Production of functional dendritic cells from mouse bone marrow
Abstract
Introduction
Dendritic cells (DCs) are professional presenting cells that can process and present antigens to both T and B cells Steinman, 1991. In vivo, DCs will trigger and initiate immune response. These cells can be differentiated from myeloid progenitors and lymphoid progenitors. Therefore, stimulation of DCs in vivo has also been considered as a potential therapy for cancer treatment. In almost all studies, there were two cytokines used to stimulate DCs, including the flt3 ligand (FL) and granulocyte macrophage colony-stimulating factor (GM-CSF) Hanada et al., 1996Maraskovsky et al., 1996Pulendran et al., 1999.
However, DCs will present suitable antigens when they are induced with antigens in vitro before injection into the body. In fact, in cancer patients, DCs interact with tumor cells, and these DCs could become tolerant and also display non-immunologic effects against tumors and the tumor microenvironment Dhodapkar et al., 2008. Therefore, DCs must be matured by antigens in an in vitro culture system prior to use.
In an early study, Inaba et al., 1992 used GM-CSF to induce maturation of hematopoietic precursors into DCs after 6–8 days Inaba et al., 1992. Subsequently, other studies optimized the procedure to produce mature DCs when combined with GM-CSF and IL-4 Labeur et al., 1999Saunders et al., 1996. Thereafter, almost all studies used both GM-CSF and IL-4 to produce DCs for DC therapy. These mature DCs must express CD80, CD86, and CD40 as well as MHC class II molecules. Moreover, DCs can also produce some interleukins (ILs), especially IL-12, or efficiently stimulate T lymphocytes in vitro and in vivo.
Here, we describe a simple method to produce large numbers of DCs in vitro from mouse bone marrow cells using GM-CSF and IL-4. Using this method, DCs completely exhibited the DC phenotypes, including both morphological and other characteristics of DCs. This method provides a model to produce and study DCs in vitro and DC therapies in mouse models.
Material and methods
Cell culture and differentiation of monocytes into dendritic cells
The mice were euthanized by cervical dislocation, in which the neck of each mouse was grasped firmly in one hand and the other hand was used to sharply pull at the base of the tail. Prior to its placement in the glass cabinet for manipulation, the mouse model was doused with 70% alcohol. A small cut was made in the abdominal area with a pair of sterile scissors, just above the mid-line. This was then followed by the removal of the membrane layer to reveal the organs in the mid-section of the body. The femurs of the mice were dislodged and submerged in PBS (phosphate buffer saline) supplemented with antibiotic-mycotic (Sigma-Aldrich, St Louis, MO).
Bone marrow cells were used to isolate mononuclear cells by centrifugation in a Ficoll gradient. All obtained mononuclear cells were maintained at 37°C with 5% CO2. We added IL-4 to the culture medium, which contains GM-CSF for culturing DCs, because a combination of GM-CSF and IL-4 is better able to stimulate mixed leukocyte reactions. The culture medium that was used for all of the experiments was RPMI-1640 (Sigma-Alrich, St Louis, MO) supplemented with 2 mM/L glutamine, 100 μg/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). To produce immature DCs (iDCs), adherent cells were cultured for 6 days in medium containing recombinant GM-CSF and IL-4 at concentrations of 20 ng/mL each. At days 7–12, these cells matured with TNF-α (20 ng/mL), a different cytokine that was added to the complete media. At day 12, mature DCs (mDCs) were confirmed to have DC phenotypes through flow cytometry detection of CD14 (for monocytes), CD40, D80, and CD86 (for DCs).
Dextran-FITC uptake assay
The phagocytic capacities of iDCs and mDCs were analyzed according to a previously published protocol Phuc et al., 2011. Briefly, 5×104 cells were incubated for 1 h with dextran-FITC (1 mg/mL; Sigma-Aldrich, St Louis, MO) in 100 μL of culture medium at 37°C and 4°C as negative control. Cells were then washed with cold PBS supplemented with 1% bovine serum albumin (BSA) four times before flow cytometry analysis. Phagocytic ability of DCs was confirmed by the appearance of cell populations that expressed FITC fluorescent signals.
Stimulation of CD4+ T lymphocyte proliferation
Stimulation of CD4+ T lymphocyte proliferation was evaluated following the previously published protocol Phuc et al., 2011. The assessment was performed in different groups with the ratio of DC/lymphocytes as follows: 0.25:100, 0.5:100, 1:100, 2:100, and 8:100. The control groups included DC+PHA (phytohemagglutinin, Sigma-Aldrich, St Louis, MO), PHA, and PHA+lymphocytes. PHA concentration was 50 mg/L, and the experiment was conducted on 96-well plates (Nunc, Roskilde, Denmark) and repeated four times. CD4+ T lymphocyte proliferation was measured by MTT assay using the Cell Growth Determination Kit, MTT based (Sigma-Aldrich, St Louis, MO). Twenty microliters of MTT was added into each well of the 96- well plates, followed by incubation for 4 h and addition of 150 μL of DMSO. Plates were then thoroughly mixed for 10 min until the crystals completely dissolved. Absorption values (A-values) for each well were measured at a wavelength of 490 nm using micro-plate reader DTX 880 (Beckman Coulter, Brea, CA). An offset value of A and absorption value of a control group (DC+PHA) reflect lymphocyte proliferation. An offset value of absorption in the lymphocyte+ PHA and PHA groups showed proliferation of lymphocytes in the control group. All results were analyzed by Staraphic 7.0 software.
Quantity of cytokine/chemokine production
To detect the secretion of cytokines, monocytes were further induced with TNF-α after induction with GMCSF and IL-4, as described above, in culture medium in 24-well plates for 24 h. Supernatant was collected and frozen at −80°C until analysis. The quantity of cytokine IL-12 in the supernatant was determined by ELISA (Abcam, Cambridge, UK) and read on a DTX 880 (Beckman Coulter, Brea, CA).
Results
Induced monocytes express DC phenotypes
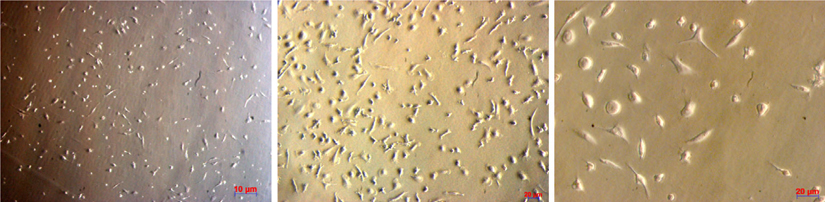
We observed that monocytes formed small groups similar to the culture of monocytes from human bone marrow Bai et al., 2002, peripheral blood Ratta et al., 1998 or umbilical cord blood Liu et al., 2002Shi et al., 2005 as well as to those observed in some previous studies addressing bone marrow-derived dendritic cells Lutz et al.,1999Lutz and Rossner, 2007Lutz et al., 2000Scheicher et al., 1992. Most of the cells had expanded cytoplasm and small dendritelike structures. Morphologically, the differentiated cells had some characteristics of DCs. These cells had relatively uniform shapes with large heterogeneity of nuclei, many mitochondria and vacuoles, and relatively few particles in the cytoplasm. Cell shape is comparable to DCs ( Figure 1 ).
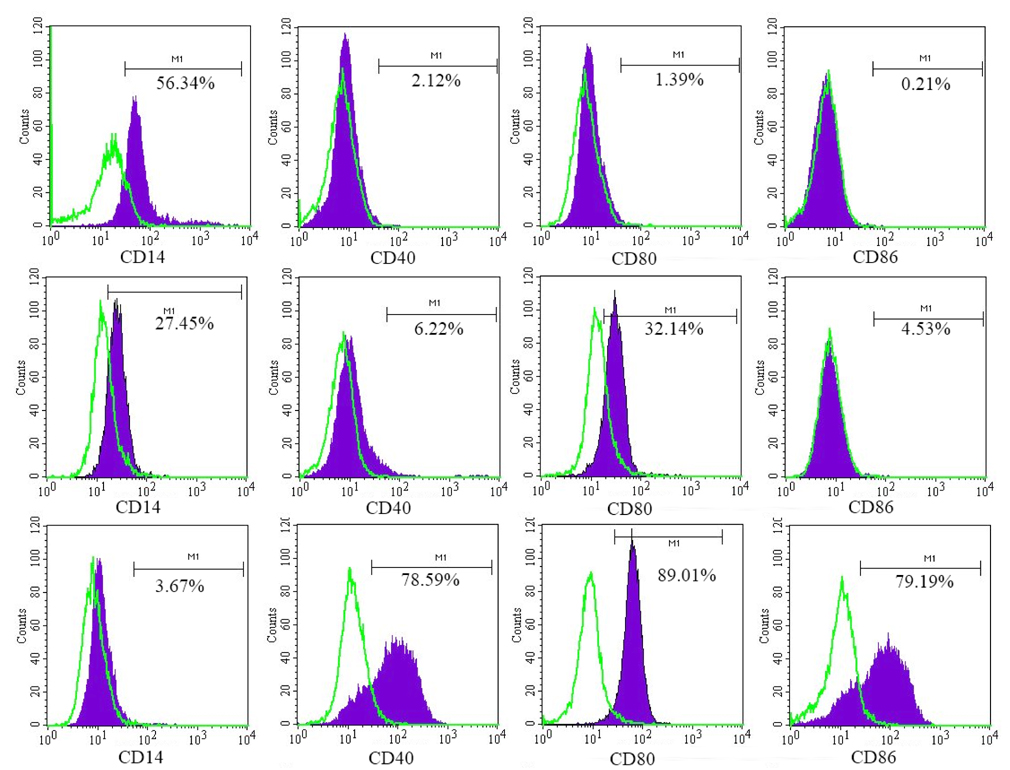
The results of DC marker analysis are presented in Figure 2 . It was observed that monocytes expressed CD14 but did not express CD40, CD80, and CD86 before induction; they expressed CD86 and CD40 but did not express CD14 and CD80 after induction of iDCs; and they expressed CD40, CD80, and CD86 but did not express CD14 after induction of mDCs.
Differentiated DCs from monocytes were functional in vitro
Antigen phagocytosis
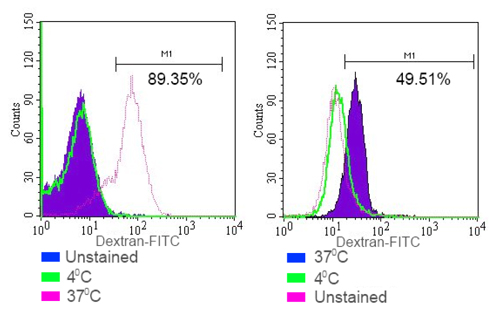
To evaluate the function of DCs, we measured the phagocytic ability of DCs in vitro. Phagocytic activity was assessed by measuring the ability of cells to consume dextran-FITC. Our data showed that monocytes after induction increased their phagocytic ability to 92.28 ± 9.25% in iDCs and 78.54 ± 11.33% in mDCs (p<0.05). Meanwhile, phagocytic ability was only 8.11 ± 3.13% (n = 3) in the group of cells that were cultured in the medium without GM-CSF, IL-4, and TNF-α. In addition, cells that were cultured at 4°C could not engulf the dextran-FITC ( Figure 3 ).
Induced monocyte-stimulated T lymphocytes
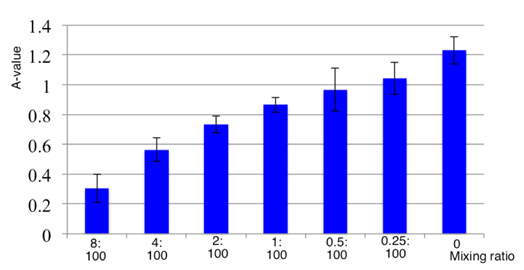
The expression of CD80 and CD86 on the surface of the DCs was associated with the activity of activated T lymphocytes. The results showed that induced DCs by monocytes with the cytokines GM-CSF, IL-4, and TNF-α triggered T cell proliferation. Figure 4 reveals that the activity of DCs was significantly different compared with that of the control group (p<0.05) and was augmented when DC concentrations increased (p<0.05).
A-values ( Figure 4 ) are offset of OD values that were measured in the lymphocyte+PHA control group and experimental groups. Because PHA is the strongest stimulant of lymphocytes, the OD value of the control sample was the highest. Therefore, the A-value was smaller when the growth capacity of experimental cells was higher. In a similar manner, the A-value was larger if the stimulation by DC was lower. The experimental results also revealed that DCs differentiated from bone marrow monocytes had the ability to stimulate lymphocyte cells in vitro. This ability depended on the ratio of mixing between DCs and lymphocyte cells; the more DCs added, more lymphocytes were stimulated. Activity of stimulating lymphocyte proliferation is a critical characteristic of DCs in vivo. This feature helps enhance immunity in cancer treatment, which is a key point for success of this approach.
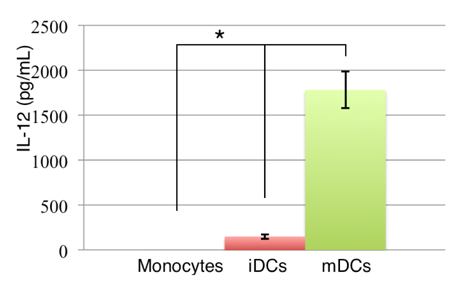
Induced monocytes produced IL-12
To determine the mechanism of activated T cell proliferation, the secretion of IL-12 was evaluated after induction because IL-12 is the most important interleukin in the activation of T lymphocytes. In fact, DCs always interact with other cells in the body. This interaction can occur directly by cell-cell communication via the interaction of surface proteins (as described above) through B7 (CD80 or CD86) with CD28 on lymphocytes; alternatively, this interaction can occur indirectly over a greater distance through cytokines. Typically, DCs produce IL-12 after induction with antigens. IL-12 is a signal that induces CD4+ T cells into the Th1 phenotype. Then, it activates the immune system to attack antigens that are presented by DCs.
IL-12 quantity was analyzed by ELISA ( Figure 5 ) and it was shown that there was a statistically significant difference (p<0.5) between monocytes with and without cytokine treatment.
Discussion
DCs are the most professional antigen-presenting cells in our bodies. Unlike macrophages, they have the ability to present antigens not only to T cells but also to B and NK cells. Within our biological system, DCs can be differentiated by hematopoietic stem cells (HSCs). When there is any stimulation by risk factors through toll-like receptors (TLRs), DCs phagocytose small portions of the membrane of the inducers through a process called nibbling. Subsequently, small fragments are processed and sent to their cell surface using MHC molecules. In the mature stage, mDCs express CD80 (B7.1), CD86 (B7.2), and CD40. These are essential coreceptors that stimulate T cell activity. By the expression of mentioned co-receptors, DCs have the power to provoke memory T and naïve T cells, as well as other forms of T cells. Hence, appearance of these surface proteins is a prerequisite for DCs to activate other cells of the immune system. Numerous studies that created DCs from monocytes or HSCs from umbilical cord blood (UCB), bone marrow (BM), or peripheral blood succeeded in enhancing the expressivity of these markers Ferlazzo et al., 2000Goriely et al., 2001Liu et al., 2002Reddy et al., 1997Reis e Sousa et al., 1997. In this report, we showed that the DCs in our study also expressed similar proteins, including, for example, CD80 and CD86. This evidence reveals that induced cells have the capacity to excite other cells in the immune system, especially naïve T and memory T cells.
This point is demonstrated by the results of our investigation of the ability of DCs to activate T cells. In the experiment to stimulate CD4+ T cells by induced monocytes, we clearly saw remarkable T cell proliferation when DCs were co-cultured with CD4+ T cells extracted from allogenic bone marrow samples. Furthermore, the increase in T cells was dosagedependent when the same amount of T cells was mixed with variable amounts of DCs. This trend was facilitated by direct interaction between DCs and CD4+ T cells through CD80 and CD86 co-receptors. It is known that the stimulatory effect of DCs on T cells is not only caused by direct interaction but also by the indirect action of cytokine signals, which are released by DCs. In the human body, IL-12, an important interleukin, is produced by either DCs or macrophages and B cells. IL-12 initiates differentiation of naïve T cells into Th0 and then into Th1 and Th2 cells. IL-12 also plays a pivotal role in NK and T cell activities; it intensifies the toxicity of NK cells and CD8+ T cells. Fortunately, DCs from bone marrow can release IL-12. Data from this study indicated that induced DCs with a suitable cocktail of cytokines had significantly elevated IL-12 secretion. This evidence again supports our conclusion that authentic DCs were derived from mouse bone marrow.
Not only did induced DCs from bone marrow have a DC-like shape and ability to activate T cells, they also had high phagocytic capacity and antigen presentation, which are indispensable for DCs to perform their functions in immune therapy. In this report, we showed that phagocytosis is feasible by DCs through dextran-FITC uptake assay. After incubation with dextran, 92.28 ± 9.25% of iDCs and 78.54 ± 11.33% of mDCs participated in dextran uptake and expressed strong fluorescent signals. Hence, we created functional DCs with all of the typical characteristics of normal DCs, and these results are similar to those of some previously published publications Burdek et al.,2010Morelli et al., 2001. These cells have DC-like shapes, phagocytic capacities, and process and present antigens to stimulate other cells in the immune system, especially T cells, through direct cell-cell interaction or indirectly by IL-12 secretion.
Conclusion
DCs are professional antigen-presenting cells. The roles of DCs are different from those of macrophages in that they can present antigens to both lymphocytes (T and B cells) as well as NK cells. Mouse DCs can be easily produced from bone marrow cells by induction in RPMI-1640 medium supplemented with 2 mM/L glutamine, 100 μg/mL penicillin, 100 μg/mL streptomycin, 10% heat-inactivated FBS, 20 ng/mL IL-4, and 20 ng/mL GM-CSF for 7 days. These DCs entirely exhibited the DC phenotypes such as specific markers of DCs and strong phagocytic abilities, IL-12 production, and T cell stimulation. This procedure can be applied to produce functional DCs in future studies.
Abbreviations
BM: Bone marrow; BSA: Bovine serum albumin; CD: Cluster of differentiation; DC: Dendritic cell; ELISA: enzyme-linked immunesorbent assay; FL: Flt3 ligand; GM-CSF: Granulocyte macrophage colony-stimulating factor; HSC: Hematopoietic stem cell; iDC: Immature DC; IL: Interleukin; MHC: Major Histocompatibility Complex; mDC: mature DC; MNC: Mononuclear cell; PBS: Phosphate buffer saline; PHA: Phytohemagglutinin; TLR: Toll like receptor; TNF-α: Tumor necrosifsa ctor-α; UCB: Umbilical cord blood
References
-
L.
Bai,
M.
Feuerer,
P.
Beckhove,
V.
Umansky,
V.
Schirrmacher.
Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. International journal of oncology.
2002;
20
:
247-253
.
-
M.
Burdek,
S.
Spranger,
S.
Wilde,
B.
Frankenberger,
D.J.
Schendel,
C.
Geiger.
Three-day dendritic cells for vaccine development: antigen uptake, processing and presentation. Journal of translational medicine.
2010;
8
:
90
.
-
M.V.
Dhodapkar,
K.M.
Dhodapkar,
A.K.
Palucka.
Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell death and differentiation.
2008;
15
:
39-50
.
-
G.
Ferlazzo,
J.
Klein,
X.
Paliard,
W.Z.
Wei,
A.
Galy.
Dendritic cells generated from CD34+ progenitor cells with flt3 ligand, c-kit ligand, GM-CSF, IL-4, and TNF-alpha are functional antigen presenting cells resembling mature monocyte-derived dendritic cells. Journal of immunotherapy.
2000;
(Hagerstown
:
Md : 1997) 23, 48-58
.
-
S.
Goriely,
B.
Vincart,
P.
Stordeur,
J.
Vekemans,
F.
Willems,
M.
Goldman,
D.
De Wit.
Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. Journal of immunology.
2001;
(Baltimore
:
Md : 1950) 166, 2141-2146
.
-
K.
Hanada,
R.
Tsunoda,
H.
Hamada.
GM-CSF-induced in vivo expansion of splenic dendritic cells and their strong costimulation activity. Journal of leukocyte biology.
1996;
60
:
181-190
.
-
K.
Inaba,
M.
Inaba,
N.
Romani,
H.
Aya,
M.
Deguchi,
S.
Ikehara,
S.
Muramatsu,
R.M.
Steinman.
Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. The Journal of experimental medicine.
1992;
176
:
1693-1702
.
-
M.S.
Labeur,
B.
Roters,
B.
Pers,
A.
Mehling,
T.A.
Luger,
T.
Schwarz,
S.
Grabbe.
Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. Journal of immunology.
1999;
(Baltimore
:
Md : 1950) 162, 168-175
.
-
A.
Liu,
M.
Takahashi,
M.
Narita,
Z.
Zheng,
N.
Kanazawa,
T.
Abe,
K.
Nikkuni,
T.
Furukawa,
K.
Toba,
I.
Fuse.
Generation of functional and mature dendritic cells from cord blood and bone marrow CD34+ cells by two-step culture combined with calcium ionophore treatment. Journal of immunological methods.
2002;
261
:
49-63
.
-
M.B.
Lutz,
N.
Kukutsch,
A.L.
Ogilvie,
S.
Rossner,
F.
Koch,
N.
Romani,
G.
Schuler.
An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of immunological methods.
1999;
223
:
77-92
.
-
M.B.
Lutz,
S.
Rossner.
Factors influencing the generation of murine dendritic cells from bone marrow: the special role of fetal calf serum. Immunobiology.
2007;
212
:
855-862
.
-
M.B.
Lutz,
R.M.
Suri,
M.
Niimi,
A.L.
Ogilvie,
N.A.
Kukutsch,
S.
Rossner,
G.
Schuler,
J.M.
Austyn.
Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. European journal of immunology.
2000;
30
:
1813-1822
.
-
E.
Maraskovsky,
K.
Brasel,
M.
Teepe,
E.R.
Roux,
S.D.
Lyman,
K.
Shortman,
H.J.
McKenna.
Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. The Journal of experimental medicine.
1996;
184
:
1953-1962
.
-
A.E.
Morelli,
A.F.
Zahorchak,
A.T.
Larregina,
B.L.
Colvin,
A.J.
Logar,
T.
Takayama,
L.D.
Falo,
A.W.
Thomson.
Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood.
2001;
98
:
1512-1523
.
-
P.V.
Phuc,
D.H.
Lam,
V.B.
Ngoc,
D.T.
Thu,
N.T.
Nguyet,
P.K.
Ngoc.
Production of functional dendritic cells from menstrual blood-a new dendritic cell source for immune therapy. In vitro cellular & developmental biology Animal.
2011;
47
:
368-375
.
-
B.
Pulendran,
J.L.
Smith,
G.
Caspary,
K.
Brasel,
D.
Pettit,
E.
Maraskovsky,
C.R.
Maliszewski.
Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proceedings of the National Academy of Sciences of the United States of America.
1999;
96
:
1036-1041
.
-
M.
Ratta,
D.
Rondelli,
A.
Fortuna,
A.
Curti,
M.
Fogli,
F.
Fagnoni,
G.
Martinelli,
C.
Terragna,
S.
Tura,
R.M.
Lemoli.
Generation and functional characterization of human dendritic cells derived from CD34 cells mobilized into peripheral blood: comparison with bone marrow CD34+ cells. British journal of haematology.
1998;
101
:
756-765
.
-
A.
Reddy,
M.
Sapp,
M.
Feldman,
M.
Subklewe,
N.
Bhardwaj.
A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood.
1997;
90
:
3640-3646
.
-
C.
Reis e Sousa,
S.
Hieny,
T.
Scharton-Kersten,
D.
Jankovic,
H.
Charest,
R.N.
Germain,
A.
Sher.
In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. The Journal of experimental medicine.
1997;
186
:
1819-1829
.
-
D.
Saunders,
K.
Lucas,
J.
Ismaili,
L.
Wu,
E.
Maraskovsky,
A.
Dunn,
K.
Shortman.
Dendritic cell development in culture from thymic precursor cells in the absence of granulocyte/macrophage colonystimulating factor. The Journal of experimental medicine.
1996;
184
:
2185-2196
.
-
C.
Scheicher,
M.
Mehlig,
R.
Zecher,
K.
Reske.
Dendritic cells from mouse bone marrow: in vitro differentiation using low doses of recombinant granulocyte-macrophage colony-stimulating factor. Journal of immunological methods.
1992;
154
:
253-264
.
-
J.
Shi,
K.
Ikeda,
N.
Fujii,
E.
Kondo,
K.
Shinagawa,
F.
Ishimaru,
K.
Kaneda,
M.
Tanimoto,
X.
Li,
Q.
Pu.
Activated human umbilical cord blood dendritic cells kill tumor cells without damaging normal hematological progenitor cells. Cancer science.
2005;
96
:
127-133
.
-
R.M.
Steinman.
The dendritic cell system and its role in immunogenicity. Annual review of immunology.
1991;
9
:
271-296
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 04 (2014)
Page No.: 126-132
Published on: 2014-09-19
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8350 times
- Download PDF downloaded - 1538 times
- View Article downloaded - 6 times
 Biomedpress
Biomedpress