Abstract
Adipose derived stem cell (ADSC) is the most popular mesenchymal stem cells (MSCs) used in the clinic in recent years. ADSC transplantation has some advantages compared to others from bone marrow, umbilical cord blood... ADSCs produced important growth factors for wound healing, modulate the immune system, decrease inflammation, and home in on injured tissues. Particularly, ADSC extraction from adipose tissue is a simple procedure with minimum invasion in the patients. Therefore, ADSCs were used in the treatment of many diseases and clinical trials since 2000s. To date, ADSC transplantation was approved in some countries to treat medical complications such graft versus host disease, osteoarthritis... This review is an overview of applications and future challenges of ADSC in clinic.
Introduction
Adipose-derived stem cells (ADSCs) were first identified by Zuk and colleagues at the David Geffen School of Medicine at UCLA in 2001. They termed these cells as processed lipoaspirate cells or “PLA” cells Zuk et al.,2001. Zuk et al. used an enzyme to isolate PLA cells from adipose tissue. PLA cells were also named stromal vascular fraction (SVF) cells that consist of various cell types, including red blood cells, fibroblasts, endothelial cells, smooth muscle cells, pericytes, and preadipocytes Poznanski et al., 1973. However, most of these cell types cannot adhere to the flask surface and are thus eliminated during culture. These adherent cells exhibit stem cell characteristics such as a multilineage differentiation potential Zuk et al., 2001. Subsequently, PLA cells have been isolated by many researchers. PLA cells have been called various names such as ADSCs, adipose-derived adult stem (ADAS) cells, adipose-derived mesenchymal stem cells (AD-MSCs), adipose MSCs (AMSCs), and adipose stromal/stem cells (ASCs).
At the IFAT conference, plastic-adherent cells derived from the SVF were termed ASCs or ADSCs. In recent studies of ADSCs, it has been demonstrated that ADSCs possess the characteristics of MSCs that are isolated from the bone marrow or umbilical cord blood. Therefore, ADSCs are considered as a type of MSC. MSCs were first isolated from the bone marrow by Friedenstein et al. in 1968 Friedenstein et al., 1968. Bone marrow MSCs are considered as the gold standard for MSCs. To unify the classification of MSCs, Dominici et al. (2006) suggested a minimum standard for MSCs. This standard states that MSCs must be plastic adherent when maintained under standard culture conditions; they must express CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14, or CD11b, CD79alpha or CD19, and HLA-DR surface molecules; MSCs must differentiate to osteoblasts, adipocytes, and chondroblasts in vitro Dominici et al.,2006. Although ADSCs satisfy these standards, some authors have argued that ADSCs are different from MSCs.
Studies have shown that adipose tissue is the richest source of MSCs. There are only 0.001-0.01% mononuclear cells in bone marrow Pittenger et al., 1999, while adipose tissue contains up to 10% stem cells in the SVF. Recent studies have documented that 1 g of adipose tissue contains approximately 1-2 x 106 SVF cells, and 10% of these cells are thought to be ADSCs Aust et al., 2004Oedayrajsingh-Varma et al., 2006Zhu et al., 2008. By comparing colony-forming units (CFUs) between umbilical cord blood, bone marrow, liposuctioned fat, and sliced fat, it has been shown that sliced fat contains the most CFUs (28,000 CFUs/g), whereas liposuctioned fat has 3600-10,700 CFUs/g, umbilical cord blood has 200-20,000 CFUs/mL, and bone marrow has 100-1,000 CFUs/mL. Therefore, ADSCs have become a promising candidate for stem cell therapy.
Adsc properties
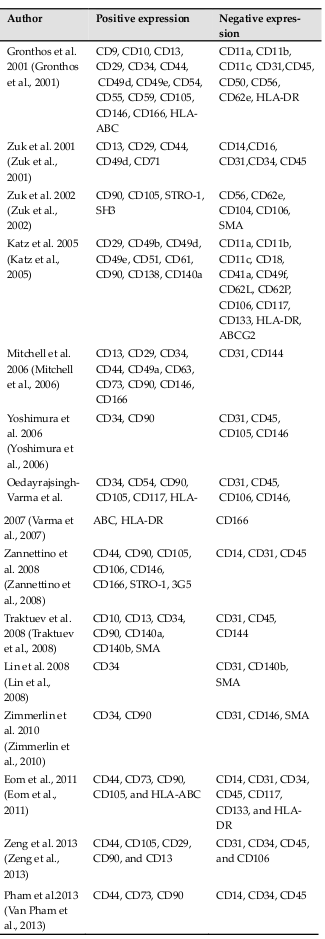
ADSCs are generally considered as MSCs in the literature. They possess MSC properties, including a fibroblast-like shape when cultured under adherent conditions, differentiation potential for mesenchymal cell lineages such as osteoblasts, chondroblasts, and adipocytes, strong expression of some MSC markers such as CD44, CD73, CD90, and CD105, and negativity for CD14 (monocytes), CD34 (HSCs), CD45 (white blood cells), and HLA-DR (mature cells). However, some studies also show that ADSCs express markers other than those expressed by MSCs ( Table 1 ). ADSCs also express hematopoietic cell markers, pericyte markers, and muscle cell markers. These differences are related to culture conditions and adipose tissue collection. In fact, adipose tissue is usually contaminated with muscular or skin tissue Basu et al., 2011Tallone et al.,2011.
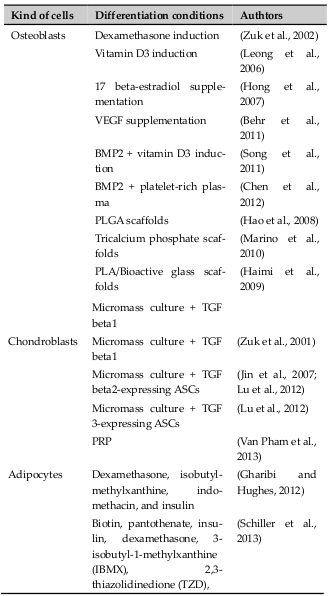
ADSCs are multipotent stem cells that can differentiate into specific kinds of mesoderm lineage cells, including osteoblasts, chondroblasts, and adipocytes ( Table 2 ). However, many studies also show that ADSCs can transdifferentiate into cell types of other lineages such as the ectoderm or endoderm. Differentiation of ADSCs into specific cells requires specific agents.
Applications of adipose stem cells in the clinic
Applications of adipose tissue grafts and lipoaspirated fat have been developed for the clinic. Initially, most applications of adipose tissue were related to plastic surgery. Subsequently, some studies used SVFs as concentrated adipose tissue containing mononuclear cells to replace whole adipose tissues. Moreover, transplantation of expanded ADSCs has been applied in the last 5 years.
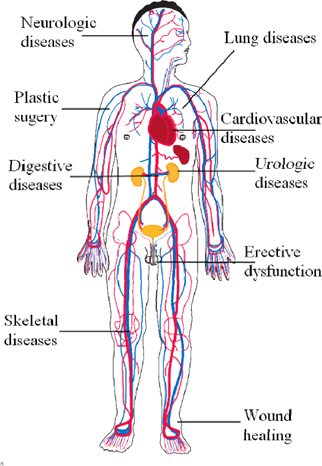
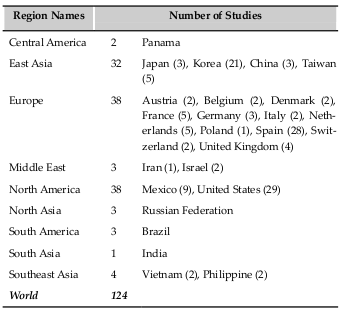
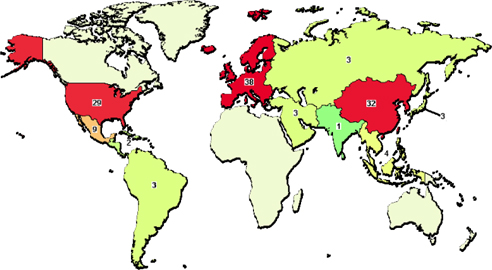
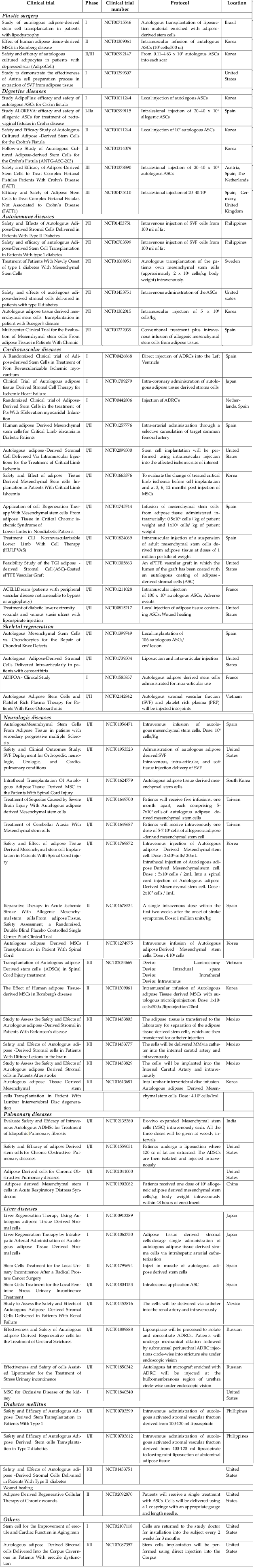
The use of transplantation of SVFs and ADSCs has rapidly increased as of 2010. Based on gross calculations according to the clinical trials recorded in clinicaltrial.gov and articles cited in PubMed, at least 3000 patients have been treated with ADSCs or SVFs for more than 10 different diseases. Such treatments are related to plastic surgery, digestive diseases, autoimmune diseases, cardiovascular diseases, skeletal regeneration, neurologic diseases, hematological and immunological disorders, diabetes mellitus, urologic disorders and diseases, and lung disorders and diseases ( Figure 1 ). We found 124 clinical trials registered in clinicaltrial.gov with some clinical trials in phase III ( Table 3 ) with a large number of patients (approximately 200 patients). Most clinical trials have been conducted in East Asia, Europe, and North America ( Figure 2 ; Supplement 1 ).
Studies have shown that ADSC transplantation for the treatment of numerous diseases is safe and effective. To date, there are about 5 clinical trials in Phase III for ADSC transplantation (NCT00475410, NCT01541579, NCT01378390, NCT01803347, andNCT00992147). Four of these clinical trials are related to perianal fistulas treatment. With more than 200 patients, the trial with registration number NCT00475410 showed that perinatal fistula can be effectively treated by ADSC grafts in platelet-rich plasma (PRP) glue with healing rates of approximately 40% at 6months and more than 50% at the 1-year follow-up Herreros et al., 2012. ADSC transplantation has also shown good results for treating many other diseases such as knee osteoarthritis Bui et al., 2014Koh and Choi, 2012, chronic ulcers Marino et al., 2013, Crohn’s fistula Cho et al., 2013de la Portilla et al., 2013Garcia-Olmo et al., 2009Lee et al., 2012, limb ischemia Lee et al.,2012, femoral head necrosis Pak, 2012, Parry-Romberg disease Koh et al., 2012, radiotherapy-induced tissue damage Rigotti et al., 2007, and maxillary and mandibulary bone tissue Kulakov et al., 2008.
Safety of adipose stem cells in the clinic
Similar to any other drug or therapy, ASC transplantation has some limitations and side effects. However, there are different risks for SVFs and ADSCs. SVFs are considered safer than ADSCs. SVFs are directly collected from adipose tissue with enzymes, and the risks of these samples are usually related to adipose tissue processing. In fact, Change et al. (2013) surveyed 100 randomly selected private plastic surgery clinics, 68 plastic surgery departments of general and university hospitals, and 5 biotechnology companies in South Korea that performed ADSC-related procedures using ADSCs they harvested themselves. They found no toxicity resulting from residual collagenase or tumorigenicity associated with the ADSCs Chang et al.,2013.
However, the use of ADSCs or cultured SVF cells to isolate MSCs can be associated with high risks if applied in the clinic. Expanded ADSCs need to be carefully processed and controlled for application to humans. It is considered that cultured ADSCs should be assessed in terms of stability, toxicity, and tumorigenicity during culture. Some recent studies show that the quality of MSCs significantly decreases after long-term culture. Bonab et al. (2006) showed that MSCs derived from bone marrow underwent senescence after 6 passages, as some properties such as population doubling, telomere length, and differentiation potential decrease after the 6th passage Bonab et al., 2006. Furthermore, extended culture of bone marrow-derived MSCs alters their ability to differentiate into hematopoietic progenitor cells without concomitant changes in their phenotype or differentiation capacity Briquet et al., 2010. Another study showed that MSCs can transform into cancer cells Rubio et al., 2005. However, this study was retracted in 2010. In fact, the researchers were unable to reproduce some of the reported spontaneous transformation events and suspected that the phenomenon had occurred because of cross-contamination artifacts de la Fuente et al., 2010Garcia et al., 2010. Rubio et al. also published two studies concerning MSC transformation Rubio et al., 2008aRubio et al., 2008b. However, many other studies show that SVF or ADSC transplantation is safe in animals and humans.
Map of clinical trials about ADSC transplantation in the world. In clinicaltrial.gov, Europe is area with the most clinical trials; and after East Asia, and North America.
In animals, SVF and ADSC transplantation by local injection Gao et al., 2011Gimble et al., 2010Kojima et al., 2011Kondo et al., 2009Van Pham et al., 2013 and intravenous transfusion Lim et al., 2013Sun et al.,2012Tajiri et al., 2014Wang et al., 2013Yanez et al., 2006 has shown high safety. In a recent long-term tumorigenic assessment of a mouse model, MacIsaas et al. injected expanded ADSCs into mice at high doses and the mice were followed up for 1 year MacIsaac et al., 2012. They found no difference in the growth/weight and lifespan of cell- and vehicle-treated animals, and no malignancies were detected in the cell-treated animals. Expanded ADSCs have also been injected into the eyes Rajashekhar et al., 2014. Expanded ADSC transplantation is safe in dogs Black et al., 2008Cui et al., 2007Haghighat et al., 2011Vilar et al., 2013, rabbits Toghraie et al., 2011, rats Tajiri et al., 2014, horses Nicpon et al., 2013Ricco et al., 2013, and pigs Gomez-Mauricio et al., 2013Niada et al., 2013.
In the clinic, most studies of SVF and ADSC transplantation show that local injection and systemic transfusion of ADSCs are safe. Non-expanded SVF cells have been clinically applied to treat multiple sclerosis Riordan et al., 2009, knee osteoarthritis Bui et al., 2014, and femoral head necrosis Namazi,2012Pak, 2012. Autologous expanded ADSCs have been isolated and in vitro-expanded to obtain enough cells for perianal fistula treatment. More than 200 patients were enrolled for intralesional treatment. The results demonstrated that this method is safe and effective de la Portilla et al., 2013Garcia-Olmo et al.,2009Herreros et al., 2012, even after 3 years Guadalajara et al., 2012. The procedure of expanded ADSC-enriched fat grafting has excellent feasibility and safety Kolle et al., 2013. Expanded ADSCs have also been injected into the myocardium to treat chronic myocardial ischemia, which showed safety after 3 years Qayyum et al., 2012.
Lee et al. (2012) showed that intramuscular injection of passage 3 ADSCs into patients with critical limb ischemia is safe, and clinical improvements were observed in 66.7% of patients after 6 months Lee et al.,2012. Koh and Choi injected ADSCs into patients with knee osteoarthritis. They also recorded a clinical improvement without adverse effects Koh and Choi, 2012. Ra et al. (2011) also showed that ADSCs could be expanded up to 12 passages while maintaining their MSC properties. These ADSCs were intravenously transfused into SCID mice, Balb/c-nu mice, and 8 male patients. The results showed that none of the mice or humans developed any serious adverse events related to ADSC transplantation during the 3-month follow-up in humans and over 26 weeks in mice. This study used extremely high doses of 2.5 ˟ 108 cells/kg in mice and 4 ˟ 108 cells in humans Ra et al., 2011b. Ra et al. also used expanded ADSCs for treating autoimmune disease by intravenous transfusion.
They also showed that there were no side effects in the 10 enrolled patients Ra et al., 2011a. Intravenous infusion of autologous expanded ADSCs has been approved as a safe method in the treatment of progressive supranuclear palsy Choi et al., 2014. Intravenous infusion of allogeneic expanded ADSCs is also safe for the treatment of acute respiratory distress syndrome Zheng et al., 2014,
In addition to the risks related to mutations and transformation of ADSC during long-term culture, adverse effects of ADSC transplantation also depend on the culture conditions. In general, GMP-compliant culture is considered to be essential to ensure ADSC quality. One of the concerns of ADSC culture relates to supplementation of fetal bovine serum (FBS) in the culture medium. FBS not only contains xenogeneic proteins that cause immune reactions, but can also transmit viruses. However, some studies have clinically used ADSCs expanded in FBS culture medium.
Svfs and adscs in the clinic
SVFs are a mixture of mononuclear cells including more than 5 kinds of cells, whereas ADSCs are a heterogeneous cell population of the SVF. This cell population is purified by adherent culture. It is easy to understand when there are a comparable mean between adipose tissue and bone marrow, SVFs and mononuclear cells (MNCs). Some studies have considered SVF cells as ADSCs; however, these cells are in reality different. Similar to MNCs and MSCs from bone marrow, there are few studies that have compared transplantation efficiencies between SVFs and ADSCs. Compared with MSCs, MNCs from bone marrow have some advantages in certain cases Karlupia et al., 2014.
Further investigations need to be performed, but it is likely that leukocytes and red blood cells contaminate SVFs or MNCs, resulting in adverse effects. Recent studies in animal models show that MNCs or SVF with leukocyte or red blood cell contamination cause graft-versus-host disease or autoimmune diseases. However, some studies demonstrate that various kinds of stem cells are included in MNCs or SVFs, which contribute to their regeneration Lv et al., 2013.
Future of adsc transplantion
ADSCs have become the main type of adult stem cell that is approved for use in humans. ADSC transplantation has been gradually developed in many countries for the treatment of chronic and degenerative diseases. Although ADSC transplantation has some clinical benefits, the specific mechanisms of ADSC-based treatment are unclear. For successful ADSC application, ADSC migration should be controlled in the human body. Moreover, there should be verification of the in vivo differentiation of ADSCs.
Some recent clinical studies have shown that ADSC transplantation shows better results when used in combination with certain therapies. In fact, a new strategy is the use of adjuvants in ADSC transplantation. Adjuvants are considered as stimulators and differentiating factors that can improve the patient’s condition. The most commonly used adjuvant is PRP. In combination with ADSCs, PRP has been successfully applied in the treatment of osteoarthritis Bui et al., 2014. Furthermore, some cytokines or vitamins may improve the quality or viability of ADSCs in the human body.
For other approaches, studies have focused on the in vitro differentiation of ADSCs into specific cell types. These specific types of cells can be used in stem cell therapy and tissue engineering to create tissues for transplantation.
Abbreviations
AMSCs: Adipose MSCs; Adipose-derived adult stem: ADAS; ADSC: Adipose-derived stem cells; CFUs: colony-forming units; FBS: fetal bovine serum; GMP: Good manufacturing practice; IFAT: International Fat Applied Technology Society; PLA: Lipoaspirate cells; MSC: Mesenchymal stem cell; MNC: Mononuclear cells; PRP: Platelet rich plasma, SVF: stromal vascular fraction
References
-
L.
Aust,
B.
Devlin,
S.J.
Foster,
Y.D.
Halvorsen,
K.
Hicok,
T.
du Laney,
A.
Sen,
G.D.
Willingmyre,
J.M.
Gimble.
Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy.
2004;
6
:
7-14
.
-
J.
Basu,
C.W.
Genheimer,
K.I.
Guthrie,
N.
Sangha,
S.F.
Quinlan,
A.T.
Bruce,
B.
Reavis,
C.
Halberstadt,
R.M.
Ilagan,
J.W.
Ludlow.
Expansion of the human adipose-derived stromal vascular cell fraction yields a population of smooth muscle-like cells cells in the clinicwith markedly distinct phenotypic and functional properties relative to mesenchymal stem cells. Tissue engineering Part.
2011;
C
:
Methods 17, 843-860
.
-
B.
Behr,
C.
Tang,
G.
Germann,
M.T.
Longaker,
N.
Quarto.
Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem cells (Dayton, Ohio).
2011;
29
:
286-296
.
-
L.L.
Black,
J.
Gaynor,
C.
Adams,
S.
Dhupa,
A.E.
Sams,
R.
Taylor,
S.
Harman,
D.A.
Gingerich,
R.
Harman.
Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Veterinary therapeutics: research in applied veterinary medicine.
2008;
9
:
192-200
.
-
M.M.
Bonab,
K.
Alimoghaddam,
F.
Talebian,
S.H.
Ghaffari,
A.
Ghavamzadeh,
B.
Nikbin.
Aging of mesenchymal stem cell in vitro. BMC cell biology.
2006;
7
:
14
.
-
A.
Briquet,
S.
Dubois,
S.
Bekaert,
M.
Dolhet,
Y.
Beguin,
A.
Gothot.
Prolonged ex vivo culture of human bone marrow mesenchymal stem cells influences their supportive activity toward NOD/SCID-repopulating cells and committed progenitor cells of B lymphoid and myeloid lineages. Haematologica.
2010;
95
:
47-56
.
-
K.H.-T.
Bui,
T.D.
Duong,
N.T.
Nguyen,
T.D.
Nguyen,
V.T.
Le,
V.T.
Mai,
N.L.-C.
Phan,
D.M.
Le,
N.K.
Phan,
P.V.
Pham.
Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study 2014. 2014;
1
.
-
H.
Chang,
B.R.
Do,
J.H.
Che,
B.C.
Kang,
J.H.
Kim,
E.
Kwon,
J.Y.
Kim,
K.H.
Min.
Safety of adipose-derived stem cells and collagenase in fat tissue preparation. Aesthetic plastic surgery.
2013;
37
:
802-808
.
-
L.
Chen,
X.
Lu,
S.
Li,
Q.
Sun,
W.
Li,
D.
Song.
Sustained delivery of BMP-2 and platelet-rich plasma-released growth factors contributes to osteogenesis of human adipose- derived stem cells. Orthopedics.
2012;
35
:
e1402-1409
.
-
Y.B.
Cho,
W.Y.
Lee,
K.J.
Park,
M.
Kim,
H.W.
Yoo,
C.S.
Yu.
Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell transplantation.
2013;
22
:
279-285
.
-
S.W.
Choi,
K.B.
Park,
S.K.
Woo,
S.K.
Kang,
J.C.
Ra.
Treatment of progressive supranuclear palsy with autologous adipose tissue-derived mesenchymal stem cells: a case report. Journal of medical case reports.
2014;
8
:
87
.
-
L.
Cui,
B.
Liu,
G.
Liu,
W.
Zhang,
L.
Cen,
J.
Sun,
S.
Yin,
W.
Liu,
Y.
Cao.
Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials.
2007;
28
:
5477-5486
.
-
R.
Fuente,
A.
Bernad,
J.
Garcia-Castro,
M.C.
Martin,
J.C.
Cigudosa.
Retraction: Spontaneous human adult stem cell transformation. Cancer research.
2010;
70
:
6682
.
-
F.
Portilla,
F.
Alba,
D.
Garcia-Olmo,
J.M.
Herrerias,
F.X.
Gonzalez,
A.
Galindo.
Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. International journal of colorectal disease.
2013;
28
:
313-323
.
-
M.
Dominici,
K.
Le Blanc,
I.
Mueller,
I.
Slaper-Cortenbach,
Krause
Marini,
R.
Deans,
A.
Keating,
D.
Prockop,
E.
Horwitz.
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy.
2006;
8
:
315-317
.
-
Y.W.
Eom,
J.E.
Lee,
M.S.
Yang,
I.K.
Jang,
H.E.
Kim,
D.H.
Lee,
Y.J.
Kim,
W.J.
Park,
J.H.
Kong,
K.Y.
Shim.
Rapid isolation of adipose tissue-derived stem cells by the storage of lipoaspirates. Yonsei medical journal.
2011;
52
:
999-1007
.
-
A.J.
Friedenstein,
K.V.
Petrakova,
A.I.
Kurolesova,
G.P.
Frolova.
Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation.
1968;
6
:
230247
.
-
W.
Gao,
X.
Qiao,
S.
Ma,
L.
Cui.
Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1alpha. Journal of cellular and molecular medicine.
2011;
15
:
2575-2585
.
-
S.
Garcia,
A.
Bernad,
M.C.
Martin,
J.C.
Cigudosa,
J.
Garcia-Castro,
R.
de la Fuente.
Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Experimental cell research.
2010;
316
:
1648-1650
.
-
D.
Garcia-Olmo,
D.
Herreros,
I.
Pascual,
J.A.
Pascual,
E.
Del-Valle,
J.
Zorrilla,
P.
De-La-Quintana,
M.
Garcia-Arranz,
M.
Pascual.
Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Diseases of the colon and rectum.
2009;
52
:
79-86
.
-
B.
Gharibi,
F.J.
Hughes.
Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem cells translational medicine.
2012;
1
:
771782
.
-
J.M.
Gimble,
F.
Guilak,
B.A.
Bunnell.
Clinical and preclinical translation of cell-based therapies using adipose tissue- derived cells. Stem cell research & therapy.
2010;
1
:
19
.
-
R.G.
Gomez-Mauricio,
A.
Acarregui,
F.M.
Sanchez-Margallo,
V.
Crisostomo,
I.
Gallo,
R.M.
Hernandez,
J.L.
Pedraz,
G.
Orive,
M.F.
Martin-Cancho.
A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV.
2013;
84
:
29-39
.
-
S.
Gronthos,
D.M.
Franklin,
H.A.
Leddy,
P.G.
Robey,
R.W.
Storms,
J.M.
Gimble.
Surface protein characterization of human adipose tissue-derived stromal cells. Journal of cellular physiology.
2001;
189
:
54-63
.
-
H.
Guadalajara,
D.
Herreros,
P.
De-La-Quintana,
J.
Trebol,
M.
Garcia-Arranz,
D.
Garcia-Olmo.
Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. International journal of colorectal disease.
2012;
27
:
595-600
.
-
A.
Haghighat,
A.
Akhavan,
B.
Hashemi-Beni,
P.
Deihimi,
A.
Yadegari,
F.
Heidari.
Adipose derived stem cells for treatment of mandibular bone defects: An autologous study in dogs. Dental research journal.
2011;
8
:
S51-57
.
-
S.
Haimi,
N.
Suuriniemi,
A.M.
Haaparanta,
V.
Ella,
B.
Lindroos,
H.
Huhtala,
S.
Raty,
H.
Kuokkanen,
G.K.
Sandor,
M.
Kellomaki.
Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue engineering Part A.
2009;
15
:
1473-1480
.
-
W.
Hao,
Y.Y.
Hu,
Y.Y.
Wei,
L.
Pang,
R.
Lv,
J.P.
Bai,
Z.
Xiong,
M.
Jiang.
Collagen I gel can facilitate homogenous bone formation of adipose-derived stem cells in PLGA-beta-TCP scaffold. Cells, tissues, organs.
2008;
187
:
89-102
.
-
M.D.
Herreros,
M.
Garcia-Arranz,
H.
Guadalajara,
P.
De-La- Quintana,
D.
Garcia-Olmo.
Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Diseases of the colon and rectum.
2012;
55
:
762-772
.
-
L.
Hong,
A.
Colpan,
I.A.
Peptan,
J.
Daw,
A.
George,
C.A.
Evans.
17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue engineering.
2007;
13
:
1197-1203
.
-
X.
Jin,
Y.
Sun,
K.
Zhang,
J.
Wang,
T.
Shi,
X.
Ju,
S.
Lou.
Ectopic neocartilage formation from predifferentiated human adipose derived stem cells induced by adenoviral-mediated transfer of hTGF beta2. Biomaterials.
2007;
28
:
2994-3003
.
-
N.
Karlupia,
N.C.
Manley,
K.
Prasad,
R.
Schafer,
G.K.
Steinberg.
Intra-arterial transplantation of human umbilical cord blood mononuclear cells is more efficacious and safer compared with umbilical cord mesenchymal stromal cells in a rodent stroke model. Stem cell research & therapy.
2014;
5
:
45
.
-
A.J.
Katz,
A.
Tholpady,
S.S.
Tholpady,
H.
Shang,
R.C.
Ogle.
Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem cells.
2005;
(Dayton
:
Ohio) 23, 412-423
.
-
K.S.
Koh,
T.S.
Oh,
H.
Kim,
I.W.
Chung,
K.W.
Lee,
H.B.
Lee,
E.J.
Park,
J.S.
Jung,
I.S.
Shin,
J.C.
Ra.
Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Annals of plastic surgery.
2012;
69
:
331-337
.
-
Y.G.
Koh,
Y.J.
Choi.
Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. The Knee.
2012;
19
:
902-907
.
-
T.
Kojima,
S.
Kanemaru,
S.
Hirano,
I.
Tateya,
S.
Ohno,
T.
Nakamura,
J.
Ito.
Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. The Laryngoscope.
2011;
121
:
1864-1869
.
-
S.F.
Kolle,
A.
Fischer-Nielsen,
A.B.
Mathiasen,
J.J.
Elberg,
R.S.
Oliveri,
P.V.
Glovinski,
J.
Kastrup,
M.
Kirchhoff,
B.S.
Rasmussen,
M.L et al.
Talman.
Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet.
2013;
382
:
11131120
.
-
K.
Kondo,
S.
Shintani,
R.
Shibata,
H.
Murakami,
R.
Murakami,
M.
Imaizumi,
Y.
Kitagawa,
T.
Murohara.
Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arteriosclerosis, thrombosis, and vascular biology.
2009;
29
:
61-66
.
-
A.A.
Kulakov,
D.V.
Goldshtein,
A.S.
Grigoryan,
A.A.
Rzhaninova,
I.S.
Alekseeva,
I.V.
Arutyunyan,
A.V.
Volkov.
Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bulletin of experimental biology and medicine.
2008;
146
:
522-525
.
-
H.C.
Lee,
S.G.
An,
H.W.
Lee,
J.S.
Park,
K.S.
Cha,
T.J.
Hong,
J.H.
Park,
S.Y.
Lee,
S.P.
Kim,
Y.D.
Kim.
Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circulation journal: official journal of the Japanese Circulation Society.
2012;
76
:
1750-1760
.
-
D.T.
Leong,
W.M.
Khor,
F.T.
Chew,
T.C.
Lim,
D.W.
Hutmacher.
Characterization of osteogenically induced adipose tissue-derived precursor cells in 2-dimensional and 3-dimensional environments. Cells, tissues, organs.
2006;
182
:
1-11
.
-
J.Y.
Lim,
J.C.
Ra,
I.S.
Shin,
Y.H.
Jang,
H.Y.
An,
J.S.
Choi,
W.C.
Kim,
Y.M.
Kim.
Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PloS one.
2013;
8
:
e71167
.
-
G.
Lin,
M.
Garcia,
H.
Ning,
L.
Banie,
Y.L.
Guo,
T.F.
Lue,
C.S.
Lin.
Defining stem and progenitor cells within adipose tissue. Stem cells and development.
2008;
17
:
1053-1063
.
-
C.H.
Lu,
K.J.
Lin,
H.Y.
Chiu,
C.Y.
Chen,
T.C.
Yen,
S.M.
Hwang,
Y.H.
Chang,
Y.C.
Hu.
Improved chondrogenesis and engineered cartilage formation from TGF-beta3-expressing adipose- derived stem cells cultured in the rotating-shaft bioreactor. Tissue engineering Part A.
2012;
18
:
2114-2124
.
-
Y.T.
Lv,
Y.
Zhang,
M.
Liu,
J.N.
Qiuwaxi,
P.
Ashwood,
S.C.
Cho,
Y.
Huan,
R.C.
Ge,
X.W.
Chen,
Z.J et al.
Wang.
Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. Journal of translational medicine.
2013;
11
:
196
.
-
Z.M.
MacIsaac,
H.
Shang,
H.
Agrawal,
N.
Yang,
A.
Parker,
A.J.
Katz.
Long-term in-vivo tumorigenic assessment of human culture-expanded adipose stromal/stem cells. Experimental cell research.
2012;
318
:
416-423
.
-
G.
Marino,
M.
Moraci,
E.
Armenia,
C.
Orabona,
R.
Sergio,
G.
De Sena,
V.
Capuozzo,
M.
Barbarisi,
F.
Rosso,
G.
Giordano.
Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. The Journal of surgical research.
2013;
185
:
3644
.
-
G.
Marino,
F.
Rosso,
G.
Cafiero,
C.
Tortora,
M.
Moraci,
M.
Barbarisi,
A.
Barbarisi.
Beta-tricalcium phosphate 3D scaffold promote alone osteogenic differentiation of human adipose stem cells: in vitro study. Journal of materials science Materials in medicine.
2010;
21
:
353-363
.
-
J.B.
Mitchell,
K.
McIntosh,
S.
Zvonic,
S.
Garrett,
Z.E.
Floyd,
A.
Kloster,
Y.
Di Halvorsen,
R.W.
Storms,
B.
Goh,
G.
Kilroy.
Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem cells (Dayton , Ohio).
2006;
24
:
376-385
.
-
H.
Namazi.
Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads: a molecular mechanism. Pain physician.
2012;
15
:
E345; author reply E345
.
-
S.
Niada,
L.M.
Ferreira,
E.
Arrigoni,
A.
Addis,
M.
Campagnol,
E.
Broccaioli,
A.T.
Brini.
Porcine adipose-derived stem cells from buccal fat pad and subcutaneous adipose tissue for future preclinical studies in oral surgery. Stem cell research & therapy.
2013;
4
:
148
.
-
J.
Nicpon,
K.
Marycz,
J.
Grzesiak.
Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Polish journal of veterinary sciences.
2013;
16
:
753-754
.
-
M.J.
Oedayrajsingh-Varma,
S.M.
van Ham,
M.
Knippenberg,
M.N.
Helder,
J.
Klein-Nulend,
T.E.
Schouten,
M.J.
Ritt,
F.J.
van Milligen.
Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissueharvesting procedure. Cytotherapy.
2006;
8
:
166-177
.
-
J.
Pak.
Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain physician.
2012;
15
:
75-85
.
-
M.F.
Pittenger,
A.M.
Mackay,
S.C.
Beck,
R.K.
Jaiswal,
R.
Douglas,
J.D.
Mosca,
M.A.
Moorman,
D.W.
Simonetti,
S.
Craig,
D.R.
Marshak.
Multilineage potential of adult human mesenchymal stem cells. Science (New.
1999;
York
:
NY) 284, 143-147
.
-
W.J.
Poznanski,
I.
Waheed,
R.
Van.
Human fat cell precursors. Morphologic and metabolic differentiation in culture. Laboratory investigation; a journal of technical methods and pathology.
1973;
29
:
570-576
.
-
A.A.
Qayyum,
M.
Haack-Sorensen,
A.B.
Mathiasen,
E.
Jorgensen,
A.
Ekblond,
J.
Kastrup.
Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): study design. Regenerative medicine.
2012;
7
:
421-428
.
-
J.C.
Ra,
S.K.
Kang,
I.S.
Shin,
H.G.
Park,
S.A.
Joo,
J.G.
Kim,
B.C.
Kang,
Y.S.
Lee,
K.
Nakama,
M.
Piao.
Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. Journal of translational medicine.
2011a;
9
:
181
.
-
J.C.
Ra,
I.S.
Shin,
S.H.
Kim,
S.K.
Kang,
B.C.
Kang,
H.Y.
Lee,
Y.J.
Kim,
J.Y.
Jo,
E.J.
Yoon,
H.J et al.
Choi.
Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem cells and development.
2011b;
20
:
1297-1308
.
-
G.
Rajashekhar,
A.
Ramadan,
C.
Abburi,
B.
Callaghan,
D.O.
Traktuev,
C.
Evans-Molina,
R.
Maturi,
A.
Harris,
T.S.
Kern,
K.L.
March.
Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PloS one.
2014;
9
:
e84671
.
-
S.
Ricco,
S.
Renzi,
M.
Del Bue,
V.
Conti,
E.
Merli,
R.
Ramoni,
E.
Lucarelli,
G.
Gnudi,
M.
Ferrari,
S.
Grolli.
Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. International journal of immunopathology and pharmacology.
2013;
26
:
61-68
.
-
G.
Rigotti,
A.
Marchi,
M.
Galie,
G.
Baroni,
D.
Benati,
M.
Krampera,
A.
Pasini,
A.
Sbarbati.
Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plastic and reconstructive surgery.
2007;
119
:
1409-1422; discussion 1423
.
-
N.H.
Riordan,
T.E.
Ichim,
W.P.
Min,
H.
Wang,
F.
Solano,
F.
Lara,
M.
Alfaro,
J.P.
Rodriguez,
R.J.
Harman,
A.N et al.
Patel.
Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. Journal of translational medicine.
2009;
7
:
29
.
-
D.
Rubio,
S.
Garcia,
T.
De la Cueva,
M.F.
Paz,
A.C.
Lloyd,
A.
Bernad,
J.
Garcia-Castro.
Human mesenchymal stem cell transformation is associated with a mesenchymal-epithelial transition. Experimental cell research.
2008a;
314
:
691-698
.
-
D.
Rubio,
S.
Garcia,
M.F.
Paz,
T.
De la Cueva,
L.A.
Lopez-Fernandez,
A.C.
Lloyd,
J.
Garcia-Castro,
A.
Bernad.
Molecular characterization of spontaneous mesenchymal stem cell transformation. PloS one.
2008b;
3
:
e1398
.
-
D.
Rubio,
J.
Garcia-Castro,
M.C.
Martin,
R.
de la Fuente,
J.C.
Cigudosa,
A.C.
Lloyd,
A.
Bernad.
Spontaneous human adult stem cell transformation. Cancer research.
2005;
65
:
3035-3039
.
-
Z.A.
Schiller,
N.R.
Schiele,
J.K.
Sims,
K.
Lee,
C.K.
Kuo.
Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem cell research & therapy.
2013;
4
:
79
.
-
I.
Song,
B.S.
Kim,
C.S.
Kim,
G.I.
Im.
Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochemical and biophysical research communications.
2011;
408
:
126-131
.
-
C.K.
Sun,
C.L.
Chang,
Y.C.
Lin,
Y.H.
Kao,
L.T.
Chang,
C.H.
Yen,
P.L.
Shao,
C.H.
Chen,
S.
Leu,
H.K.
Yip.
Systemic administration of autologous adipose-derived mesenchymal stem cells alleviates hepatic ischemia-reperfusion injury in rats. Critical care medicine.
2012;
40
:
1279-1290
.
-
N.
Tajiri,
S.A.
Acosta,
M.
Shahaduzzaman,
H.
Ishikawa,
K.
Shinozuka,
M.
Pabon,
D.
Hernandez-Ontiveros,
D.W.
Kim,
C.
Metcalf,
Mv et al.
Staples.
Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. The Journal of neuroscience: the official journal of the Society for Neuroscience.
2014;
34
:
313-326
.
-
T.
Tallone,
C.
Realini,
A.
Bohmler,
C.
Kornfeld,
G.
Vassalli,
T.
Moccetti,
S.
Bardelli,
G.
Soldati.
Adult human adipose tissue contains several types of multipotent cells. Journal of cardiovascular translational research.
2011;
4
:
200-210
.
-
F.S.
Toghraie,
N.
Chenari,
M.A.
Gholipour,
Z.
Faghih,
S.
Torabinejad,
S.
Dehghani,
A.
Ghaderi.
Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. The Knee.
2011;
18
:
71-75
.
-
D.O.
Traktuev,
S.
Merfeld-Clauss,
J.
Li,
M.
Kolonin,
W.
Arap,
R.
Pasqualini,
B.H.
Johnstone,
K.L.
March.
A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation research.
2008;
102
:
77-85
.
-
P.
Van Pham,
K.H.
Bui,
D.Q.
Ngo,
N.B.
Vu,
N.H.
Truong,
N.L.
Phan,
D.M.
Le,
T.D.
Duong,
T.D.
Nguyen,
V.T.
Le.
Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem cell research & therapy.
2013;
4
:
91
.
-
M.J.
Varma,
R.G.
Breuls,
T.E.
Schouten,
W.J.
Jurgens,
J.
Bontkes,
G.J.
Schuurhuis,
S.M.
van Ham,
F.J.
van Milligen.
Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem cells and development.
2007;
16
:
91-104
.
-
J.M.
Vilar,
M.
Morales,
A.
Santana,
G.
Spinella,
M.
Rubio,
B.
Cuervo,
R.
Cugat,
J.M.
Carrillo.
Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC veterinary research.
2013;
9
:
131
.
-
Y.L.
Wang,
G.
Li,
X.F.
Zou,
X.B.
Chen,
T.
Liu,
Z.Y.
Shen.
Effect of autologous adipose-derived stem cells in renal cold ischemia and reperfusion injury. Transplantation proceedings.
2013;
45
:
3198-3202
.
-
R.
Yanez,
M.L.
Lamana,
J.
Garcia-Castro,
I.
Colmenero,
M.
Ramirez,
J.A.
Bueren.
Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem cells.
2006;
(Dayton
:
Ohio) 24, 2582-2591
.
-
K.
Yoshimura,
T.
Shigeura,
D.
Matsumoto,
T.
Sato,
Y.
Takaki,
E.
Aiba-Kojima,
K.
Sato,
K.
Inoue,
T.
Nagase,
I.
Koshima.
Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. Journal of cellular physiology.
2006;
208
:
64-76
.
-
A.C.
Zannettino,
S.
Paton,
A.
Arthur,
F.
Khor,
S.
Itescu,
J.M.
Gimble,
S.
Gronthos.
Multipotential human adipose- derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. Journal of cellular physiology.
2008;
214
:
413-421
.
-
G.
Zeng,
K.
Lai,
J.
Li,
Y.
Zou,
H.
Huang,
J.
Liang,
X.
Tang,
J.
Wei,
P.
Zhang.
A rapid and efficient method for primary culture of human adipose-derived stem cells. Organogenesis.
2013;
9
:
287-295
.
-
G.
Zheng,
L.
Huang,
H.
Tong,
Q.
Shu,
Y.
Hu,
M.
Ge,
K.
Deng,
L.
Zhang,
B.
Zou,
B.
Cheng.
Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory research.
2014;
15
:
39
.
-
Y.
Zhu,
T.
Liu,
K.
Song,
X.
Fan,
X.
Ma,
Z.
Cui.
Adipose-derived stem cell: a better stem cell than BMSC. Cell biochemistry and function.
2008;
26
:
664-675
.
-
L.
Zimmerlin,
V.S.
Donnenberg,
M.E.
Pfeifer,
E.M.
Meyer,
B.
Peault,
J.P.
Rubin,
A.D.
Donnenberg.
Stromal vascular progenitors in adult human adipose tissue. Cytometry Part A: the journal of the International Society for Analytical Cytology.
2010;
77
:
2230
.
-
P.A.
Zuk,
M.
Zhu,
P.
Ashjian,
D.A.
De Ugarte,
J.I.
Huang,
H.
Mizuno,
Z.C.
Alfonso,
J.K.
Fraser,
P.
Benhaim,
M.H.
Hedrick.
Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell.
2002;
13
:
4279-4295
.
-
P.A.
Zuk,
M.
Zhu,
H.
Mizuno,
J.
Huang,
J.W.
Futrell,
A.J.
Katz,
P.
Benhaim,
H.P.
Lorenz,
M.H.
Hedrick.
Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering.
2001;
7
:
211-228
.
-
P.
Pham.
Adipose stem cells in the clinic. Biomedical Research And Therapy.
2014;
1(2)
:
57-70
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 02 (2014)
Page No.: 57-70
Published on: 2014-05-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 8455 times
- Download PDF downloaded - 2016 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress