Human umbilical cord blood derived mesenchymal stem cells were differentiated into pancreatic endocrine cell by Pdx-1 electrotransfer
Abstract
Diabetes mellitus type 1 is an autoimmune disease with high incidence in adolescents and young adults. A seductive approach overcomes normally obstacles treatment is cell-replacement therapy to endogenous insulin production. The pancreatic duodenal homeobox 1 (Pdx1) is essential transcription factor pancreatic development, beta cell differentiation and other metabolic processes. This study aimed to orient umbilical cord blood-derived mesenchymal stem cell (UCB-MSCs) to pancreatic endocrine cells by Pdx1 electrotransfer. The results showed that the low voltage of electrotransfer significantly increased in the efficiency of electrotransfer and survival cells compared with other high voltages. Pdx-1 transfected UCB-MSCs over-expressed pancreatic related genes as Ngn3, Nkx6.1. These results suggested that Pdx1 transfected UCB-MSCs were successfully oriented pancreatic endocrine cells. Different to lentiviral vectors, electrotransfer is a safer method to transfer Pdx-1 to UCB-MSCs and a useful tool in translational research.
Introduction
Diabetes mellitus type 1 autoimmune disease is caused by insulin deficiency in which insulin-producing beta cells were destroyed by immune systems and developed severe metabolic abnormalities Vudattu and Herold, 2014. At the present, diabetes mellitus type 1 was treated by either insulin administration or pancreatic/islet of Langerhans transplantation Beltrand and Robert, 2013Oberholzer and Morel, 2002. Although the efficiency and feasibility of these therapies were recognized, the obstacles were still not deleted absolutely. The patients have been burdened in treatment and daily life such as stringent injection of insulin, side effects of immunosuppressant drugs in transplantation and even thought the shortage of replace pancreatic Wu and Mahato, 2014. Hence, a novel therapy is required for public treatment.
The embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs) are the potential candidates in stem cell therapies in diabetes mellitus treatment. ESCs can be differentiated into all cell type originated from three germ layers of the embryo, including beta cells. However, ESC based applications were barricaded by ethical reasons and the high rate of malignant tumor formation Abdelalim et al., 2014Su et al., 2011. MSCs possess the ability of high self-renewal, easily develop into various cells, especially modulate immune system; so they were especially interested in diabetes mellitus type 1 treatment Helledie et al., 2008Wu and Mahato, 2014. To date, MSCs were successfully isolated from abundant sources such as bone marrow, umbilical cord blood, adipose tissues Wu and Mahato, 2014.
MSCs could be differentiated into PECs by some different ways. MSCs could be in vivodifferentiated into insulin producing cells (IPCs) by direct injection of MSCs into pancreatic gland Ianus et al., 2003Karaoz et al., 2013Phuc et al., 2011. In vitro, MSCs were successfully induced by chemical exposure Chao et al., 2008Ngoc et al.,2011Phuc et al., 2011Wei et al., 2012 or co-culture with pancreatic cells to IPCs Karaoz et al., 2013. However, beta cell differentiation of MSCs also low, and that was the main difficulty to use them in clinic. Therefore, the combination of gene therapy to improve differentiation efficacy of MSCs into PECs is interested in much recent research groups.
Pancreatic duodenal homeobox 1 transcription factor (Pdx-1) holds an important role in pancreatic development Oliver-Krasinski, 2008. In the early phase of embryo, Pdx-1activates the forming process of all cell types in pancreatic such as endocrine, exocrine and duct cells. In the human, Pdx-1 was highly expressed in progenitor pancreatic to not only orienting the differentiation, but also maintaining the function of beta cells and activation of other insulin-related genes. The Pdx-1 mutated mice were impaired the pancreatic development and died after several days. Pdx-1 deficiency lead to the disorders of pierce pancreatic function, the death of beta cell and diabetes mellitus in both human and rodent.
In fact, by Pdx-1 transfection, some authors successfully differentiated and trans-differentiated of some kinds of cells into IPCs or PECs. Ferber et al were trans-differentiated mouse hepatic cells to IPCs by Rattus Pdx-1 adenovirus infection Ferber et al., 2000. The same results were reported by Kojima et al (2003) combined infection of NeuroD and BTC Kojima et al., 2003, Sapir et al (2005) combined infection of Pdx-1 and endothelium growth factors to reborn the new islet in the liver, after that transplant to treat diabetes mellitus mice Sapir et al., 2005. The combination of Pdx-1 with other growth factors was believed in decreasing the effect of exocrine and maintain the phenotype of endocrine. In addition, after Pdx-1 and BTC transfection, the MSCs were induced in pancreatic ductal epithelium progenitor cell which positive with both of Ck19 and Nestin markers Li et al., 2008. The gene therapy delivery systems can be used for transgene transfer into MSCs comprises viral and non-viral methods. Viral vectors allow gene delivery efficient and long-term expression in MSCs, but safety concerns were considered in clinical applications Cone and Mulligan, 1984Nayerossadat et al., 2012. To overcome these drawbacks, non-viral methods have been substituted for delivering therapeutic genes into MSCs. Electrotransfer techniques were determined most efficient for gene delivering into MSCs with highs of percentages of gene-transfected cells. A recent study showed that by electrotransfer MSCs had still maintained “stem” characteristics such as the ability of proliferative, differentiation after gene manipulation Jordan et al., 2008. So that, in view of these advantages, this study aimed to differentiate UCB-MSCs into PECs by Pdx-1 electrotransfer.
Materials – Methods
Isolation and characterization of UCB-MSCs
UCB-MSCs were isolated according to the previous published publication Pham et al.,2014. In briefly, UCB was collected to prepare activated platelet-rich plasma (aPRP) and mononuclear cells (MNCs). aPRP was applied as a supplement at 5% in Iscove modified Dulbecco medium (IMDM) together with antibiotics. MNCs were cultured in complete IMDM to isolate UCB-MSCs. Isolated cells were sub-cultured for 3 passages before used in further experiments.
Plasmid construction of pcDNA3.1-Pdx1 and purified plasmid preparation
The full-length human Pdx-1 sequence was isolated from pWPT vector (9.5kb) (Addgene, Cambridge, MA). Both of Pdx-1 gene and pcDNA3.1 was prepared by double digestion of BamHI and NotI enzymes to remove Pdx-1 gene from pWPT and open pcDNA3.1 plasmid. Then the purified Pdx1 was inserted into opened pcDNA3.1 plasmid (8.6kb) by T4 ligase (all bought from Invitrogen, NY). The ligation reaction was objected to electrotransfer into Ecoli. DH5α and colony screening in ampicillin-contained LB-agar. The candidate colonies which grew on Ampicilin LB-agar was amplified on LB medium to isolate interested plasmid. The insert of Pdx1 into pcDNA3.1 plasmid was confirmed by double-digestion reaction and DNA sequencing. The recombinant pcDNA3.1 plasmid extracted in large-scale by Qiagen Kit with abundant circular form, high purified A280/260 >1.8 and free-endotoxin would be used to for further experiments.
Buffer and voltage selection for electrotransfer
The electrotransfer process was performed under the instruction of electroporation system (Eppendorf, Germany). To facilitate electrotransfer, both the cells and their nucleus assume rounded form and the cell membrane detached from the cytoskeleton. The osmotic concentration lower than physiological buffer solutions was suggested. We seek to the lowest osmotic concentration of electrotransfer buffer which stable cell membrane and survival. The cells were de-attached by Trypsin-EDTA 0.25%, and the cell pellets were resuspended in Hypoosmolar buffer (Eppendorf, Germany). The cell stability and viability were evaluated by under microscope after cells were incubated at 0, 10, 20, 30, and 40 minutes in the Hypoosmolar buffer. The diameters of rounded cells were measured at 0, 10, 20, 30, and 40 minutes to determine optimal voltage for the electrotransfer. The field strength was calculated based on the formular Ec = Vc / (0.75 x d) and V = Ec x l with Ec: Critical field strength [V / cm]; Vc: Permeation voltage of the membrane (1 V at 20 °C) and d: Cell diameter [cm]; l : gap width of the cuvette (0.2 cm). In the case of suspension cells, the ideal value for introducing plasmid DNA into the cell is usually 1 to 3 times that of Ec (follow in Eppendorf’s manual).
Plasmid electrotransfer
The cell pellet was washed twice with PBS and resuspended in hypoosmolar buffer at concentration 2.106/ml of cells. A mixture of 400μl of UCB-MSCs suspension and the pcDNA3.1-Pdx-1 (10μg/ml) was added into an electrocuvette with 2-mm gap in width. After pulsing the cell suspension was allowed to incubate in the cuvette for 5-10 minutes. The cell suspension was collected from the cuvette and cultured in 3 ml DMEM in a 60-mm culture dish. After 72 hours, the G418 (200μg/ml) was added to the culture medium to select successfully Pdx-1 transfected UCB-MSCs. This concentration of G418 was investigated in previous assays. The selection medium was changed every 3-day. At day 10 of the selection, the UCB-MSCs were isolated for further assays. Electrotransfer efficacy was recorded in three voltages 200V, 350V, and 550V.
Annexin-V and PI analysis
The efficacy of pcDNA3.1-Pdx-1 electrotransfer was performed by the amount of viable cells after selecting with G418 basing Annexin-V and PI analysis (BD Bioscience, Franklin Lakes, NJ). Viable cells after selected with G418 reflected that they were successfully transfected with Pdx-1 plasmid. The UCB-MSCs were detached by Trypsin-EDTA 0.25% and washed twice with cold PBS. The cells were resuspended in 100μl of binding buffer and add 5μl of FITC Annexin-V and add 10μl PI solution. The UCB-MSCs were gently vortexed and incubated for 15 min at room temperature in the dark. Flow cytometry analysis was occurred within 1 hour, followed by added 400μl of binding buffer to each tube. The percentage of Annexin-V and PI negative cells were used to evaluate efficient electrotransfer in various voltages 200V, 350V, 550V, respectively. The experiment was triplicated independently.
Pancreatic endocrine related gene expression
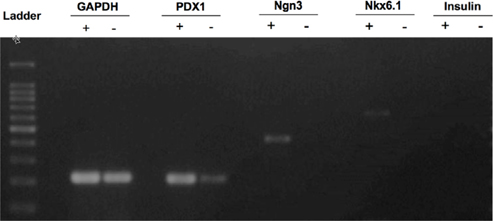
Total RNA of pcDNA3.1-Pdx-1 transferred UCB-MSCs was extracted using easyBlue (Intron biotech, Korea) with high quality A280/260>1.8 confirmed by photometer machine (Eppendorf, Germany). About 1000 ng of total RNA was synthesized into cDNA by reverse transcriptase superscript II (Invitrogen, Carlsbad, CA) following the manufacturer’s instruction. The cDNA were amplified by polymerase chain reaction (PCR) with following primer pairs to detect the expression of pancreatic endocrine related-genes, GAPDH, forward, 5’ TGCTGGCGCTGAGTACGTCG 3’ and reverse, 5’ TGACCTTGGCCAGGGGTGCT-3’; Pdx1, forward, 5’ GGATGAAGTCTACCAAAGCTCACGC-3’, reverse, 5’ CCAGATCTTGATGTGTCTCTCGGTC-3’; Ngn3, forward, 5’ TCGCTGCTCATCGCTCTC-3’, reverse 5’ CCAACTCGCTCTTAGGCC-3’; Nkx6.1, forward, 5’ TCTTCTGGCCCGGAGTGA-3’, reverse, 5’ CCAACAAAATGGATCCTTGATGA-3’; Insulin, forward, 5’ CTACCTAGTGTGCGGGGAAC-3’, reverse, 5’ CACAATGCCACGCTTCTG-3’. TheGAPDH gene served as an internal control. Polymerase chain reaction (PCR) amplification was performed by Intron pfu according to the following cycling parameters: 94˚C for 30 second, 58˚C for 30 second, 72˚C for 30 second, repeating for 40 cycles. PCR products were separated by electrophoresis on a 2% agarose gel containing ethidium bromide and visualize under gel reader machine system (UVP, Upland, CA).
Statistical analysis
The mean and SD were calculated for each experimental group. Differences between groups were analyzed by oneway ANOVA in SPSS 16.0 software. Significant differences among groups were calculated at indicated P < 0.05.
Results
UCB-MSCs isolation and proliferation
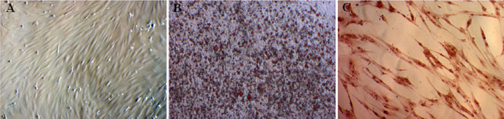
Similar to published publication, we successfully isolated UCB-MSCs. These cells also confirmed as MSCs according to MSC criteria suggested by Dominici et al. Dominici et al.,2006. These cells exhibited the particular shape like fibroblasts. They expressed the MSC marker profiles such as positive with CD44, CD73, CD90, and CD105; negative with CD14, CD34, CD45 and HLA-DR. In the induced conditions, UCB-MSCs were differentiated into osteoblasts, chondrocytes and adipocytes ( Figure 1 ).
pcDNA3.1-Pdx-1 vector
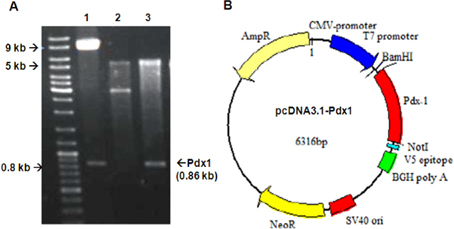
The recombinant pcDNA3.1-Pdx-1 plasmid was double digested with BamHI and NotI enzyme. The results showed that the two bands of linear pcDNA3.1 5.5 kb and Pdx-10.86 kb were isolated. These results indicated that Pdx-1 full- length was inserted accurately into the expression pcDNA3.1 vector ( Figure 2 ). The BamHI and NotI double digestion of pWPT and empty pcDNA3.1 vector were used as control. Furthermore, thePdx-1 gene inserted into pcDNA3.1-Pdx-1 vector was confirmed to be completely identical to the sequence of Pdx-1 (data not shown).
The influence of osmosis buffer on UCB-MSC diameter and field strength
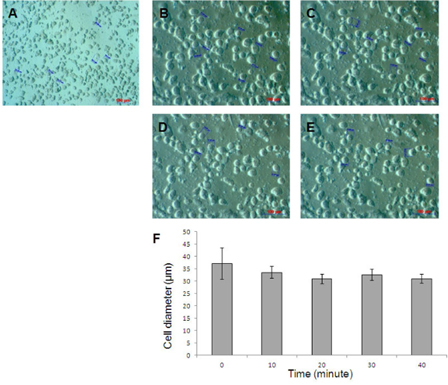
The results showed that the UCB-MSCs still maintained rounded cell shape after 40 minutes in 90 mOsmol/kg osmosis buffer. At this osmosis buffer, cells did not changes in size in electrotransfer condition (P<0.05). In fact, the cell diameters were 37.14±6.38, 33.51±2.41; 30.83±1.85; 32.51±2.25; 30.92±1.87 μm (n = 5) after incubation of 0, 10, 20, 30, 40 minutes, respectively ( Figure 3 ). Based on cell diameter, we calculated the voltage pulses for UCB-MSC electrotransfer is 200V, 350V, 550V respectively.
The efficiency of Pdxl electrotransfer in UCB-MSCs
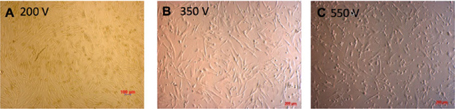
After 24 hours of the electrotransfer, UCB-MSCs was observed under microscope to determine the effect of voltage on cell viability ( Figure 4 ). The Annnexin-V and PI analysis was performed to evaluate of electrotransfer efficacy followed by G418 selection for 10 days. Cell viability of UCBMSCs after selection was altered in 200V, 350V, and 550V. The adherent cells in 200V were higher than others. The cell viability after 10-day G418 selection was 82.94 ± 4.17, 39.93 ± 12.7, 49.5 ±3.02 at 200V, 350V, 550 V, respectively ( Figure 4 ).
Transfected UCB-MSC phenotypes
The Pdx1-UCB-MSCs over-expressed Pdx-1 at 24 h (data not shown) and prolong the expression until day 10 of G418 selection. The expression of the pancreatic endocrine-related Ngn3, Nkx6.1 genes was detected after G418 selection. Expression of theInsulin gene was not observed at the end of the experiment ( Figure 5 ).
Discussion
Nowadays, the non-viral vector transfection methods are broken down into two major approaches including liposome and electrotransfer. The non-viral vector based methods are more advantages than the viral based vector systems Kamimura et al.,2011. Despite fast and efficient way to deliver genes of viral method, non-viral vectors must be seriously considered for their safety in clinical trials Manjila et al.,2013. The non-viral liposome methods were acknowledged in improvement of gene transfected efficient by the positive charge of commercial liposomes such as FuGENE 6 (Promega), Lipofectamine 2000 or DMRIE-C (Life Technologies), Lipoflex (Takara). However, these liposomes could damage cells and change cell phenotype when cultured in high concentration of cationic lipid. Compared to liposome methods, the electrotransfer efficacy was autonomous and low toxic. More importantly, the electrotransfer was demonstrated that did not impair the characteristics of stem cells and maintained multipotent and proliferation of cells after transfection Helledie et al.,2008. Furthermore, the electric capacitance is directly proportional the efficiency of transformation and independent on DNA concentration Oldak et al.,2002. In previous research, the efficacy of pCMV-DsRed transfected MSCs by electrotransfer, Fugene5, Lipofectamine 2000, Lipofectamine Plus methods were compared Helledie et al.,2008. The results showed that the electrotransfer method achieved more 90% transfected cells than lipofection Helledie et al.,2008. From this advantage, electrotransfer was dominant applied to orienting the differentiation of stem cell to functional cell in regenerative medicine.
In this study, we induced the differentiation of UCB-MSCs into endothelial pancreatic cells by Pdx-1 electrotransfer. Electric field strength and the osmosis solution take a crucial role to achieve the highest efficiency of transformation as well as maintaining cell viability Amiri Yekta et al., 2013Jordan et al., 2008Mir, 2014. In our results, the average diameter and the survival of UCB-MSCs in Hypoosmolar solution were used to determine the electric field strength in the optimal osmosis buffer. The 90mOsm hypoosmolar was suitable for UC-MSCs membrane stability with more than 90% of cell viability. This alternation of UCB-MSCs membrane was consistent with Marko et al. in evaluation of CHO, V79, B16-F1’s cell diameter in 100 mOsm hypotonic solution Marko Ušaj, 2009. Thus, UCB-MSCs were optimal ready to electrotransfer followed by the incubation in Hypoosmolar solution within 10 minutes to achieve the largest size. From these results, we calculated the voltage range 200V, 350V, and 550V based on the average diameter at 10-minute incubation. Besides that, electroplated efficiency also depends on the condition, density of cells, nucleic acid concentration, as well as the nucleic acid and depends on each cell line, will have the voltage for different the highest transformed efficiency Jordan et al., 2008Weecharangsan et al., 2007.
After 10-day G418 selection, the Pdx-1 transfected UCBMSCs was objected in Annexin-V and PI analysis. Annexin V and PI stains were used to discriminate the early apoptotic cells (Annexin-V positive only) and late apoptotic/necrotic cells (both Annexin V and PI positive) from the integrity cells (Annexin–V and PI negative) Bakar et al.,2014Balaji et al., 2013Zhou et al., 2012. The percentage of Annexin V and PI negative UCB-MSCs was the percentage of survival Pdx-1-UCB-MSCs under G418 selection. The results showed that the number of survival Pdx-1-UCB-MSCs at 200 V voltage were significantly higher (82.94 ± 5.1) than at 350V and 550V voltages (P<0.05). In theory, the damaged cell membrane could not recover and underwent necrosis because of high voltage and long pulse (100μs in this experiment). The low voltage and long pulse can bring out the high survival rate of cells Zhou et al., 2012. In another experiment, the epithelial cells of embryonic mouse also showed a high proportion of cells (5%-20%) expressed foreign gene without cell death by using low-voltage square wave electrotransfer Abud et al., 2004. Therefore, low voltage (200V) and longpulse (100μs) was an appropriate value to electrotransfer into UCB-MSCs.
Pdx-1 is the crucial transcription factor in pancreatic formation, can turn on the differentiation and development of stem cells to pancreatic buds. Our results showed that the over-expression of Pdx-1 could induce UCB-MSCs to express pancreatic endocrine related-genes in culture medium in vitro. These cells were detected to express Pdx-1, Ngn3, and Nkx6.1 which are insulin gene transcription-regulating mechanism of pancreatic β-cells. Pdx-1 has been demonstrated to take an initial role in regulating insulin gene expression and in the activation of the differentiation process of pancreas precursor cells to endocrine pancreas cells. Our results showed that endogenous Pdx-1 also detected, but unable to trigger the expression of Ngn3, Nkx6.1to induce cell differentiation in original UCB-MSCs. Our results were consistent with previous reports in which Pdx-1 expression activated Ngn3, Nkx6.1 expression Hermann et al., 2011Pedersen et al., 2005, in which Pdx-1, Ngn3 and Nkx6.1 were important for the differentiation of pancreatic endocrine cells Nelson et al., 2007. After 10 days of selection, the Pdx-1-UCB-MSCs were only oriented to endocrine pancreatic cells but defeated in insulin expression. It suggested that the Pdx-1 transfected UCB-MSCs may be switched to insulinproducing cells completely whenever were triggered by induction factors such as the medium containing high glucose, β-mercaptoethanol, as well as HGF and EGF.
Conclusion
In summary, our findings indicated that Pdx-1-electrotransfer UCB-MSCs could be oriented to differentiate into endocrine pancreatic cells, which expressed β-cell-related genes such as Ngn3, Nkx6.1 in vitro. Therefore, Pdx-1 electrotransfer of UCB-MSCs can become an emerging candidate in β-cell replacement therapy of diabetes. However, the proliferative and functional stability of endocrine pancreatic induced UCB-MSCs needs further investigations.
Abbreviations
aPRP: Activated platelet-rich plasma, BTC: Betacellulin, Ck19: Cytokeratin 19, DMEM: Dulbecco's modified Eagle's medium, Ec: Critical field strength, ESCs: Embryonic stem cells, IMDM: Iscove modified Dulbecco medium, IPCs: Insulin producing cells, MNCs: mononuclear cells, MSCs: Mesenchymal stem cells, Ngn3: Neurogenin 3, Nkx6.1: NK6 homeobox 1, Pdx-1: Pancreatic duodenal homeobox 1 transcription factor, PECs: Pancreatic endocrine cells, PI: Propidium Iodide, UCB-MSCs: Umbilical cord-derived mesenchymal stem cells, UCB: Umbilical cord blood, Vc: Permeation voltage of the membrane.
Authors’ contributions
Phuoc Thi-My Nguyen and Anh Thai-Quynh Nguyen made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data. Phuoc Thi-My Nguyen made drafting the article and Anh Thai-Quynh Nguyen reviewed and revise critically for important intellectual content and final approval of the version to be published. Nhung Thi Nguyen, Nguyet Thi-Minh Nguyen, Thu Thi Duong contributed in conduct plasmid construct experiment and acquisition of data. Nhung Hai Truong and Ngoc Kim Phan are research leaders of this study.
Thi Duong contributed in conduct plasmid construct experiment and acquisition of data. Nhung Hai Truong and Ngoc Kim Phan are research leaders of this study.
References
-
E.M.
Abdelalim,
A.
Bonnefond,
A.
Bennaceur-Griscelli,
P.
Froguel.
Pluripotent Stem Cells as a Potential Tool for Disease Modelling and Cell Therapy in Diabetes. Stem cell reviews.
2014
.
-
H.E.
Abud,
P.
Lock,
J.K.
Heath.
Efficient gene transfer into the epithelial cell layer of embryonic mouse intestine using lowvoltage electroporation. Gastroenterology.
2004;
126
:
1779-1787
.
-
A.
Amiri Yekta,
A.
Dalman,
M.H.
Sanati,
N.
Fatemi,
H.
Vazirinasab,
A.
Zomorodipour,
M.
Chehrazi,
H.
Gourabi.
Optimization of The Electroporation Conditions for Transfection of Human Factor IX into The Goat Fetal Fibroblasts. Cell journal.
2013;
14
:
270-275
.
-
F.
Bakar,
U.
Unluturk,
N.
Baskal,
S.
Nebioglu.
Annexin V Expression and Anti-Annexin V Antibodies in Type 1 Diabetes. The Journal of clinical endocrinology and metabolism, jc20132592.
2014
.
-
N.
Balaji,
A.S.
Devy,
M.K.
Sumathi,
S.
Vidyalakshmi,
G.S.
Kumar,
S.
D’Silva.
Annexin v - affinity assay - apoptosis detection system in granular cell ameloblastoma. Journal of international oral health : JIOH.
2013;
5
:
25-30
.
-
J.
Beltrand,
J.J.
Robert.
[Insulin treatment in children with type one diabetes]. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie 20 Suppl.
2013;
4
:
S131-135
.
-
K.C.
Chao,
K.F.
Chao,
Y.S.
Fu,
S.H.
Liu.
Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PloS one.
2008;
3
:
e1451
.
-
R.D.
Cone,
R.C.
Mulligan.
High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proceedings of the National Academy of Sciences of the United States of America.
1984;
81
:
6349-6353
.
-
M.
Dominici,
K.
Le Blanc,
I.
Mueller,
I.
Slaper-Cortenbach,
F.
Marini,
D.
Krause,
R.
Deans,
A.
Keating,
D.
Prockop,
E.
Horwitz.
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy.
2006;
8
:
315-317
.
-
S.
Ferber,
A.
Halkin,
H.
Cohen,
I.
Ber,
Y.
Einav,
I.
Goldberg,
I.
Barshack,
R.
Seijffers,
J.
Kopolovic,
N.
Kaiser.
Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nature medicine.
2000;
6
:
568-572
.
-
T.
Helledie,
V.
Nurcombe,
S.M.
Cool.
A simple and reliable electroporation method for human bone marrow mesenchymal stem cells. Stem cells and development.
2008;
17
:
837-848
.
-
G.
Hermann,
B.
Konukiewitz,
A.
Schmitt,
A.
Perren,
G.
Kloppel.
Hormonally defined pancreatic and duodenal neuroendocrine tumors differ in their transcription factor signatures: expression of ISL1, PDX1, NGN3, and CDX2. Virchows Archiv : an international journal of pathology.
2011;
459
:
147-154
.
-
A.
Ianus,
G.G.
Holz,
N.D.
Theise,
M.A.
Hussain.
In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. The Journal of clinical investigation.
2003;
111
:
843-850
.
-
E.T.
Jordan,
M.
Collins,
J.
Terefe,
L.
Ugozzoli,
T.
Rubio.
Optimizing electroporation conditions in primary and other difficult-to-transfect cells. Journal of biomolecular techniques : JBT.
2008;
19
:
328-334
.
-
K.
Kamimura,
T.
Suda,
G.
Zhang,
D.
Liu.
Advances in Gene Delivery Systems. Pharmaceutical medicine.
2011;
25
:
293-306
.
-
E.
Karaoz,
A.
Okcu,
Z.S.
Unal,
C.
Subasi,
O.
Saglam,
G.
Duruksu.
Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy.
2013;
15
:
557-570
.
-
H.
Kojima,
M.
Fujimiya,
K.
Matsumura,
P.
Younan,
H.
Imaeda,
M.
Maeda,
L.
Chan.
NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nature medicine.
2003;
9
:
596-603
.
-
L.
Li,
F.
Li,
H.
Qi,
G.
Feng,
K.
Yuan,
H.
Deng,
H.
Zhou.
Coexpression of Pdx1 and betacellulin in mesenchymal stem cells could promote the differentiation of nestin-positive epitheliumlike progenitors and pancreatic islet-like spheroids. Stem cells and development.
2008;
17
:
815-823
.
-
S.B.
Manjila,
J.N.
Baby,
E.N.
Bijin,
I.
Constantine,
K.
Pramod,
J.
Valsalakumari.
Novel gene delivery systems. International journal of pharmaceutical investigation.
2013;
3
:
1-7
.
-
K.T.
Marko Ušaj,
Maša Kandušer
Rosana Hudej.
Cell size dynamics and viability of cells exposed to hypotonic treatment and electroporation for electrofusion optimization. Radiol Oncol.
2009;
43
:
108-119
.
-
L.M.
Mir.
Electroporation-based gene therapy: recent evolution in the mechanism description and technology developments. Methods in molecular biology.
2014;
1121
:
3-23
.
-
N.
Nayerossadat,
T.
Maedeh,
P.A.
Ali.
Viral and nonviral delivery systems for gene delivery. Advanced biomedical research.
2012;
1
:
27
.
-
S.B.
Nelson,
A.E.
Schaffer,
M.
Sander.
The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development (Cambridge, England).
2007;
134
:
2491-2500
.
-
P.K.
Ngoc,
P.V.
Phuc,
T.H.
Nhung,
D.T.
Thuy,
N.T.
Nguyet.
Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells. Human cell.
2011;
24
:
86-95
.
-
J.
Oberholzer,
P.
Morel.
[Perspectives for diabetes treatment through pancreas transplantation or islet transplantation]. Diabetes & metabolism.
2002;
28
:
2S27-22S32
.
-
T.
Oldak,
M.
Kruszewski,
E.K.
Machaj,
A.
Gajkowska,
Z.
Pojda.
Optimisation of transfection conditions of CD34+hematopoietic cells derived from human umbilical cord blood. Acta biochimica Polonica.
2002;
49
:
625-632
.
-
J.M.
Oliver-Krasinski.
Novel roles for Pdx1 in the endocrine pancreas. Genetics.
2008;
181
.
-
J.K.
Pedersen,
S.B.
Nelson,
M.C.
Jorgensen,
K.D.
Henseleit,
Y.
Fujitani,
C.V.
Wright,
M.
Sander,
P.
Serup.
Endodermal expression of Nkx6 genes depends differentially on Pdx1. Developmental biology.
2005;
288
:
487-501
.
-
P.V.
Pham,
N.B.
Vu,
V.M.
Pham,
N.H.
Truong,
T.L.
Pham,
L.T.
Dang,
T.T.
Nguyen,
A.N.
Bui,
N.K.
Phan.
Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. Journal of translational medicine.
2014;
12
:
56
.
-
P.V.
Phuc,
T.H.
Nhung,
D.T.
Loan,
D.C.
Chung,
P.K.
Ngoc.
Differentiating of banked human umbilical cord bloodderived mesenchymal stem cells into insulin-secreting cells. In vitro cellular & developmental biology Animal.
2011;
47
:
54-63
.
-
T.
Sapir,
K.
Shternhall,
I.
Meivar-Levy,
T.
Blumenfeld,
H.
Cohen,
E.
Skutelsky,
S.
Eventov-Friedman,
I.
Barshack,
I.
Goldberg,
S.
Pri- Chen.
Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proceedings of the National Academy of Sciences of the United States of America.
2005;
102
:
7964-7969
.
-
W.
Su,
M.
Zhou,
Y.
Zheng,
Y.
Fan,
L.
Wang,
Z.
Han,
D.
Kong,
R.C.
Zhao,
J.C.
Wu,
R.
Xiang.
Bioluminescence reporter gene imaging characterize human embryonic stem cellderived teratoma formation. Journal of cellular biochemistry.
2011;
112
:
840-848
.
-
N.K.
Vudattu,
K.C.
Herold.
Treatment of new onset type 1 diabetes with teplizumab : successes and pitfalls in development. Expert opinion on biological therapy.
2014;
14
:
377-385
.
-
W.
Weecharangsan,
P.
Opanasopit,
R.J.
Lee.
In vitro gene transfer using cationic vectors, electroporation and their combination. Anticancer research.
2007;
27
:
309-313
.
-
A.H.
Wei,
W.J.
Wang,
X.P.
Mu,
H.M.
Li,
W.Q.
Yan.
Enhanced differentiation of human adipose tissue-derived stromal cells into insulin-producing cells with glucagon-like peptide-1. Experimental and clinical endocrinology & diabetes : official.
2012;
journal
:
German Society of Endocrinology [and] German Diabetes Association 120, 28-34
.
-
H.
Wu,
R.I.
Mahato.
Mesenchymal stem cell-based therapy for type 1 diabetes. Discovery medicine.
2014;
17
:
139-143
.
-
W.
Zhou,
Z.
Xiong,
Y.
Liu,
C.
Yao,
C.
Li.
Low voltage irreversible electroporation induced apoptosis in HeLa cells. Journal of cancer research and therapeutics.
2012;
8
:
80-85
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 02 (2014)
Page No.: 50-56
Published on: 2014-04-26
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5439 times
- Download PDF downloaded - 1433 times
- View Article downloaded - 3 times
 Biomedpress
Biomedpress