Met-UHO than based model (Met-HO). Taking into consideration that Met-UHO is considered a powerful risk factor of type 2 diabetes mellitus, we suggest that increased number of CD31+/ Annexin V+ and CD144+/ Annexin V+ EMPs related to HOMA-IR in both Met-UHO and Met-HO may predict probably obesity-related complications including higher risk of t2dm. Thus, early detection of abnormality in circulating levels of apoptotic EPCs may be a biomarker of IR and predictor of Met-UHO in patients with abdominal obesity when other metabolic disturbances are absent or evidence regarding them is fare limited.
Recently it has been suggested that Met-HO is a stage of development of Met-UHO and T2DM Mongraw-Chaffin et al., 2016. However, our results revealed that the most important factor that affects metabolic dysregulation in obesity is IR, which probably appears to be predominantly early stage of the Met-HO. There is evidence that an accumulation of visceral adiposity tissue (VAT) might associate with over-production of pro-inflammatory cytokines including hs-CRP, leptin and vistafin and induce IR Brede et al., 2016. Therefore, infiltration of the sub-intima by LDL cholesterol may induce production of free radicals, oxidation of cytoskeleton and membrane vesiculation of endothelial cells. Finally, membrane vesiculation of endothelial cells is enhanced by inflammatory cytokines in conveying of VAT accumulation Mause and Weber, 2010. Interestingly, the circulating number of apoptotic EMPs has well associated with conventionally obesity biomarkers (adiponectin, leptin, vistafin) in Met0UHO patients, but did not in Met-HO individuals. Indeed, in Met-HO patients we did not find severe metabolic abnormalities apart from leptin elevation compared with Met-UHO, however, IR was determined as common finding for both Met-UHO and Met-HO individuals without a difference in BMI.
The increased amount of VAT together with a chronic inflammation and IR predisposes to the development of endothelial dysfunction through attenuation synthesis and secretion of apoptotic EMPs. Indeed, pro-inflammatory cytokines, i.e. interleukin-6, tumor factor necrosis-alpha, leptin, and vistafin, may directly influence structure of endothelial cells and trigger a secretion of apoptotic EMPs Berezin, 2016bRautou et al., 2011. The main biological function of this process is attenuation of endothelial cell repair and recovery of vascular function Jansen et al., 2015. Unfortunately, co-existing IR affects endothelial progenitor cells and they are not able to differentiate into functionally mature endothelial cells even after stimulation by apoptotic EMPs Martinez and Andriantsitohaina, 2011Tetta et al., 2011. As a result, apoptotic EMP-induced endothelial dysfunction and IR may become an early predictor of shaping Met-UHO.
Recently we have reported that apoptotic EMPs may independent predict asymptomatic atherosclerosis and CV disease in T2DM patients Berezin, 2016aBerezin et al., 2016a, while their role in individuals with different phenotypes of obesity has remained controversial Montoro-García et al., 2011. First, it is not clear whether increased number of apoptotic EMPs is adaptive mechanism of vascular repair or factor of endothelial injury. Indeed, circulating EPMs, which are enhanced in a large number of metabolic disorders including abdominal obesity, associated with IR and this has been linked to deleterious effects on endothelial cells Martinez and Andriantsitohaina, 2011. At the same time, apoptotic EPMs are powerful factor contributing in endothelial progenitor cell mobbing and differentiation Montoro-García et al., 2011. Secondary, it is not fully understand the innate molecular mechanisms, which correspond to triggers of secretion of these apoptotic MPs. Apoptotic MPs as cargo microvesicles consist of a variety of biomolecules including regulated proteins, DNA, mRNA, and non-coding RNA. The proportion of these components as well as an entire secretome is under a tight control of autocrine / paracrine mechanisms and inflammatory factors (i.e. tumor necrosis factor-alpha, interleukin-2, -6), which induces EMP formation in a time-dependent manner Lee et al., 2014. Consequently, the final reply of the recipient cells, such as endothelial progenitor cells, is depends on epigenetic regulation of secretome secretion and primary trigger, which affects vesiculation Berezin, 2016bBerezin et al., 2015c. Obviously, an ability of apoptotic EMPs to modulate immune and inflammatory processes, coagulation and vascular function, angiogenesis and vascular injury may interact with other regulatory mechanisms the role of witch in the pathogenesis of abdominal obesity requires still being determined. It is no excluded that release of apoptotic EMPs might act as a direct endogenous survival signal for target cells Lichtenauer et al., 2015.
The present results exhibited first the interrelationship between increased number of circulating apoptotic EMPs and IR in patient with Met-HO. Extrapolating these findings on entire obese population, we can suggest that a clinical diagnosis of abdominal obesity irrespective to its phenotypes (Met-UHO or Met-HO) is probably not sufficient to assess a risk of T2DM and CV disease Berezin et al., 2015c. In this context, measurement of circulating apoptotic EMP number would be useful tool for stratification amongst obesity individuals at higher risk of T2DM and CV Berezin, 2016a, bMontoro-García et al., 2011, especially when conventional biomarkers of obesity are not detected in appropriate diagnostic level. Large investigations are required to understand the role of apoptotic EMPs in pathogenesis of different phenotypes of abdominal obesity, because they may be a target of the therapy as well as predictive biomarkers.
Study limitations
This study has some limitations. The first limitation is low number of the patient involved in the investigation. Another limitation is lack of standardization of MP measurements, while commercial flow cytometers are existed. It is necessary to note that a large pool of MPs might be produced after blood sampling due to destruction of platelets and blood cells. In this study we used platelet free plasma to prevent of contamination of samples with MPs originated from platelets. Therefore, preparation of MP isolates from samples is the most sophisticated step for further examination. The next limitation might relate to complicated assay and suffers from resolution of MP detection technique that is worth considering. Indeed, there were several technical-related difficulties in the measurement of MPs affected centrifugation of samples, labeling of MPs, using HD-FACS methodology and final assay of results obtained. Although HD-FACS methodology is widely used, theoretically overlap between two or more fluorochromes might reflect some obstacles for further interpretation of obtained results, especially including size gating in MP determination. Therefore, rotor type and centrifugation time theoretically may influence on purity of MPs. However, flow cytometery and HD-FACS methodology are commonly used procedure to determine and measure MPs.
Another limitation of the present study is that a specific role of MPs is also possible and has not been characterized. However, the authors suppose that these optionally technically restrictions might have no significant impact on the study data interpretation. Additionally, retrospective, relative small sample size may limit the significance of the present study.
Conclusion
In this investigation we first determined the increased apoptotic EMP number may predict transformation of Met-HO into Met-UHO. The evidence allow suggesting the apoptotic EMPs might serve as a biomarker higher risk of T2DM and CV disease. Future investigations are needed to confirm these suggestions and clear situation around discriminative value of apoptotic EMP number in abdominal obesity individuals.
Abbreviations
AUC: area under the curve; BMI: body mass index; CI: confidence interval; CV: cardiovascular: GFR: glomerular filtration rate; EMPs: endothelial cell-derived mocroparticles; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high sensitive C-reactive protein; IDI: integrated discrimination index; LDL-C: low-density lipoprotein cholesterol; Met-HO: metabolically healthy obesity; MetS: metabolic syndrome; Met-UHO: metabolically unhealthy obesity; MPs: microparticles; NCEP: National Cholesterol Education Program; NRI: net reclassification index; OR: odds ratio.
Author contribution
AEB gave the concept, designed experiment, analyzed data and gave final approval of the manuscript and was the supervisor of the project. AAK performed experiment, analyzed data and wrote draft of manuscript. TAB helped in data acquisition and experiment conditions optimization. YVM and TAS helped in editing and literature review for the paper TAS did data acquisition, organization and data analysis and helped in write up of final draft.
References
-
A.
Agouni,
R.
Andriantsitohaina,
M.
C Martinez.
Microparticles as biomarkers of vascular dysfunction in metabolic syndrome and its individual components. Current vascular pharmacology.
2014;
12
:
483-492
.
-
N.
Alexandru,
E.
Badila,
E.
Weiss,
D.
Cochior,
E.
Stępień,
A.
Georgescu.
Vascular complications in diabetes: Microparticles and microparticle associated microRNAs as active players. Biochemical and biophysical research communications.
2016;
472
:
1-10
.
-
F.
Antwi,
N.
Fazylova,
M.-C.
Garcon,
L.
Lopez,
R.
Rubiano,
J.T.
Slyer.
The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: A systematic review. JBI Database of Systematic Reviews and Implementation Reports.
2012;
10
:
1-14
.
-
M.
Ashwell,
P.
Gunn,
S.
Gibson.
Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity reviews.
2012;
13
:
275-286
.
-
F.
Basterra-Gortari,
M.
Bes-Rastrollo,
M.
Ruiz-Canela,
A.
Gea,
M.
Martinez-Gonzalez.
Trends in the prevalence of obesity and diabetes in Spanish adults 1987-2012. Medicina clinica.
2017
.
-
A.
Berezin,
A.
Zulli,
S.
Kerrigan,
D.
Petrovic,
P.
Kruzliak.
Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clinical biochemistry.
2015a;
48
:
562-568
.
-
A.E.
Berezin.
Biomarkers for cardiovascular risk in patients with diabetes. BMJ Publishing Group Ltd and British Cardiovascular Society.
2016a
.
-
A.E.
Berezin.
Cardiac biomarkers in diabetes mellitus: New dawn for risk stratification?. Diabetes & Metabolic Syndrome: Clinical Research & Reviews.
2016b
.
-
A.E.
Berezin,
A.A.
Kremzer,
T.A.
Berezina,
Y.V.
Martovitskaya.
Pattern of circulating microparticles in chronic heart failure patients with metabolic syndrome: Relevance to neurohumoral and inflammatory activation. BBA clinical.
2015b;
4
:
69-75
.
-
A.E.
Berezin,
A.A.
Kremzer,
T.A.
Berezina,
Y.V.
Martovitskaya.
The pattern of circulating microparticles in patients with diabetes mellitus with asymptomatic atherosclerosis. Acta clinica Belgica.
2016a;
71
:
38-45
.
-
A.E.
Berezin,
A.A.
Kremzer,
G.
Cammarota,
A.
Zulli,
D.
Petrovic,
N.
Martell-Claros,
J.
Sabo,
P.
Kruzliak.
Circulating endothelial-derived apoptotic microparticles and insulin resistance in non-diabetic patients with chronic heart failure. Clinical Chemistry and Laboratory Medicine (CCLM).
2016b;
54
:
1259-1267
.
-
A.E.
Berezin,
A.A.
Kremzer,
Y.V.
Martovitskaya,
T.A.
Berezina,
E.A.
Gromenko.
Pattern of endothelial progenitor cells and apoptotic endothelial cell-derived microparticles in chronic heart failure patients with preserved and reduced left ventricular ejection fraction. EBioMedicine.
2016c;
4
:
86-94
.
-
A.E.
Berezin,
A.A.
Kremzer,
T.A.
Samura,
T.A.
Berezina,
P.
Kruzliak.
Immune phenotypes of endothelial-derived microparticles in dysmetabolic patients. Journal of Proteomics & Bioinformatics.
2015c;
8
:
60
.
-
S.
Brede,
G.
Serfling,
J.
Klement,
S.M.
Schmid,
H.
Lehnert.
Clinical Scenario of the Metabolic Syndrome. Visceral medicine.
2016;
32
:
336-341
.
-
W.E.
Consultation.
Waist circumference and waist-hip ratio. Report of a WHO. Expert Consultation Geneva: World Health Organization.
2008;
:
8-11
.
-
D.J.
Cuthbertson,
T.
Steele,
J.P.
Wilding,
J.C.
Halford,
J.A.
Harrold,
M.
Hamer,
F.
Karpe.
What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications. 2017
.
-
A.
Cvjetkovic,
J.
Lötvall,
C.
Lässer.
The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. Journal of extracellular vesicles.
2014;
3
.
-
M.M.
Finucane,
G.A.
Stevens,
M.J.
Cowan,
G.
Danaei,
J.K.
Lin,
C.J.
Paciorek,
G.M.
Singh,
H.R.
Gutierrez,
Y.
Lu,
A.N.
Bahalim.
National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9· 1 million participants. The Lancet.
2011;
377
:
557-567
.
-
K.M.
Flegal,
M.D.
Carroll,
C.L.
Ogden,
L.R.
Curtin.
Prevalence and trends in obesity among US adults, 1999-2008. Jama.
2010;
303
:
235-241
.
-
S.M.
Grundy,
J.I.
Cleeman,
S.R.
Daniels,
K.A.
Donato,
R.H.
Eckel,
B.A.
Franklin,
D.J.
Gordon,
R.M.
Krauss,
P.J.
Savage,
S.C.
Smith.
Diagnosis and management of the metabolic syndrome. Circulation.
2005;
112
:
2735-2752
.
-
S.M.
Grundy,
J.I.
Cleeman,
C.N.B.
Merz,
H.B.
Brewer,
L.T.
Clark,
D.B.
Hunninghake,
R.C.
Pasternak,
S.C.
Smith,
N.J.
Stone.
A summary of implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Am Heart Assoc.
2004
.
-
F.
Jansen,
X.
Yang,
K.
Baumann,
D.
Przybilla,
T.
Schmitz,
A.
Flender,
K.
Paul,
A.
Alhusseiny,
G.
Nickenig,
N.
Werner.
Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. Journal of cellular and molecular medicine.
2015;
19
:
2202-2214
.
-
H.-N.
Kim,
S.-H.
Kim,
Y.-M.
Eun,
S.-W.
Song.
Obesity with metabolic abnormality is associated with the presence of carotid atherosclerosis in Korean men: a cross-sectional study. Diabetology & metabolic syndrome.
2015;
7
:
68
.
-
R.
Lacroix,
C.
Judicone,
M.
Mooberry,
M.
Boucekine,
N.S.
Key,
F.
Dignat-George.
Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. Journal of Thrombosis and Haemostasis.
2013;
11
:
1190-1193
.
-
S.K.
Lee,
S.-H.
Yang,
I.
Kwon,
O.
Lee,
J.H.
Heo.
Role of tumour necrosis factor receptor-1 and nuclear factor-B in production of TNF--induced pro-inflammatory microparticles in endothelial cells. Thrombosis and haemostasis.
2014;
112
:
580-588
.
-
A.S.
Levey,
L.A.
Stevens,
C.H.
Schmid,
Y.L.
Zhang,
A.F.
Castro,
Feldman
3rd,
J.W.
Kusek,
P.
Eggers,
F.
Van Lente,
T.
Greene.
A new equation to estimate glomerular filtration rate. Annals of internal medicine.
2009;
150
:
604-612
.
-
M.
Lichtenauer,
B.
Goebel,
M.
Fritzenwanger,
M.
Förster,
S.
Betge,
A.
Lauten,
H.-R.
Figulla,
C.
Jung.
Simulated temporary hypoxia triggers the release of CD31+/Annexin+ endothelial microparticles: A prospective pilot study in humans. Clinical hemorheology and microcirculation.
2015;
61
:
83-90
.
-
N.
Lindson-Hawley,
R.
Begh,
M.S.
McDermott,
A.
McEwen,
D.
Lycett.
The importance of practitioner smoking status: A survey of NHS Stop Smoking Service practitioners. Patient education and counseling.
2013;
93
:
139-145
.
-
M.C.
Martinez,
R.
Andriantsitohaina.
Microparticles in angiogenesis. Circulation Research.
2011;
109
:
110-119
.
-
D.
Matthews,
J.
Hosker,
A.
Rudenski,
B.
Naylor,
D.
Treacher,
R.
Turner.
Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia.
1985;
28
:
412-419
.
-
S.F.
Mause,
C.
Weber.
Microparticles. Circulation research.
2010;
107
:
1047-1057
.
-
M.
Mongraw-Chaffin,
M.C.
Foster,
R.R.
Kalyani,
D.
Vaidya,
G.L.
Burke,
M.
Woodward,
C.A.
Anderson.
Obesity severity and duration are associated with incident metabolic syndrome: Evidence against metabolically healthy obesity from the Multi-Ethnic Study of Atherosclerosis. The Journal of Clinical Endocrinology & Metabolism.
2016;
101
:
4117-4124
.
-
S.
Montoro-García,
E.
Shantsila,
F.
Marín,
A.
Blann,
G.Y.
Lip.
Circulating microparticles: new insights into the biochemical basis of microparticle release and activity. Basic research in cardiology.
2011;
106
:
911-923
.
-
A.F.
Orozco,
D.E.
Lewis.
Flow cytometric analysis of circulating microparticles in plasma. Cytometry Part A.
2010;
77
:
502-514
.
-
P.-E.
Rautou,
A.-C.
Vion,
N.
Amabile,
G.
Chironi,
A.
Simon,
A.
Tedgui,
C.M.
Boulanger.
Microparticles, vascular function, and atherothrombosis. Circulation research.
2011;
109
:
593-606
.
-
J.
Rey-López,
L.
Rezende,
M.
Pastor-Valero,
B.
Tess.
The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obesity reviews.
2014;
15
:
781-790
.
-
L.
Ryden,
P.J.
Grant,
S.D.
Anker,
C.
Berne,
F.
Cosentino,
N.
Danchin,
C.
Deaton,
J.
Escaned,
H.P.
Hammes,
H.
Huikuri.
ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). European heart journal.
2013;
34
:
3035-3087
.
-
S.A.
Sterling,
A.E.
Jones,
R.D.
Cox.
Longitudinal Trends in the Prevalence of Diabetes Mellitus in an Urban Emergency Department. Southern medical journal.
2016;
109
:
222-227
.
-
R.
Sturm.
Increases in morbid obesity in the USA: 2000-2005. Public health.
2007;
121
:
492-496
.
-
C.
Tetta,
S.
Bruno,
V.
Fonsato,
M.C.
Deregibus,
G.
Camussi.
The role of microvesicles in tissue repair. Organogenesis.
2011;
7
:
105-115
.
-
S.
Valdés,
F.
García-Torres,
C.
Maldonado-Araque,
A.
Goday,
A.
Calle-Pascual,
F.
Soriguer,
L.
Castaño,
M.
Catalá,
R.
Gomis,
G.
Rojo-Martínez.
Prevalence of obesity, diabetes and other cardiovascular risk factors in Andalusia (southern Spain). Comparison with national prevalence data. The Diabetes study. Revista Española de Cardiología (English Edition).
2014;
67
:
442-448
.
-
D.
Vanuzzo,
L.
Pilotto,
R.
Mirolo,
S.
Pirelli.
Cardiovascular risk and cardiometabolic risk: an epidemiological evaluation. Giornale italiano di cardiologia (2006).
2008;
9
:
6S-17S
.
-
L.
Williams.
Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation.
2002;
106
:
3143-3143
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 1 (2017)
Page No.: 1110-1128
Published on: 2017-01-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6397 times
- Download PDF downloaded - 1983 times
- View Article downloaded - 21 times
 Biomedpress
Biomedpress