Preliminary evaluation of intravenous infusion and intra-pancreatic injection of human umbilical cord blood-derived mesenchymal stem cells for the treatment of diabetic mice
Abstract
Type 1 diabetes mellitus is characterized by the destruction of pancreatic islet beta cells, which leads to insulin insufficiency, hyperglycemia, and reduced metabolic glucose level. Insulin replacement is the current standard therapy for type 1 diabetes mellitus but has several limitations. Pancreatic islet transplantation can result in the production of exogenous insulin, but its use is limited by immune-rejection and donor availability. Recent studies have shown that mesenchymal stem cells (MSCs) can trans-differentiate into insulin-producing cells (IPCs), which could be utilized for diabetes mellitus treatment. Previously published reports have demonstrated that MSC or IPC transplantation could produce significant improvement in mouse models of diabetes mellitus. This study was aimed at determining the effects of two different methods of MSC transplantation on the efficacy of diabetes mellitus treatment in mouse models. The MSCs were isolated from umbilical cord blood and were proliferated following a previously published procedure. Diabetes mellitus was induced in mice by streptozotocin (STZ) injection. Thirty days after transplantation, the weight of the mice treated by intra-venous infusion and intra-pancreatic injection was found to be 22% and 14% higher than that of the un-treated mice. The blood glucose concentrations in both intra-venous infusion and intra-pancreatic injection groups decreased and remained more stable than those in the control group. Moreover, insulin was detected in the serum of the treated mice, and the pancreas also showed gradual recovery. Based on the results of this preliminary investigation, intra-venous infusion seems more suitable than intra-pancreatic injection for MSC transplantation for diabetes mellitus treatment.
Introduction
Type 1 diabetes mellitus is a chronic metabolic disorder caused by the gradual destruction of beta cells, resulting in insulin deficiency and hyperglycemia. This disorder can also lead to chronic vascular complications. Insulin therapy can significantly reduce the severity and complications of the disease; however, patients can develop resistance to insulin after long-term usage of this therapy. Islet transplantation is a new therapy for type 1 diabetes mellitus; it has yielded good results clinically, with several patients being successfully treated Al-Adra et al., 2014Bruni et al., 2014. However, this therapy still has some limitations, including the shortage of islet donors (usually at least two donors are needed per patient) and post-transplantation rejection problems Linn et al., 2006Naftanel and Harlan, 2004.
Mesenchymal stem cells (MSCs) have a high potential for use in therapeutic applications. MSCs have the ability to differentiate into cells of all three germ layers, and they also have high proliferative potential ex vivo. They are promising candidates for use in cell transplantation and tissue engineering Das et al., 2013Kim and Cho,2013Zander et al., 2011. Recent studies show that MSC transplantation may improve metabolism in animal models of diabetes mellitus Pan et al., 2013Tang et al., 2014Xiao et al., 2013. Some researchers have suggested that MSCs should be transdifferentiated into insulin-producing cells (IPCs) before transplantation Gabr et al.,2013Wu et al., 2007, while others have used un-differentiated MSCs Dong et al.,2008. MSCs could stimulate the production of cytokines and growth factors. The paracrine effects of these secreted factors can greatly influence the surrounding microenvironment of the MSCs and promote the survival of the surrounding cells Dormady et al., 2001Ohnishi et al., 2007. MSCs can “home” to the damaged area and help initiate recovery Vija et al., 2009. Therefore, when transplanted into type 1 diabetes mellitus patients, MSCs could help restore beta cell numbers, support metabolism, and improve the medical condition of the patient.
The MSCs has been assessed to be non-immunogenic. Therefore, MSCs can be used in allo-transplantation without immunosuppression. Many reports have demonstrated the immunosuppression potential of MSCs. Specifically, MSCs can modulate many functions of T cells, including cell activation Bartholomew et al., 2002Le Blanc et al.,2003Rasmusson et al., 2005. MSC-mediated immune modulation inhibits the maturation and function of antigen-presenting cells Fibbe et al., 2007.
MSCs can be obtained from many sources such as the bone marrow Prockop et al.,2001, adipose tissue Boquest et al., 2006, amniotic fluid Tsai et al., 2004, and umbilical cord blood Erices et al., 2000. Umbilical cord blood is a rich source of MSCs, and the isolation from this source is not clinically invasive and does not involve ethical violations. Therefore, this study was aimed at evaluating the effects of MSC transplantation on mice with diabetes mellitus by using 2 different delivery methods—intravenous infusion and intra-pancreatic injection.
Materials – Methods
Mouse models of diabetes mellitus
Male mice aged 6-8 weeks with body weights of 20-25 grams were intravenously injected with streptozotocin (STZ, Sigma- Aldrich, St Louis, MO) at a dose of 50 mg·kg-1·day-1 for 5 days. The STZ-treated mice were monitored for blood glucose levels, and only the mice with blood glucose levels higher than 200 mg/dl after 20 days were considered to have type 1 diabetes mellitus. All the procedures were approved by the Institutional Ethical Committee of the Laboratory of Stem Cell Research and Application, University of Science, Vietnam National University, HCMC, VN.
Isolation of MSCs
Umbilical cord blood was collected from donors after obtaining informed consent. Only samples from full-term subjects free of human immunodeficiency virus and hepatitis B virus were used for this study. All procedures related to the handling of the blood samples were approved by the Ethical Committee of our university. MSCs from human umbilical cord blood were cultured according to previously published procedure Phuc et al., 2011. Briefly, mononuclear cells (MNCs) were isolated from umbilical cord blood by centrifuging with Ficoll 1.077 (GE-Healthcare). MNCs were cultured in Iscove’s modified Dulbecco’s medium (IMDM) containing 15% fetal bovine serum (FBS) and 1% antibiotics and antimycotics (all purchased from Sigma-Aldrich, St Louis, MO). The fresh medium was added every 3 days. The cells were sub-cultured after reaching 70-80% confluence. These cells were then used for all further experiments.
MSC transplantation
For the study, the mice were divided into 5 groups: group 1 (Normal) — normal mice that were not injected with STZ; group 2 (PBS-I.V) — diabetic mice that were intravenously injected with PBS; group 3 (PBS-PAN) — diabetic mice in which PBS was injected into the pancreas; group 4 (MSC-I.V) — diabetic mice that were treated with one intravenous injection of 5 ×106 MSCs/mouse; and group 5 (MSC-PAN) — mice that were treated with one injection of 5 ×106 MSCs/mouse into the pancreas.
Quantitative treatment assessments
Blood glucose levels and weights were continuously measured every 3 days for 30 days after transplantation. At day 30, the mice were anesthetized with ketamine (Solupharm GmbH, Germany), and blood was collected from the heart. Serum was collected from the blood, and insulin was detected by high performance liquid chromatography (HPLC). The pancreas was collected, and the pancreatic cells were isolated using a 100-μm cell strainer. The pancreatic cells were stained with dithizone (DTZ) to determine the recovery of the insulin-secreting cells in the pancreases of mice in all groups.
Statistical Analysis
Data were analyzed and processed using Microsoft Excel 2007 and Statgraphics Centurion software (XV.I version). Data are expressed in mean values and standard error values (Standard Error_SD), with P < 0.05 considered statistically significant.
Results
Isolation of UCB-MSCs
UCB-MSCs were successfully isolated from the UCB. These cells exhibited the particular phenotypes of MSCs such as fibroblast like shape when adhered into the flask surface ( Figure 1A ); successfully differentiated into adipocytes ( Figure 1B ) and osteoblasts ( Figure 1C ). They also expressed the MSC marker profile included positive with CD13, CD44, CD73, CD90; and negative with CD14, CD34, CD45 and HLA-DR ( Figure 1D-L ).
Characteristics of mice after transplantation
From 7–30 days after injection of MSCs, mice in the MSC-I.V and MSC-PAN groups exhibited visible signs of recovery including flexibility, smooth and shining hair, increase in weight, and a decrease in urine output. Diabetic mice in the PBS-I.V group exhibited a decrease in strength, denuded hair, polyuria, and stress, and 2 mice in the group died on days N12 and N24.
Weight of mice after transplantation
The mice in the two treated groups had higher weights than those in the control groups and lower weights than those in the normal group. In the MSC-I.V group, from days 0–6, the mice initially gained weight. From days 9–30, the weights of the mice became stable (32.66 ± 1.20 g, 31.58 ± 0.89 g, 32.25 ± 1.45 g, and 32.92 ± 1.0 g, respectively, at days 9, 21, 27, and 30). At 30 days after MSC transplantation, the MSC-I.V mice showed a 22% increase in their weights compared to a 27% increase in the weights of the normal mice.
In the MSC-PAN group, the mice gained weight from days 0–6 and then stably maintained the weight. At day 30, the weight of the mice increased by 14% compared to that before MSC transplantation. The mice in the MSC-I.V group gained more weight than those in the MSC-PAN group, especially during days 9 to 30. The weight of the mice in the control groups, PBS-I.V and PBS-PAN, decreased to 24% and 28%, respectively.
The results also showed that mice in both the MSC transplantation groups did not completely recover their weights like the normal mice. The weight of the mice in the MSC-I.V and MSC-PAN groups was significantly higher than that of the mice in the PBS-I.V and PBS-PAN groups, respectively.
Blood glucose levels of mice after transplantation
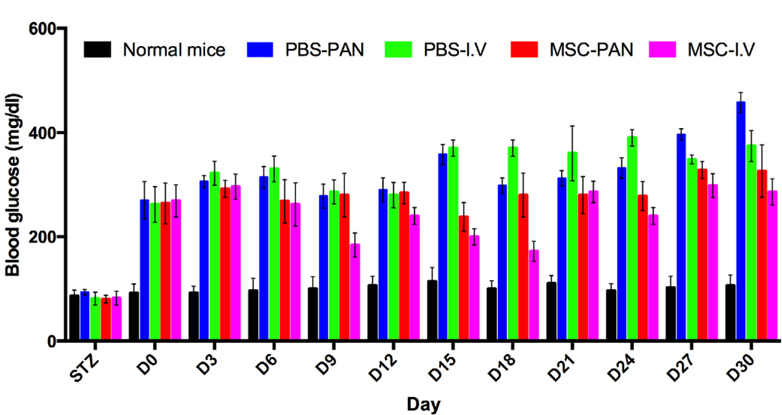
The blood glucose levels of mice in both the treated groups were higher than those of the mice in the normal group, but they were lower than those of the mice in the PBS-I.V and PBS-PAN groups. There was a slight decrease in blood glucose level at day 3 in the treated mice, after which it remained stable ( Figure 2 ).
The blood glucose level of the mice in the MSC-I.V group was significantly lower than that of mice in the un-treated groups on days 15 and 18; the blood glucose level was also lower than that observed before transplantation. At day 18, the blood glucose level in the MSC-I.V group decreased to 37%. From days 6–18, the blood glucose level in the MSC-I.V group was found to be significantly lower than that in the un-treated group and reached the level found in the normal mice (MSC-I.V group—176.00 ± 72.64 at day 9 and 164.33 ± 75.57 at day 18; normal group—75.53 ± 10.84 at day 9 and 98.87 ± 11.24 at day 18). However, 30 days after transplantation, the blood glucose levels gradually increased and reached the same level as those before transplantation (293.33 ± 93.82 vs. 259.00 ± 0.00, respectively). The blood glucose level of the PBS-I.V (untreated control) group showed a drastic increased from days 0 to 30 reaching 420.00 ± 78.00 at day 30, which was 62% greater than the glucose level on day 0 ( Figure 2 ).
In the MSC-PAN group, the blood glucose level was found to gradually increase after MSC transplantation. The level was also lower than that in the PBS-PAN (untreated control) group. The blood glucose level in both treated mice (MSCPAN) and un-treated mice (PBS-PAN) increased following the injection of MSCs or PBS into the pancreas; however, a maximum increase of 50% was recorded in the blood glucose level of the MSC-PAN mice, and the PBS-PAN mice.
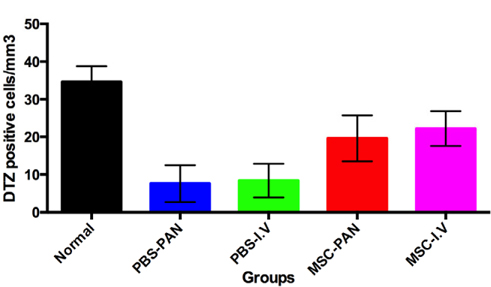
Figure 4 Figure 4 DTZ positive cells of four experiment groups compared to normal mice There was a recovery of DTZ positive cells (beta cells) in the MSC treated mice in both MSC-PAN or MSC-I.V. Normal: normal mice; PBS-PAN: control, PBS intra-pancreatic injection; PBS-I.V: control, PBS intra-vein injection; MSC-PAN: MSC intrapancreatic injection; MSC-I.V: MSC intra-vein injection.
Presence of insulin in serum after transplantation
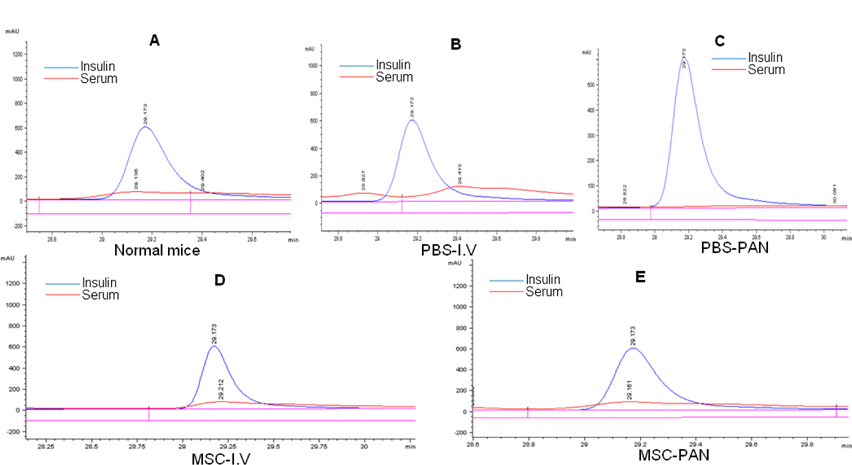
We compared the insulin peaks of the serum samples of the mice in our study with those of standard insulin samples through exist periods of protein peaks and detected the presence of insulin in the mouse serum ( Figure 3A ). Insulin was not detected in the serum of mice in the two un-treated groups ( Figure 3B,C ). The results observed in the two treated groups (MSC-I.V and MSC-PAN) were the same, and the presence of insulin in the serum of these mice was similar to that in the normal mice ( Figure 3D,E ). It has been demonstrated that transplantation of umbilical cord blood-MSCs can result in the release of insulin in the blood stream.
Determining the level of pancreatic recovery by DTZ staining
There was a significantly greater number of cells that stained with the DTZ dye in both of the treated groups (MSC-I.V and MSC-PAN) when compared to the un-treated groups (PBS-I.V and PBS-PAN). By counting the number of DTZpositive cells per mm3 of the pancreas, we found that that there were 17 ± 3 cells/ mm3 in the MSC-I.V group, and 3 ± 1 cells/ mm3 in the PBS-I.V group, and 16 ± 4 cells/ mm3 in the MSC-PAN group and 3 ± 1 cells/ mm3 in the PBS-PAN group (P < 0.05). However, in both groups that were injected with MSCs, the number of DTZ-positive cells was less than that in the normal mice (32 ± 5 cells/ mm3 ). MSC transplantation resulted in a 6.8- and 6.4-fold increase in the number of DTZ-positive cells in MSC-I.V and MSC-PAN groups, respectively, as compared to the un-treated groups.
Discussion
MSCs have a high potential for use in diabetes mellitus treatment. In this study, MSC transplantation elicited recovery in a diabetic mouse model. The mice recovered their weight (by 22% (MSC-I.V group) and 14% (MSC-PAN group)) and also showed partly restored blood glucose levels. The levels remained stable for 30 days. There was a slight increase of about 13% in the blood glucose level in the MSC-I.V group, and an increase of about 50% was observed in the MSC-PAN group. At some time points, the blood glucose levels in the MSC-I.V group were similar to those in the normal mice. In transplanted mice, the beta cells were recovered, and insulin was detected in the serum. There were significant differences between the mice transplanted with MSCs and the un-treated mice, indicating that the transplantation of MSCs could be a contributing factor.
There are some hypotheses about the effects of transplanted MSCs on diabetes recovery. MSCs have been shown to be capable of “homing” to the damaged area of the body and replacing injured cells Vija et al., 2009. Xu et al. reported that MSC transplantation could help in recovery of the pancreas that was injured by injection of STZ or attacked by T cells, through paracrine mechanisms Xu et al., 2008. In fact, MSCs can secrete a variety of cytokines, growth factors, and stimulating factors that come in contact with the surrounding cells. This positive paracrine effect on the damaged beta cells could help to protect these cells from apoptosis and to promote the proliferation of intrinsic pancreatic progenitor cells. This effect is also related to angiogenesis, cell protection, anti-inflammatory activities, promotion of mitosis, and apoptosis resistance Bell et al., 2012Gao et al., 2014Xu et al., 2008. Furthermore, MSCs can trans-differentiate into insulin-producing cells (IPCs) in vivo in a suitable microenvironment Chang et al., 2007. In addition, these cells also have the ability to fuse with other pancreatic cells (as well as hepatic cells and brain cells) and promote insulin production Ying et al., 2002.
MSCs intravenously injected into mice could migrate to all the organs through the blood stream, selectively “homing” to the site of injury. They can affect different organs of the body, especially all injured sites (including the liver cells and brain cells damaged by STZ). Therefore, the efficiency of treatment by this method could be higher than that of treatment by localized MSC injection into the pancreas.
Previously published reports have also shown that MSC transplantation could be used to treat diabetic mice. Filiuzzi et al. demonstrated that the injection of 1 ×106 MSCs (obtained from the bone marrow) and 2 × 103 islets in the renal cortex of the Lewis rats, in which diabetes mellitus was induced by 65 mg/kg STZ, effectively reduced blood glucose level to under 200 mg/dl after 15 days, this levels remained stable until day 39 Figliuzzi et al., 2009. Lin et al. provided evidence of in vivo trans-differentiation of MSCs into IPCs in mice when 1 × 107 MSCs were injected into the tail vein of Wistar rats in which diabetes mellitus was induced by injection with 60 mg/kg STZ Lin et al.,2009. This research group also showed that, at only 14 days after MSC transplantation, the blood glucose level decreased to lower than 200 mg/dl; and, after 28 days, it was reduced to that of normal mice (< 126 mg/dl). The transplantation of MSCs also simultaneously increased the number of IPCs and the insulin level in the blood Bell et al., 2012.
UrBan et al. also injected 1 × 105 MSCs and 1 ×106 bone marrow cells into the veins of diabetic C57B1/6 mice, in which diabetes was induced by injection with 50 mg/kg STZ,; the mice were immune-suppressed by irradiation at a dose of 900 cGy. The blood glucose level and serum insulin levels in mice transplanted with both cell types quickly returned to normal levels. This study also showed that MSCs induced endogenous insulin secretion and inhibited T-cell–mediated immune responses against newly formed beta cells Urban et al., 2008.
In this study, intravenous MSC transfusion was more effective than the intra-pancreatic injection. Some factors affected the efficacy of intra-pancreatic injection method of MSC transplantation. The first may be related to MSC migration— intravenously-injected MSCs could home to any injured tissues by migration through the blood stream, while MSCs injected into the pancreas could only remain in the pancreas. In the mice with STZ-induced diabetes mellitus, STZ could have affected tissues such as the spleen, liver, stomach, and brain. Therefore, the recovery of the pancreas may not be enough to heal the disease. Secondly, the pancreas is a complex organ with two different roles, including an endocrine function that relates to the production of hormones such as insulin and an exocrine function that relates to the production of enzymes such as proteases. An injured pancreas could release proteases that could be harmful to local tissue and reduce the efficacy of therapy.
Another organ has also been considered a “house” for MSCs or ICPs in diabetes mellitus treatment. Xu et al. showed that intrahepatic transplantation of murine MSCs expressing human insulin in diabetic mice could be effective for up to 6 weeks. Intra-hepatic transplantation of MSCs in a larger animal model (dog), also gave positive results Zhu et al., 2011.
Conclusion
MSC transplantation is considered a promising therapy for type 1 diabetes mellitus. MSC transplantation can decrease blood glucose levels and trigger pancreatic recovery in diabetic mice. This study showed that intravenous injection of MSCs into the peripheral blood is more efficient than intrapancreatic injection. Intravenous MSC infusion efficiently reduced and stabilized blood glucose levels, stimulated pancreatic regeneration, and increased the viability of diabetic mice. These results suggested that intravenous injection of MSCs is a suitable, a minimally invasive method for the study of diabetes mellitus treatment.
Abbreviations
CD: Cluster of differentiation
IPCs: Insulin-producing cells
IV: Intravenous
MSC: Mesenchymal stem cells
PAN: Pancreatic
PBS: Phosphate buffer saline
STZ: Streptozotocin
UCB: Umbilical cord blood
Authors’ contributions
All authors read and approved the final manuscript. NKP, TTD, PVP performed isolation of UCB-MSCs; TLBP, LTTD, ANTB carried out the in vivo assays; VMP, NCT evaluated and created the diabetes mellitus models; PVP suggested the ideas, wrote the manuscripts.
References
-
D.P.
Al-Adra,
R.S.
Gill,
S.
Imes,
D.
O’Gorman,
T.
Kin,
S.J.
Axford,
X.
Shi,
P.A.
Senior,
A.M.
Shapiro.
Single-Donor Islet Transplantation and Long-term Insulin Independence in Select Patients With Type 1 Diabetes Mellitus. Transplantation 10.1097/tp.0000000000000217.
2014
.
-
A.
Bartholomew,
C.
Sturgeon,
M.
Siatskas,
K.
Ferrer,
K.
McIntosh,
S.
Patil,
W.
Hardy,
S.
Devine,
D.
Ucker,
R.
Deans.
Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental hematology.
2002;
30
:
42-48
.
-
G.I.
Bell,
M.T.
Meschino,
J.M.
Hughes-Large,
H.C.
Broughton,
A.
Xenocostas,
D.A.
Hess.
Combinatorial human progenitor cell transplantation optimizes islet regeneration through secretion of paracrine factors. Stem cells and development.
2012;
21
:
1863-1876
.
-
A.C.
Boquest,
A.
Shahdadfar,
J.E.
Brinchmann,
P.
Collas.
Isolation of stromal stem cells from human adipose tissue. Methods in molecular biology.
2006;
(Clifton
:
NJ) 325, 35-46
.
-
A.
Bruni,
B.
Gala-Lopez,
A.R.
Pepper,
N.S.
Abualhassan,
A.J.
Shapiro.
Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes, metabolic syndrome and obesity : targets and therapy.
2014;
7
:
211-223
.
-
C.
Chang,
D.
Niu,
H.
Zhou,
F.
Li,
F.
Gong.
Mesenchymal stem cells contribute to insulin-producing cells upon microenvironmental manipulation in vitro. Transplantation proceedings.
2007;
39
:
3363-3368
.
-
M.
Das,
I.B.
Sundell,
P.S.
Koka.
Adult mesenchymal stem cells and their potency in the cell-based therapy. Journal of stem cells.
2013;
8
:
1-16
.
-
Q.Y.
Dong,
L.
Chen,
G.Q.
Gao,
L.
Wang,
J.
Song,
B.
Chen,
Y.X.
Xu,
L.
Sun.
Allogeneic diabetic mesenchymal stem cells transplantation in streptozotocin-induced diabetic rat. Clinical and investigative medicine Medecine clinique et experimentale.
2008;
31
:
E328-337
.
-
S.P.
Dormady,
O.
Bashayan,
R.
Dougherty,
X.M.
Zhang,
R.S.
Basch.
Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. Journal of hematotherapy & stem cell research.
2001;
10
:
125-140
.
-
A.
Erices,
P.
Conget,
J.J.
Minguell.
Mesenchymal progenitor cells in human umbilical cord blood. British journal of haematology.
2000;
109
:
235-242
.
-
W.E.
Fibbe,
A.J.
Nauta,
H.
Roelofs.
Modulation of immune responses by mesenchymal stem cells. Annals of the New York Academy of Sciences.
2007;
1106
:
272-278
.
-
M.
Figliuzzi,
R.
Cornolti,
N.
Perico,
C.
Rota,
M.
Morigi,
G.
Remuzzi,
A.
Remuzzi,
A.
Benigni.
Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplantation proceedings.
2009;
41
:
1797-1800
.
-
M.M.
Gabr,
M.M.
Zakaria,
A.F.
Refaie,
A.M.
Ismail,
M.A.
Abou-El-Mahasen,
S.A.
Ashamallah,
S.M.
Khater,
S.M.
El-Halawani,
R.Y.
Ibrahim,
G.S.
Uin.
Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell transplantation.
2013;
22
:
133-145
.
-
X.
Gao,
L.
Song,
K.
Shen,
H.
Wang,
M.
Qian,
W.
Niu,
X.
Qin.
Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Molecular and cellular endocrinology.
2014;
388
:
41-50
.
-
N.
Kim,
S.G.
Cho.
Clinical applications of mesenchymal stem cells. The Korean journal of internal medicine.
2013;
28
:
387-402
.
-
K.
Le Blanc,
L.
Tammik,
B.
Sundberg,
S.E.
Haynesworth,
O.
Ringden.
Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scandinavian journal of immunology.
2003;
57
:
11-20
.
-
P.
Lin,
L.
Chen,
N.
Yang,
Y.
Sun,
Y.X.
Xu.
Evaluation of stem cell differentiation in diabetic rats transplanted with bone marrow mesenchymal stem cells. Transplantation proceedings.
2009;
41
:
1891-1893
.
-
T.
Linn,
J.
Schmitz,
I.
Hauck-Schmalenberger,
Y.
Lai,
R.G.
Bretzel,
H.
Brandhorst,
D.
Brandhorst.
Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clinical and experimental immunology.
2006;
144
:
179-187
.
-
M.A.
Naftanel,
D.M.
Harlan.
Pancreatic islet transplantation. PLoS medicine.
2004;
1
:
e58; quiz e75
.
-
S.
Ohnishi,
T.
Yasuda,
S.
Kitamura,
N.
Nagaya.
Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem cells.
2007;
(Dayton
:
Ohio) 25, 1166-1177
.
-
X.H.
Pan,
Q.Q.
Song,
J.J.
Dai,
X.
Yao,
J.X.
Wang,
R.Q.
Pang,
J.
He,
Z.A.
Li,
X.M.
Sun,
G.P.
Ruan.
Transplantation of bone marrow mesenchymal stem cells for the treatment of type 2 diabetes in a macaque model. Cells, tissues, organs.
2013;
198
:
414-427
.
-
P.V.
Phuc,
T.H.
Nhung,
D.T.
Loan,
D.C.
Chung,
P.K.
Ngoc.
Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In vitro cellular & developmental biology Animal.
2011;
47
:
54-63
.
-
D.J.
Prockop,
I.
Sekiya,
D.C.
Colter.
Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy.
2001;
3
:
393-396
.
-
I.
Rasmusson,
O.
Ringden,
B.
Sundberg,
K.
Le Blanc.
Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Experimental cell research.
2005;
305
:
33-41
.
-
K.
Tang,
X.
Xiao,
D.
Liu,
Y.
Shen,
Y.
Chen,
Y.
Wang,
B.
Li,
F.
Yu,
D.
Ma,
J.
Yan.
Autografting of bone marrow mesenchymal stem cells alleviates streptozotocininduced diabetes in miniature pigs: real-time tracing with MRI in vivo. International journal of molecular medicine.
2014;
33
:
1469-1476
.
-
M.S.
Tsai,
J.L.
Lee,
Y.J.
Chang,
S.M.
Hwang.
Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Human reproduction.
2004;
(Oxford
:
England) 19, 1450-1456
.
-
V.S.
Urban,
J.
Kiss,
J.
Kovacs,
E.
Gocza,
V.
Vas,
E.
Monostori,
F.
Uher.
Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem cells.
2008;
(Dayton
:
Ohio) 26, 244-253
.
-
L.
Vija,
D.
Farge,
J.F.
Gautier,
P.
Vexiau,
C.
Dumitrache,
A.
Bourgarit,
F.
Verrecchia,
J.
Larghero.
Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes & metabolism.
2009;
35
:
85-93
.
-
X.H.
Wu,
C.P.
Liu,
K.F.
Xu,
X.D.
Mao,
J.
Zhu,
J.J.
Jiang,
D.
Cui,
M.
Zhang,
Y.
Xu,
C.
Liu.
Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World journal of gastroenterology : WJG.
2007;
13
:
3342-3349
.
-
N.
Xiao,
X.
Zhao,
P.
Luo,
J.
Guo,
Q.
Zhao,
G.
Lu,
L.
Cheng.
Co-transplantation of mesenchymal stromal cells and cord blood cells in treatment of diabetes. Cytotherapy.
2013;
15
:
1374-1384
.
-
Y.X.
Xu,
L.
Chen,
R.
Wang,
W.K.
Hou,
P.
Lin,
L.
Sun,
Y.
Sun,
Q.Y.
Dong.
Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Medical hypotheses.
2008;
71
:
390-393
.
-
Q.L.
Ying,
J.
Nichols,
E.P.
Evans,
A.G.
Smith.
Changing potency by spontaneous fusion. Nature.
2002;
416
:
545-548
.
-
A.R.
Zander,
C.
Lange,
C.
Westenfelder.
Mesenchymal stromal cells: main factor or helper in regenerative medicine?. Kidney international supplements.
2011;
1
:
74-76
.
-
S.
Zhu,
Y.
Lu,
J.
Zhu,
J.
Xu,
H.
Huang,
M.
Zhu,
Y.
Chen,
Y.
Zhou,
X.
Fan,
Z.
Wang.
Effects of intrahepatic bone-derived mesenchymal stem cells autotransplantation on the diabetic Beagle dogs. The Journal of surgical research.
2011;
168
:
213-223
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 03 (2014)
Page No.: 98-105
Published on: 2014-08-08
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 7882 times
- Download PDF downloaded - 1892 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress