Abstract
Immunotoxins are chimeric molecules embodied with a protein toxin and a ligand which is either a growth factor or an antibody. The ligand part of the immunotoxin recognizes and binds to an antigen of the target cell, allowing the internalization of the toxin,moiety and permitting its drift to the cytoplasm where it can destroy the cell. Target specificity of the chimeric protein is determined via the binding attributes of the chosen antibody. Predominantly, immunotoxins are purposefully constructed to slay cancer cells as part of novel treatment approach. In addition they are also used for various autoimmune, viral and other infectious diseases. With the advent of biotechnology, recombinant immunotoxins have been created and are clinically tested to target malignant cells. Our article summarizes foremost progress in the development of immunotoxin based therapeutics and presents a comprehensive portrayal of the immunotoxin generation.
Introduction
Agents with the narrow therapeutic efficacy have limited utility, particularly for treating aggressive diseases, as the dose escalation is restricted by the toxicity of the agent Vedi and Ziegler, 2014. In order to widen therapeutic efficacy of such agents, they are used to be linked to targeting moieties, such as antibodies and cytokines, intending their selective delivery. By the virtue of their specificity, targeted therapeutics are potentially less toxic and more efficacious than conventional therapeutics Carter,2001.
Protein toxins having target specificity are the latest trend of the therapeutic market. It works in three different way: by damaging the cell through membrane integrity disruption; by disturbing the activity of the nervous system of the intoxicated organism; and by interfering with the cellular processes (e.g. enzymatic activity) leading to the death of the intoxicated cell. During the last two decades, scientific advances have facilitated the processing and manoeuvring of biological substances; among which are toxic polypeptides and their encoding genes. These protein toxins gave an innovative insight to the therapeutics and are frequently referred as ‘Toxin based Therapy’. Based on the targeting strategy used, toxin based therapy is of three types namely surface antigen/receptor (immunotoxin therapy), transcriptional (suicide genes therapy) and protease explicit targeting (protease stimulated toxins therapy, Shapira and Benhar,2010). The present article has been prepared on toxin based therapeutics termed as immunotoxins.
Our immune system has various lines of defense system to combat diseases. The antibodies generated by our immune system against the cellular and noncellular antigens and their specific chemical interaction termed as antigen-antibody reaction, is one such fundamental line of host defense. Antibodies or immunoglobulins are large Y-shaped glycoproteins produced by plasma cell which identifies and neutralizes foreign or toxic particles by recognizing a unique part of the foreign target termed as ‘antigen’ or ‘antibody generator’. Each antibody binds to a specific antigen because of the disparity in the antibody’s complementary determining regions. The antigenic determinant or epitope is recognized by the paratope of the antibody, situated at the variable region of polypeptide chain containing hypervariable regions unique to amino acid sequences of each antibody. Antigens truss to antibodies by weak and non-covalent bonds including hydrogen bonds, van der waal’s forces, electrostatic and hydrophobic interactions Winau et al., 2004.
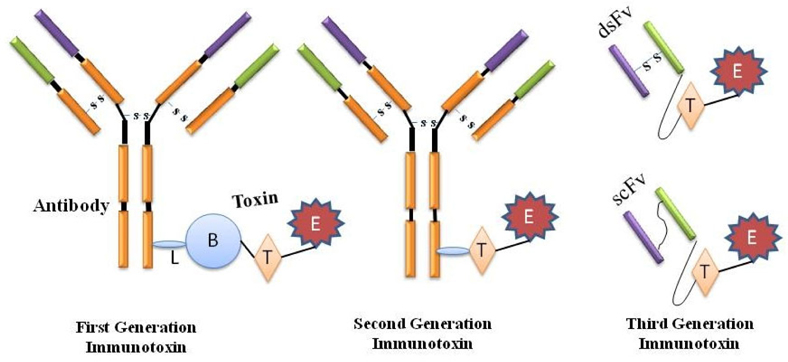
Classically, the immunotoxins have been created by conjugating an antibody to a whole protein toxin and for more discerning activity, by means of a protein toxin deprived of its natural binding domain. Paul Ehrlich was the first to suggest the use of these magic bullets (Zauberkugeln) for therapeutic function and referring them as first generation immunotoxins. However, these constructs were heterogenous and indistinct due to the assortment of probable sites accessible for chemical conjugation and intoxication of normal cells Bosch and Rosich, 2008Strebhardt and Ullrich, 2008Kreitman, 2006.
The second generation of immunotoxin was also emanated on chemical conjugation amid the intended target and the toxins. However, collective acquaintance on the structure and function of the toxins facilitated the elimination of their inhabitant non-specific cellbinding domain and engender additional targetspecific immunotoxins whilst conjugated to monoclonal antibodies. Although more specific and thus better tolerated by animals, immunotoxins from the second generation were chemically heterogeneous experiencing the drawback of great size and hinderance in piercing solid tumors. In order to avoid heterogeneity, improve diffusion into tissues and reduce production complexity and costs, recombinant DNA techniques were employed in the production of third generation immunotoxins wherein the gene constructs are equipped in such a way that the cell binding domain of the toxin is genetically swapped with a ligand or with the Fv portion of an antibody in which its light and heavy chain variable fragments are either genetically linked (scFv) or apprehended together by a disulfide bond (dsFv) and these constructs are expressed within the pro- or eukaryotic host cells such as E. coli, S. cerevisiae and CHO cells etc ( Figure 1 ) Kreitman, 2006Kreitman, 2009Pastan et al.,2007Potala et al., 2008.
Immunologic proteins smaller to monoclonal antibodies (MAbs, e.g. growth factors and cytokines), have also been chemically conjugated and hereditarily fused to protein toxins. Growth factor toxin fusions or conjugates are not considered to be immunotoxins by some researchers although they bind to target cells and contain a toxin that kills cells as that of the classical immunotoxins Chames et al., 2009.
Many plant toxins (eg. Ricin, abrin, Pokeweed antiviral proteins etc.), bacterial toxins (Pseudomonas toxin, Diphtheria toxin, Anthrax toxin) and ribosome inactivating proteins (RTI, eg. Gelonin, saporin) have been used to create immuntoxins. Other proteins of mammalian and human origins such as RNases, Proapototic proteins etc. are the latest developing trends which are used to produce immunotoxins with a précised activity. High potency towards the target makes the immunotoxins a potent tool for cancer therapeutics. Along with the cancers, various viral and autoimmune diseases can be treated with the help of immunotoxins Dosio et al., 2014Mathew and Verma, 2009Wu, 1997De Lorenzo and D'Alessio, 2008.
The binding moieties of immunotoxins
Monoclonal Antibodies and other Immunologic Conjugates
Monoclonal antibodies are the repertoire of the antibodies generated by the same clone of plasma cells and thus are monospecific antibodies abbreviated either as mAb or moAb. Human immune system spawns heterogeneous or polyclonal antibodies against each and every epitopic region of an antigen. On the other hand mAb are generated to be having a common epitopic determinant. They are isotypically (differences in the constant regions of the heavy and light chains), idiotypically (unique set of variable portion) and allotypically (unique gene sequence particular to the individual’s genome that manifest its constant region) similar and so are able to counter react against a specific epitope of an antigen. These special features of the mAbs made it the most swiftly growing class of the therapeutics with a compounded annual growth rate of 36% in comparison with a CAGR of 8% for the pharmaceutical as a whole.
The mAbs are produced in vitro using Hybridoma technology discovered by (Kohler and Milstein, 1975). The technology uses the fusion of the myeloma cells from a mouse and the invasive β-cells generated by the antigen exposure. The cell that results from this fusion is called the hybridoma. The property of recognizing a particular antigen derived from β-cell and immortality of myeloma cells makes the hybridoma cell a kind of perpetual antibody producing factory. As the antibodies are all identical clones produced from a single cell, they are called monoclonal antibodies. Most antibodies used in cancer diagnosis and therapy are derived from the IgG (immunoglobulin G) isotype. An IgG isotype antibody consists of two antigenbinding fragments (Fabs), which are connected via a flexible region (the hinge) to a constant (Fc) region. This structure includes two pairs of polypeptide chains; each pair comprises a heavy and a light chain of dissimilar sizes. Both heavy and light chains are crinkled into immunoglobulin domains. The ‘variable domains’ in the amino-terminal part of the molecule are the domains that recognize and bind antigens; the rest of the molecule is poised of ‘constant domains’ that vary amid immunoglobulin classes. The Fc portion of the immunoglobulin doles out to connect a variety of effector molecules of the immune system, as well as molecules that ascertain the antibody biodistribution.
Since last two decades, mAbs has become vital tool in pharmaceutical armory, accessible to physicians for the treatment of a growing number of human diseases. mAbs are the most widely used toxin-delivery vehicles used unswervingly for treatment since they can persuade killing through antibody arbitrated cytotoxicity Jurcic et al.,1995. Despite being in vital use, these antibodies suffer limitations due to their larger size and their non-functional receptor binding capacity within the human cell. Numerous strategies have been developed over the years in order to reduce or eliminate the immunogenicity of non-human antibodies. Manipulation of the genes has allowed significant modification which led to the formation of humanized antibodies, chimeric antibodies, CDR (complementary determining regions) grafted antibodies, deimmunized and above all human-sequence recombinant antibodies. The chimeric antibodies consist of 33% mouse protein and the remaining human protein. CDR grafted antibodies have 5-10% mouse protein within the variable region of light and heavy chains of the antibody. The other changes included the variation of the antigen- binding domains and that of the effector functions by distressing both antibody-dependent and complement-dependent cytotoxicity (Igawa et al., 2011).
To overcome the structural drawbacks, single domain fragments, diabodies, minibodies (single chain Fv (variable region) fused to a -CH3 domain) and single chain Fv (only variable light and heavy domains, connected via a peptide linker) are prepared as the conjugate. Other conjugates used to develop the immunotoxins include the cytokines, interleukins, Growth factors, MHC-I tetramers and protein scaffolds Kreitman, 2006Nelson, 2010(Igawa et al., 2010)Madhumathi and Verma, 2012.
The Toxin Moiety
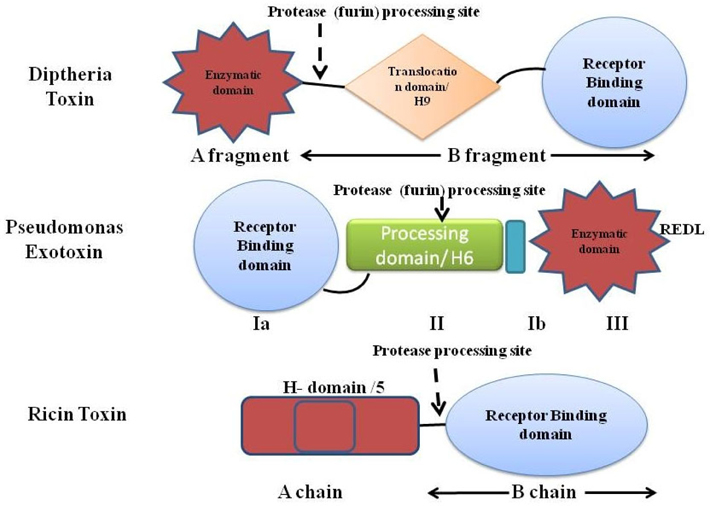
The second major part of the immunotoxin is the protein (toxins) which is used to destroy the target cells. The protein toxin employed for the immunotoxin construction is one among the proteins derived from bacteria (Pseudomonas toxin or Diptheria toxin), fungus (Restrictocin or α-sarcin) or it could be a plant holotoxin (ricin, abrin, mistletoe lectin, and modeccin) or hemitoxins (PAP, saporin, bryodin 1, bouganin, and gelonin). The toxins derived either from the bacterial origin or the plant origin; usually contain two subunits- the binding domain and the catalytic/enzymatic domain Shapira and Benhar,2010Antignani and FitzGerald, 2013 as depicted in Figure 2 with different mode of actions ( Figure 3 ).
Bacterial Toxins used in Immunotoxin Production
Diptheria toxin (DT) and the Pseudomonas exotoxin (PE) are the two bacterial toxins widely used in the immunotoxin construction. Both PE and DT catalyze the ADP-ribosylation of histidine-699 of Elongation Factor-2, which is post-translationally modified to a diphthimide residue. In spite of their analogous action, PE and DT vary significantly in their amino acid sequence, and in fact PE’s enzymatic domain is near the carboxyl terminus, while DT’s is at the amino terminus. On the contrary, PE’s binding domain is at its amino terminus and DT’s to its carboxyl terminus Shapira and Benhar, 2010.
Mechanism of action of Diptheria Toxin Shapira and Benhar, 2010Potala and Verma, 2010Antignani and FitzGerald, 2013
DT, a single-chain 58 kD protein secreted by bacterial pathogen Corynebacterium diptheriae, is the prototype for the family of ADP-ribosylating toxins and belongs to the AB group of toxins composed of an enzymatic A domain (amino acids 1-193) and a binding B domain (amino acids 482-535). Fragment B is accountable for cell entry (cell surface receptor binding and ensuing translocation in the cytoplasm) while fragment A intoxicates the cell by calibre of its enzymatic action. A third domain known as the translocation or transmembrane (T) domain is located in the center of the molecule and sometimes is considered as the part fragment B domain. Supported by the 3-D structure of DT’s in the existence and lack of NAD, DT undergoes the following steps to kill the cells:
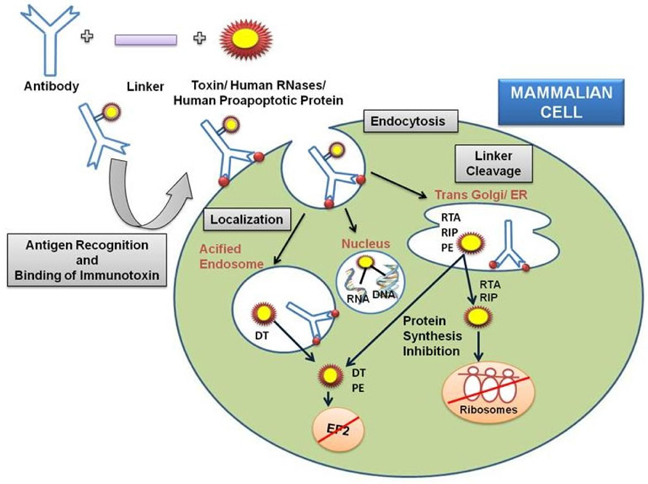
The native DT binds, to complex of heparin binding EGF precursor on the cell membrane and CD9 via its residues 482 to 535 on the receptor binding domain, where it is hewed extracellularly by cellsurface furin or furin like proteases between the residues Arg193 and Ser194, which is present within a disulfide loop formed by Cys186 and Cys201.
The di-chain DT is subsequently internalized into clathrin coated pits and reaches the lumen of budding endosome and unfolds at low pH,via reduction of the disulfide bond between amino acids 186 and 201, exposing the hydrophobic residues TH8 (amino acids 326-347) and TH9 (amino acids 358- 376) of the translocational domain to shape a hairpin, which slots the membrane of the endosome and forms a channel through which the enzymatic fragment translocates to the cytosol dodging the endosome, possibly with the aid of cytosolic factors.
The active-site crevice of DT (amino acids 34-52) binds NAD in the cytosol and the ADP ribose of NAD is shifted to a modified histidine residue (diphthamide) at position 715 in the eukaryotic translation elongation factor (eEF-2) ensuing reticence of protein synthesis and programmed cell death Shapira and Benhar, 2010Potala and Verma, 2010Antignani and FitzGerald, 2013Choe et al., 1992Tsuneoka et al.,1993Yamaizumi et al., 1978
To develop specificity, toxins for tagging mAbs are mutated to avert their binding to normal cells. Imitative of DTs that are used to make immunotoxins have the C-terminus tainted by mutations (e.g., at position 508 in DTcrm103 or at position 390/525 in DTcrm107) or partially obliterated (e.g. as in case of DT388, DAB389, DAB486) but hold the translocation and ADP-ribosylation commotion of DT.
Immunotoxins based on Diptheria toxin
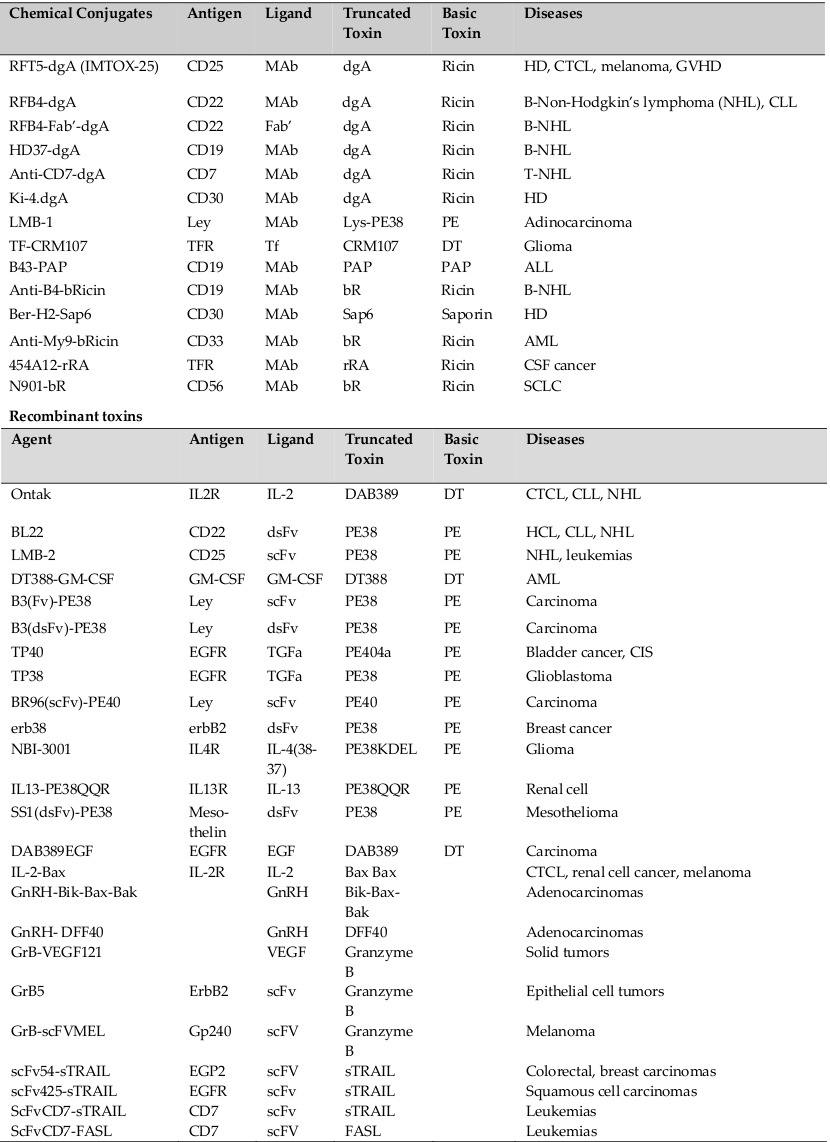
An assorted range of immunotoxins have been designed with DT or their truncated parts targeting receptors of various diseases. Among them Denileukin Diftitox, also known as ‘DAB389 IL2’ or ‘Ontak’ is a fusion protein designed to unswerving a truncated form of DT to cells that articulate the high-affinity IL2 receptor (consisting of the following subunits: CD25 (IL2Rα), CD122 (IL2Rβ), and CD132 (IL2Rγ)) exist in different hematologic malignancies like adult T cell leukemia (ATL), chronic lymphocytic leukemia (CLL), Hodgkin’s and non-Hodgkin’s lymphomas (HL/NHL), cutaneous T cell lymphoma (CTCL) and other leukemias and lymphomas. It encompasses genetic fusion between a truncated form of DT (first 388 amino acids, DAB389) where the natural receptor binding domain of the toxin was swapped by the cytokine interleukin 2 (IL2). Ontak is the first FDA approved immunotoxin for treatment of advanced CTCL in 1999. It has also been tested for the treatment of other malignant and non-malignant diseases including β cell chronic lymphocytic leukemia (CCL),B-cell NHL, panniculitic lymphoma, psoriasis and graft versus host disease GVHD Shapira and Benhar, 2010Pastan et al., 2007.
DT388-GM-CSF is a DT- based immunotoxin which has been created targeting Granulocyte-Macrophage colony stimulating factor receptor subsist on the acute myeloid leukemia (AML) cells and has been put under clinical trials Shapira and Benhar, 2010Kreitman and Pastan, 1997.
Tf-CRM107 (TransMID) is another class of DT based immunotoxin aiming transferrin receptor found on tumor cells of glioblastoma multiforme (GBM). It is a conjugate protein of a mutant DT lacking receptorbinding activity (CRM107) fastened by a thioester bond to human transferrin (Tf stands for transferrin, Laske et al., 1997).
Mechanism of action of Pseudomonas Exotoxin A
Pseudomonas exotoxin A, a single-chain 66-kDa molecule secreted by Pseudomonas aeruginosa (one of its virulence factor), is composed of three major domain chipping different functions. The N-terminal domain Ia (amino acids 1-252) mediates binding to the α2- macroglobulin receptor and hence termed as ‘binding domain’. The small domain Ib lies amid domain II and domain III has no known function. Domain II (amino acids 253-364) mediates translocation of domain III (the carboxyl-terminal ADP-ribosylating domain containing amino acids 400-613) into the cytosol of target cells. The catalytic procedure of ADP ribosylation engrosses residues His440 and Glu553. His440 binds to NAD via ribose moiety of AMP and Glu553 (carboxyl group of side chain) bind with Tyr481 and Glu546, allowing Tyr481 to truss NAD by a ring-stacking mechanism. Translocation occurs following internalization of toxin and after an array of additional steps such as pH-induced conformational change, proteolytic cleavage at a specific site in domain II, and a reductive step that detach the amino and carboxyl fragments. Eventually, the carboxyl fragment of PE is translocated from the endoplasmic reticulum into the cytosol and the ADP-ribosylating enzyme inactivates EF-2 (at amino acids 400-602) facilitating cell death via apoptosis Shapira and Benhar,2010Iglewski and Kabat, 1975(Masato and Charlotte, 1992).
Regardless of a similar mode of action (ADPribosylation), an alike primary pathway of cell entry (internalization via coated pits and endocytic vesicles) and processing (proteolytic cleavage and a reductive step) both PE and DT share no sequence homology. The lone similarity is the spatial display of key residues in their active sites that are arranged around residue Glu553 in PE and Glu148 in DT.
Mutated form of PE Toxins
Truncated and most recently used form of PE toxin includes PE40 (amino acids 253-613) include PE38, (amino acids 253-364 and 381-613 of PE) and PE35 (amino acids 281-364 and 381-613). To allow the domain (ADP-ribosylating) to transfer to the cytosol without the ligand, the latter is positioned at the amino terminus of PE. A different type of PE has a tainted carboxyl terminus from the REDLK (arginine-glutamic acid-aspartic acid-leucine-lysine) to the KDEL sequence that binds with high affinity to the receptor and results in augmented cytotoxicity. Immunotoxins enclosing PE mutants with KDEL finale (i.e. PE38KDEL or PE40KDEL) are more cytotoxic analogous to immunotoxins with REDLK sequence. Since the translocated fragment of PE38 (35 kDa in length beginning with Gly280) do not alter the activity due to the positioning of methionine, a new mutant PE35 has been created with methionine at position 280. Though it would not be appropriate for fusing to ligands, but may be ideal for chemically conjugating to ligands as it contains a single disulfide bond Shapira and Benhar, 2010Pastan et al.,2007Kreitman and Pastan, 1995Pranchevicius and Vieira, 2013.
Immunotoxins based on Pseudomonas Exotoxin
LMB-2 is a PE based immunotoxin targeting the CD25 Subunit of IL2 receptor where in PE38 was fused to a single-chain form of the anti-CD25 mAb anti-Tac. The resultant LMB-2 (Anti-TacFv-PE38) immunotoxin, demonstrated potential results in CD25+ cells and tumor xenografts model in nude mice Kreitman et al., 1994Robbins et al., 2000.
RFB4 (dsFv)-PE38 (BL22) is a stable immunotoxin targeted against CD22 expressing cells. CD22 is a 135- kDa phosphoglycoprotein adhesion molecule present on the surface of B-cells, including lymphomas and leukemias. It is composed of disulfide stabilized Fv regions (dsFv) of the anti-CD22 monoclonal antibody RFB4 fused to PE38 Kreitman et al., 2009.
LMB-1 is an immunotoxin composed of anti-Lewis Y mAb B3 conjugated to PE38. The LeY is a type 2 blood group associated oncofetal carbohydrate antigen manifested on almost 70% of human epithelial carcinomas Kuan et al., 1995.
Ribosome Inactivating Protein Toxins
Ribosome inactivating proteins (RIPs) are a cluster of glycosylated and non-glycosylated enzymes with N-glycosidase activity originally noticed in higher plants, but have also been present in algae, bacteria and fungi. They are usually classified as plant based toxins due to their extensive distribution in the tissues of the plant and their expression is enhanced in senescence, infections and during a variety of stress conditions. Based on the existence of the two domains, RIPs can either be holotoxins or hemitoxins. The binding domains of holotoxins ought to be removed by diminution of the disulfide bond preceding translocation of enzymatic counterpart into the cytosol Shapira and Benhar, 2010Nielsen and Boston, 2001; Pieumans et al., 2001. On the basis of their structure and mode of activation RIPs can be divided into three sets.
RIP Class I: Hemitoxins, comprise of single chain catalytic domain (~30kD). Examples of this class include saporin (Saponaria officinalis), pokeweed antiviral protein (PAP, Phytolacca americana) and gelonin (Gelonium multiforum).
RIP Class II: Holotoxins, includes an active A chain (~30 kDa) linked through a disulfide bond to a Bchain (~35 kDa) with the properties of a lectin. Example includes the Ricin protein from Riccinus communis.
RIP Class III: Maize and barley proteins (b-32 and JIP60) synthesized as inactive precursors (proRIPs) lacking a lectin moiety, are stimulated by proteolytic processing including the elimination of a short inhibitory internal peptide and terminal sequences.
The accurate phenomenon of translocation of the plant toxins from the cell surface to the cytosol is mysterious. However, it is couched that the process most likely differs for each plant toxin. Initially, antibodies were chemically conjugated via disulfide bond to the catalytic subunits of holotoxins (ricin or abrin) from which the binding domain was chopped out.
Ricin Toxin
The ricin toxin derived from the seed of Ricinus communis, is a RIP composed of two chains linked by a disulfide bond. The chain A (RTA) includes the Nglycosidase enzyme that blocks ribosomal activity, while chain B (RTB) is responsible for binding. The chain B binds to the galactose or Nacetylgalactosamine residues present on glycoproteins and glycolipids moiety of cell membrane of most eukaryotic cells via galactose receptors and acts to translocate the chain A into the cytosol by both clathrindependent as well as clathrin-independent endocytosis. The toxin moves through the golgi complex to the ER, where its’ disulfide linked chains becomes detached and the resulting catalytic RTA is retrotranslocated to the cytoplasm via Sec61p translocon and irreversibly damages ribosome by eliminating adenine at residue 4324 from a conserved 28S rRNA loop (‘sarcin/ricin loop’–SRL) that instigate inhibition of translation process and subsequently cell death. Ricin also removes the guanine at 4323 position and the intracellular transport of ricin is reliant on sorting receptors cycling between ER and the terminal compartments of the golgi complex. It has been revealed that glycolipids that bind ricin could be transported to golgi complex from endosomes and the deliverance of ricin to the cytosol is augmented by the addition of KDEL-ER. The RTA chain by itself is non-toxic due to the lack of cell binding ability, however even without its binding domain, the RTA was taken up nonspecifically by macrophages and non-parenchymal Kupffer cells owing to the glycosylation of side residues of RTA strapping to mannose receptors. The most triumphant procedure for plummeting nonspecific uptake of RTA was through chemical deglycosylation. The additional significant variation that leads to the high therapeutic index was configuration of the disulfide bond between the MAb and the toxin using the derivatizing agent (SMPT, 4-succinimidyloxycarbonyl-a-methyl-a (2-pyridyldithio) toluene) in a mired fashion. The whole ricin toxin has been targeted after blocking its oligosaccharide binding sites via ligands prepared by chemical alteration of glycopeptides comprising triantennary N-linked oligosaccharides to avert normal cell. The resultant blocked ricin (bR) was afterward chemically conjugated to antibodies to construct immunotoxins Pastan et al.,2007.
RFT5-dgA and ki-4.dgA: Immunotoxins developed from ricin proteins targeting CD25 and CD30 were developed namely RFT5-dgA and ki-4.dgA. These immunotoxins are chemically deglycosylated form of RTA (dgA) concurrent to the targeting moieties RFT5 (anti-CD25) and ki-4 (anti-CD30) mAbs respectively.
For targeting B-cell lymphoma cells, the anti-CD22 mAb RFB4 and the anti-CD19 mAb HD37 were conjugated to dgA forming IMTOX-22 and IMTOX-19 correspondingly.
Fusion toxins with integrated plant toxins
The cytotoxicity of both plant and bacterial toxins is most favorable when the catalytic domain alone transfer to the cytosol. To construct a fusion toxin enclosing RTA, IL-2 is fused to recombinant RTA by a linker that enfolds a proteolytic cleavage site for DT. Even if the recombinant toxin can be smited extracellularly, it might not selectively target cells since the ligand and toxins were no longer associated. Afterward, IL-2 was fused to a PAP mutant but the fusion toxin was not purified and non-cytotoxic. Ligands fused to plant toxins have produced recombinant toxins with noteworthy cytotoxic activity, including one having a CD40 single-chain antibody and bryodin 1, another enclosing urokinase binding domain and saporin, and the last containing hFGF and saporin. For these molecules, it is unclear that whether the recombinant toxin goes into the cytosol of target cells intact or the ligand was rickety after internalization, allowing the catalytic domain only to translocate to the cytosol. The aptitude of yet unwavering ligands to inevitably detach from the catalytic realm is an imperative trait of recombinant toxins and a distinctive characteristic amid all toxins from the bacterial toxins PE and DT Shapira and Benhar, 2010Pastan et al., 2007 Wu, 2007 De Lorenzo and D'Alessio, 2008May et al., 2012.
Other Mammalian Enzymes and Proteins
Pedestal to the immunotoxin principle, a novel class of molecules termed as immunoRNases (IR) have been projected where the toxin moiety is replaced by a nontoxic RNases. Infact, IR is an immunoprotoxin that trek in the bloodstream without causing any mutilation to the cells bereft of the targeted antigen, despite magically opting the cells beleaguered by the immune moiety. The RNase moiety wields its RNA degrading action after internalization guiding the death of the targeted cell. By selecting a human RNase and an antibody fragment of human as immune moiety, the resultant IR would be non-toxic and non-immunogenic De Lorenzo and D'Alessio, 2008.
Similarly, pro-apoptotic proteins such as Bcl-2 proteins (Bax, Bik, Bak), DNA fragmentation factor 40, Granzyme B, FASL (Fas-Ligand), sTRAIL (soluble tumor necrosis factor-related apoptosis-inducing ligand) have also been conjugated to IL-2, GRH (gonadotrophin releasing hormone), Anti-CD7scFV, Anti-EGP2 scFv, Anti-ErbB2 scFv, Anti-EGFR scFv, VEGF etc. to develop immunoconjugates targeting antigens of immune diseases and cancer Kreitman, 2009Mathew and Verma, 2009.
Production of immunotoxins
Chemical conjugates of GF and toxin commonly engross either reducible disulfide (S-S) or non-reducible thioether (S-C) bonds. A S-C bond is apposite if the ligand is coupled to a bacterial toxin in the component (binding domain) that does not translocate to the cytosol and S-S bond is mundane for the enzymatic domain. In exposition of RTA and its mutant along with the Pseudomonas exotoxin PE35, derivatization of the toxin entailed by a single reduction of disulfide bond as both accommodate only one cysteine moiety. Hence, derivatization of the ligand also necessitates heed to create sulfhydryls without impairing the molecule, unless the ligand has a single cysteine. As soon as both the ligand and toxin are derivatized, they must be purified and conjugated followed by repurification of correct toxin-ligand ratio. The cost and complexity of the procedure has suppressed the progress of recombinant toxins, however, harvesting recombinant protein from insoluble bacterial inclusion bodies is a common method of producing material for clinical trials. After washing extensively with detergent to remove endotoxin and solubilization, denaturation and reduction in GDTT (guanidine-dithioerythritol) solution, the recombinant protein is subsequently renatured by rapid dilution into refolding redox buffer containing arginine and glutathione, and the dialyzed renatured protein is further purified by size exclusion and anion exchange chromatography. Since the EF-2 of the eukaryotes is exceedingly vulnerable to toxin, the expressionof recombinant toxins by and large fail within eukaryotic host. Nonetheless, plant and insect cells resistant to the toxins have been developed, which are able to produce active recombinant toxins. The benefit of immunotoxins includes an exceptionally small amount of the protein needed to exterminate both resting and dividing cells and the chattels is obtained from the catalytic assests of the fusion proteins; a single molecule can assail multiple intracellular targets Binyamin et al., 2006.
Immunotoxins as therapeutic agents
The target- specificity of the immunotoxins makes them a major tool to combat an assortment of malignancies, autoimmune diseases and viral infections such as HIV. Cancer, an uncontrolled proliferation of the cells has been reported as a major cause of lethality and economic imbalance of the society. A number of treatment regimens are available against this deadly ailment but none of the therapies have established themselves to be prolific singly. The markers present extra ordinarily on the tumor and viral infected cells serve as the target to engender the immunotoxin against them. Immunotoxins have been found to be most advantageous for treating hematologic malignancies as the malignant cells are frequently intravascular and accessible to intravenously administered drug and also the patients repeatedly lack adequate immunity to make antibodies against the toxin. Thus, few antigens have been used to target hematologic malignancies by immunotoxins Dosio et al., 2014Wu, 1997Pranchevicius and Vieira, 2013May et al., 2012Binyamin et al., 2006.
ONTAX: The first FDA approved immunotoxin
Ontax targets the IL2R present on a wide variety of hematologic malignancies such as cutaneous T-cell lymphoma (CTCL), adult T-cell leukemia (ATL), Hodgkin’s Disease (HD), and other B- and T-cell leukemias and lymphomas (anti-DAB 389 IL-2, anti-DAB 486 IL-2). Phase I testing conducted on patients with CTCL (n = 35), NHL (n = 17), HD (n = 21) by administering the drug via intravenous infusion produces five complete and eight partial remissions in patients with CTCL, one complete and two partial remissions in NHL. No retort was documented in patients with HD. The dose-limiting toxicity in these trials was asthenia. In the key phase III trial, 71 patients with CTCL (30%) treated with Denileukin Diftitox showed an intent response (20% partial, 10% complete). This is the first and solitary immunotoxin approved by FDA for the treatment of advanced CTCL Shapira and Benhar, 2010.
LMB-2: An immunotoxin against CD25
In order to target CD25 in spite of the existence of other subunits of the IL2R, the anti-CD25 MAb anti-Tac was used as a ligand as an alternative of IL-2 [anti-Tac (Fv)-PE40, anti-Tac(Fv)-PE38 (called LMB-2)]. Primary ATL and hairy cell leukemia (HCL) cells were much more sensitive than β-Chronic lymphocytic leukemia (CLL) cells, possibly due to lower CD25 expression in the latter, and CLL cells have been shown to upregulate CD25 by phosphorothioate oligodeoxynucleotides. In a Phase I Trial, 35 patients (with chemotherapy- resistant leukemia, lymphoma and HD) administrated intravenously with LMB-2 results in 3% (1/35) complete and 20% (7/35) partial remissions. Six patients prepared neutralizing antibodies after one cycle, averting themselves from receiving re-treatment. In Phase II Trials, administration of LMB-2 to patients with metastatic melanoma has led to a transient prejudiced reduction in mingling and tumor-infiltrating CD25+ T-regulatory (Treg) cells that suppress the aptitude to vaccinate against self/tumor antigens. However, LMB-2 therapy did not enhance the immune response to peptide based cancer vaccines. Other Phase II Trials are now underway in patients with CD25+ CLL, CTCL and HCL Shapira and Benhar,2010.
LL2 and BL22: The first anti-CD22 immunotoxin with PE encompass the MAb LL2 and incited comprehensive regression in human xenograft models. Though as a single-chain, LL2 Fv was wobbly, and a recombinant immunotoxin cannot be made, variable domains from RFB4 were cloned and an unwavering recombinant immunotoxin RFB4(Fv)-PE38 was made and revealed to be cytotoxic toward CD22+ cell lines. To perk up the steadiness of RFB4 (Fv)-PE38, the variable domains were linked by a disulfide bond as a replacement for peptide linker, and V H was amalgamated to PE38, resulting in BL22. BL22 is the foremost agent since purine analogs stated to persuade complete remission in the preponderance of patients with HCL. Clinical trials with BL22 (administered intravenously) in adults with HCL resistant to purine analogue therapy, produced promising results with 61% CR (19) and 19% PR (6) in 31 patients in Phase I, and 25% CR, 25% PR after one cycle of treatment in Phase II (n = 36). The success of BL22 in chemo resistant patients is evidently related to the fact that CD22 is exceedingly conserved at high density on HCL cells even with purine analog resistance.
To target the GM-CSFR expressed in AML cells from most patients, human GM-CSF was fused to truncated bacterial toxins (DT388-GM-CSF and GM-CSFPE38KDEL). CD19, IL-3, JL1, CD64, CD86, CD80 and CD30 were additional targets for the hematological malignancies for which the immunotoxins were produced and has been put under clinical trials Shapira and Benhar, 2010. A summary of immunotoxins and other immunoconjugates have been listed in Table 1 .
Due to tight cellular junction and compactly packed cells, it is difficult for immunotoxins to target solid tumors than aiming hematologic tumors. Also the patients are slighter immunosuppressed and liable to make counteracting antibodies to the toxin. Several chemical conjugates and recombinant fusion toxins enclosed either EGF or TGF (both ligands for the EGF receptor) and Pseudomonas exotoxin (212 DAB 389 EGF). To target the carbohydrate antigen Lewis Y expressed on breast, ovarian and gastrointestinal tract carcinomas, a range of immunoconjugates and immunotoxins as LMB-1, LMB-7, LMB-9 and LMB-2 have been clinically tested.
The erbB2 antigen expressed in poor-prognosis breast cancer and other carcinomas have been another target against which several mAbs that differ in affinity have been cloned to produce recombinant single-chain or disulfide-stabilized immunotoxins. One of these, erb- 38, containing a disulfide-stabilized Fv fused to PE38, was tested in patients with carcinomas, mostly breast cancer. Diverse targets such as IL4R (extensively expressed by solid tumors and hematologic malignancies), IL13R, Transferrin receptors, N901 (expressed on small cell lung cancer), urokinase receptor (also called μPAR or CD87), and other tumor specific antigens are employed to generate immune- conjugates and immunotoxins.
Exceedingly dynamic antiretroviral therapy has lessened the morbidity and mortality of human immunodeficiency virus (HIV) infection, but quiescent infected cells stay for extended periods. CD45RO+ T-cells are the foremost dormant virus reservoir in persons infected with HIV. Quiescent HIV-infected T-cells competent in replication could be generated in vitro by transmitting infection to peripheral blood mononuclear cells (pBMC) with HIV and subsequently obliterating the HIV-producing cells with an anti-CD25 immunotoxin (IT). After that the CD25- quiescent infected cells can be annihilated with an anti-CD45RO immunotoxin May et al., 2012Binyamin et al., 2006Campbell and Marcus, 2003.
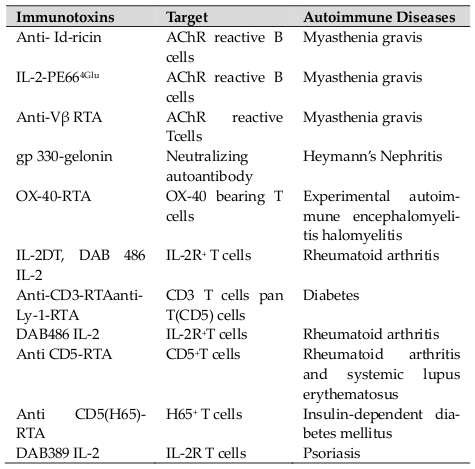
Autoimmunity is considered to be a result of abnormal numbers or function of one or two lymphocyte subgroups. Most of the autoimmune endocrinopathies appear to be directly conciliated by auto-reactive CD8+ cytotoxic T lymphocytes (CTLs) with help from the CD4+ cells, while others emerge to be mediated by auto- antibodies to cellular receptors that take place as part of a Th2 response. In any particular disease, it is expected that assorted cellular and antibody autoimmune responses are pathogenic. The fundamental strategy to cure autoimmune diseases through immunologic methodology is to remove or suppress the T or B-cells which are possibly involved in the autoimmune response. The idea of using it for treatment of autoimmunity is to pernicketly abolish the lymphocytes which cause autoimmune attack, resulting in better clearance of the diseased cells and augment clinical benefit to the patients over mAbs or cytokines alone. ITs have been used in clinical trials (Phase I and II) for the treatment of various autoimmune diseases including rheumatoid arthritis, systemic lupus erythematosus, insulin-dependent diabetes mellitus and psoriasis ( Table 2 ). ITs also helps in alleviating the symptoms of myasthenia gravis and rheumatoid arthritis in animal models Wu, 1997.
Other uses and applications of ITs include eradication of T-regulatory cells, modulation of the immune responses and removal of T-cells from grafts. Advancements have been made in producing ITs with antiparasitic and anti-viral activity too. Ex-vivo uses of ITs are also expected through killing of unwanted cells before infusing bone marrow or stem cell preparations (Madhumati and Verma, 2012).
Problems in immunotoxin development
The vital use of ITs for the treatment purpose still lags behind due to problems associated which includes immunogenicity, unwanted toxicity, difficulty in production, limited half-life and resistance Dosio et al., 2014Wu, 1997.
Immunogenicity
Extensive clinical trials signify the incidence of immunogenicity after a solitary cycle of IT ranges from 50- 100% for solid tumors and from 0-40% for hematologic tumors for which the IT therapy are usually offered. The existence of neutralizing antibodies lowers the levels of biologically active IT and compromises efficacy. Numerous approaches are used to prevent immunogenicity and the most useful is PEGylation for biological agents such as interferon and Lasparaginase, which not only blocks immunogenicity but also prolongs half-life. Limited success has been achieved in pre-clinical studies of a PEGylated form of LMB-2. PEGylating a toxin appears much more challenging than PEGylating simpler molecules, since modifying sites on a toxin diminish its activity. Immunologic studies have found a large number of B-cell and T-cell epitopes on PE implying, the “humanization of the molecule” awfully difficult.
Unwanted Toxicity
An assorted series of toxicities has been experiential with ITs that has restricted its dose and efficacy. The most common toxicity observed is Vascular Leak Syndrome (VLS), which is due to the passage of the cytotoxic protein through endothelial cells to exit the blood vessels. Report suggests that RTA binds unswervingly to endothelial cells, while truncated PE necessitates a complexing agent (ligand) that crossreacts with the endothelium. Other report indicates that definite residues on RTA and also curtailed PE can connect to endothelial cells and educe VLS by an autonomous mechanism besides the normal toxininduced cell death. VLS is characterized by hypotension, gain in weight, augmented vascular permeability, mild renal and pulmonary insufficiency, myalgias, hypoalbuminemia and in few cases pulmonary edema and aphasias. After targeted toxin treatment, the syndrome arises transiently but is sometimes very severe.
Hepatotoxicity, an emblematic side effect of recombinant ITs, pitched up to be related to cytokine production by Kupffer cells and ascribed as a result to fastening of basic residues on the Fv to negatively charged hepatic cells. Although recombinant ITs that exclusively truss to antigens expressed in liver cells are not well endured systemically, recombinant ITs such as LMB-2 and BL22 causing transaminase elevations are not allied with decreased hepatic function. ITs related renal toxicity could be non-specific and less well-defined for being the kidneys are the leading route of excretion of recombinant ITs.
Production limitations
In the beginning, chemical conjugates was prepared for the clinical trials because producers of recombinant toxins faced problems of low yield and endotoxin contamination. Productions of other recombinant proteins for clinical use have permitted large-scale production of recombinant toxins with high purity and reasonable cost and solved many problems. It is anticipated that corporate development will further improve yield and cut costs.
Current status of immunotoxins in India
Various research groups in India are too working for the development of novel ITs derived from bacteria and/or plant. These groups particularly work on the development of the ITs targeting malignancies. The developed ITs are recombinant variants and effort has been concentrated to enhance their efficacy by removing toxicity. Gadadhar and Karande, 2013 from Indian Institute of Sciences, Bangalore generated mAb F1G4- rABRa-A using abrin toxin from plant Abrus precatorius. Abrin toxin is a RIP-II protein which inhibits protein synthesis specifically on cells expressing the gonadotropin releasing hormone receptor (grhr). The group used the recombinant A chain of abrin to conjugate to antibodies raised against the human grhr. The conjugate induces cell death in cells articulating the receptor by inhibiting protein synthesis. Contrast to abrin, the conjugate exhibited differences in the kinetics of protein synthesis inhibition that has been attributed to the differences in internalization and trafficking of the conjugate within the cells. Furthermore, explanation of sequestration of the A chain into the nucleus of abrin treated cells but not in conjugate treated cells reveal a novel pathway for the association of the conjugate in the cells Gadadhar and Karande, 2013.
Professor R.S. Verma from Indian Institute of Technology, Madras have designed a number of novel construct which are truncated version of known ITs and novel humanized toxin exploiting the pre-apoptotic segment of human origin to conflict the problems associated with the immunogenicity of the toxin fragment and the non-targeted toxicity noted while treating certain form of cancers. The major constructs included by this group includes Diphtheria toxin-HN-1 peptide (DT/HN-1), which is a fusion toxin designed to target the head and neck squamous cell carcinoma (HNSCC). They have also developed a fusion toxin Diphtheria toxin-stem cell factor (DT-SCF) targeting malignancies expressing c-kit. c-kit over-expression has been reported in many forms and types of cancers including CML (K562), ALL (MOLT4), pancreatic carcinoma (PANC-1), and cervical carcinoma (HeLa 229). Various other ITs were developed and tested for targeting low affinity IL2Rα Potala and Verma, 2011.
Immunotoxin linking an anti-hCGβ antibody and PE38 has been developed by a team of research group from Institutional Talwar Research Foundation Core and National Institute of Immunology, New Delhi, India targeting histiocytic lymphoma (U937 cells) which is a rare type of NHL characterized by the occurrence of large tumor cells resembling histiocytes.
A set of ITs targeting transferrin receptors with recombinant Restrictocin, a toxin produced by the fungus Aspergillus restrictus, and a potent inhibitor of eukaryotic protein synthesis were also developed by Dr. Batra from National Institute of Immunology, India Rathore and Batra, 1996.
A team of Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Mumbai developed scFv(MUC1)-ETA immunotoxin targeting the mucin protein MUC1; over-expressed in more than ninty percent of breast cancers in an underglycosylated form, revealing the core peptides of the extracellular domain that act as a prospective target for antibody-mediated therapy Singh et al., 2007.
Nevertheless, a lot of efforts have been put forth on the development and characterization of these ITs still they have to pass the barrier of Phase Trials and FDA approval to reach the therapeutic markets.
Potential for future development
ITs are unlikely to work by themselves in many forms of diseases including cancer as their half-lives may be too limited for diffusion to occur into solid tumors. Clinical trials of ITs administered by an incessant infusion have thus non-significantly efficacious over the bolus infusion route. It is likely possible that the combination of ITs with other therapeutic agents having non-overlapping toxicities will result in better responses. Likewise, treatment of microscopic disease by ITs may be useful after cytoreduction by surgery, chemotherapy and/or radiotherapy. More than two dozen of antibody-drug conjugates and one dozen of ITs as well as various newly approved drugs, support the mellowness of the use of ITs with the antigen and disease target. As the amalgamation of diseases and antigen targets have been elected, we can anticipate exciting successes in the future development of ITs as a therapeutic agent for treating numerous diseases and disorders.
Abbreviations
MAb: monoclonal antibody; dsFv: disulfide-stabilized Fv antibody fragment; scFv: A single chain (genetically linked) variable fragment; bisFv: two Fv fragments connected via a disulfide bond; Fab: fragment antigenbinding (one constant/one variable domain of each heavy and light chain connected by a disulfide bond); IL(R): interleukin (receptor); DT: diphtheria toxin; DAB389, DAB486, DT388, DT390: truncated forms of DT that lack receptor-binding activity; CRM107: a mutated full-length diphtheria toxin that lack receptorbinding activity; PE: Pseudomonas exotoxin A; PE38, PE40: truncated forms of PE that lack the receptorbinding domain Ia; RTA: ricin toxin A; HIV: human immunodeficiency virus; CTCL: cutaneous T cell lymphoma; NHL: non-Hodgkin’s lymphoma; MDS: myelodysplastic syndrome; ALL: acute lymphoblastic leukemia; SLL: small lymphocytic lymphoma; GVHD: graft versus host disease; CNS: central nervous system; EGF(R): epidermal growth factor (receptor); TGF(R): transforming growth factor (receptor) AML: acute myelogenous leukemia; CML: chronic myelogenous leukemia; CLL: chronic lymphocytic leukemia; HD: Hodgkin’s disease; HCL: hairy cell leukemia; PLL: prolymphocytic leukemia; (N)SCLC: (non) small cell lung cancer; TAA: tumor associated antigen; TfR: transferrin receptor; CSF: cerebrospinal fluid, PAP: pokeweed antiviral protein; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; CR: complete remission (s); PR: partial response (s); GM-CSFR: granulocyte- macrophage colony stimulating factor receptor.
References
-
A.
Antignani,
D.
FitzGerald.
Immunotoxins: the role of the toxin. Toxins.
2013;
5
:
1486-1502
.
-
L.
Binyamin,
H.
Borghaei,
L.M.
Weiner.
Cancer therapy with engineered monoclonal antibodies. Update on Cancer Therapeutics.
2006;
1
:
147-157
.
-
F.
Bosch,
L.
Rosich.
The contributions of Paul Ehrlich to pharmacology: a tribute on the occasion of the centenary of his Nobel Prize. Pharmacology.
2008;
82
:
171-179
.
-
P.
Campbell,
R.
Marcus.
Monoclonal antibody therapy for lymphoma. Blood Review.
2003;
17
:
143-152
.
-
P.
Carter.
Improving the efficacy of antibody-based cancer therapies. Nature Review Cancer.
2001;
1
:
118-129
.
-
P.
Chames,
M.V.
Regenmortel,
D.
Baty.
Therapeutic antibodies: successes, limitations and hopes for the future. British Journal of Pharmacology.
2009;
157
:
220-233
.
-
S.
Choe,
M.J.
Bennett,
G.
Fujii,
P.M.G.
Curmi,
K.A.
Kantardjieff,
R.J.
Collier,
D.
Eisenberg.
The crystal structure of diphtheria toxin. Nature.
1992;
357
:
216-222
.
-
F.
Dosio,
B.
Stella,
S.
Cerioni,
D.
Gastaldi,
S.
Arpicco.
Advances in anticancer antibody-drug conjugates and immunotoxins. Recent Patents on Anti-Cancer Drug Discovery.
2014;
9
:
35-65
.
-
S.
Gadadhar,
A.A.
Karande.
Abrin immunotoxin: targeted cytotoxicity and intracellular trafficking pathway. PLoS One..
2013;
8
:
e5830-4
.
-
T.
Igawa,
H.
Tsunoda,
T.
Kuramochi,
Z.
Sampei,
S.
Ishii,
K.
Hattori.
Engineering the variable region of therapeutic IgG antibodies. MAbs.
2011;
3
:
243-252
.
-
B.H.
Iglewski,
D.
Kabat.
NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proceedings of the National Academy of Science, USA..
1975;
72
:
2284-2288
.
-
J.G.
Jurcic,
P.C.
Caron,
D.A.
Scheinberg.
Monoclonal antibody therapy of leukemia and lymphoma. Advance Pharmacology.
1995;
33
:
287-314
.
-
G.
Köhler,
C.
Milstein.
Continuous cultures of fused cells secreting antibody of predefined specificity. Nature.
1975;
256
:
495-497
.
-
R.J.
Kreitman.
Immunotoxins for targeted cancer therapy. The American Association of Pharmaceutical Scientists Journal.
2006;
8
:
Article 63, E532-E551,
.
-
R.J.
Kreitman.
Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs.
2009;
23
:
1-13
.
-
R.J.
Kreitman,
P.
Bailon,
P.K.
Chaudhary,
D.J.P.
FitzGerald,
I.
Pastan.
Recombinant immunotoxins containing anti-Tac (Fv) and derivatives of Pseudomonas exotoxin produce complete regression in mice of an interleukin-2 receptor-expressing human carcinoma.. Blood.
1994;
83
:
426-34
.
-
R.J.
Kreitman,
I.
Pastan.
Recombinant toxins containing human granulocyte-macrophage colony-stimulating factor and either pseudomonas exotoxin or diphtheria toxin kill gastrointestinal cancer and leukemia cells. Blood.
1997;
90
:
252-259
.
-
R.J.
Kreitman,
M.S.
Stevenson,
I.
Margulies,
P.
Noel,
D.J.P.
FitzGerald,
W.H.
Wilson,
I.
Pastan.
Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. Journal of Clinical Oncology.
2009;
27
:
2983-90
.
-
R.K.
Kreitman,
I.
Pastan.
Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochemistry Journal.
1995;
307
:
29-37
.
-
C.T.
Kuan,
L.H.
Pai,
I.
Pastan.
Immunotoxins containing Pseudomonas exotoxin that target LeY damage human endothelial cells in an antibody-specific mode: relevance to vascular leak syndrome. Clinical Cancer Research.
1995;
1
:
1589-1594
.
-
D.W.
Laske,
E.H.
Oldfield,
R.J.
Youle.
Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nature Medicine.
1997;
3
:
1362-1368
.
-
C.D.
Lorenzo,
G.
D'Alessio.
From immunotoxins to immunoRNases. Current Pharmaceutical Biotechnology.
2008;
9
:
210-4
.
-
J.
Madhumathi,
R.S.
Verma.
Therapeutic targets and recent advances in protein immunotoxins. Current Opinion in Microbiology.
2012;
15
:
300-309
.
-
M.
Mathew,
R.S.
Verma.
Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer Science.
2009;
100
:
1359-1365
.
-
C.
May,
P.
Sapra,
H.P.
Gerber.
Advances in bispecific biotherapeutics for the treatment of cancer. Biochemical Pharmacology.
2012;
84
:
1105-1112
.
-
A.L.
Nelson.
Antibody fragments: hope and hype. MAbs.
2010;
2
:
77-83
.
-
K.
Nielsen,
R.S.
Boston.
Ribosome-inactivating proteins: A Plant Perspective. Annual Review of Plant Physiology and Plant Molecular Biology.
2001;
52
:
785-816
.
-
M.S.
Ogata,
C.M.
Fryling,
I.
Pastan,
D.J
FitzGerald.
Cellmediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. Journal of Biological Chemistry.
1992;
267
:
25396-25401
.
-
I.
Pastan,
R.
Hassan,
D.J.
FitzGerald,
R.J.
Kreitman.
Immunotoxin treatment of cancer. Annual Review of Medicine.
2007;
58
:
221-237
.
-
W.J.
Peumans,
Q.
Hao,
Damme E.J.M.
Van.
Ribosomeinactivating proteins from plants: more than RNA N-glycosidases?. FASEB Journal.
2001;
15
:
1493-1506
.
-
S.
Potala,
S.K.
Sahoo,
R.S.
Verma.
Targeted therapy of cancer using diphtheria toxin-derived immunotoxins. Drug Discovery Today.
2008;
13
:
807-815
.
-
S.
Potala,
R.S.
Verma.
A novel fusion protein diphtheria toxinstem cell factor (DT-SCF)-purification and characterization. Applied Biochemistry and Biotechnology.
2010;
162
:
1258-69
.
-
S.
Potala,
R.S.
Verma.
Targeting head and neck squamous cell carcinoma using a novel fusion toxin-diphtheria toxin/HN-1. Molicular Biology Reports.
2011;
38
:
1389-1397
.
-
M.C.S.
Pranchevicius,
T.R.
Vieira.
Production of recombinant immunotherapeutics for anticancer treatment: the role of bioengineering. Bioengineered.
2013;
4
:
305-312
.
-
D.
Rathore,
J.K.
Batra.
Generation of active immunotoxins containing recombinant restrictocin. Biochem. Biophysics Research Communication.
1996;
222
:
58-63
.
-
D.H.
Robbins,
I.
Margulies,
M.S.
Stevenson,
R.J.
Kreitman.
Hairy cell leukemia, a B-cell neoplasm that is particularly sensitive to the cytotoxic effect of anti-Tac(Fv)-PE38 (LMB-2). Clinical Cancer Research.
2000;
6
:
693-700
.
-
A.
Shapira,
I.
Benhar.
Toxin-based therapeutic approaches. Toxins.
2010;
2
:
2519-2583
.
-
R.
Singh,
U.
Samant,
S.
Hyland,
P.R.
Chaudhari,
W.S.
Wels,
D.
Bandyopadhyay.
Target-specific cytotoxic activity of recombinant immunotoxin scFv(MUC1)-ETA on breast carcinoma cells and primary breast tumors. Molecular Cancer Therapeutics.
2007;
6
:
56-2
.
-
K.
Strebhardt,
A.
Ullrich.
Paul Ehrlich's magic bullet concept: 100 years of progress. Nature Review Cancer.
2008;
8
:
473-480
.
-
M.
Tsuneoka,
K.S.
Nakayama,
K.
Hatsuzawas,
M.
Komadall,
N.
Kitamurall,
E.
Mekada.
Evidence for involvement of furin in cleavage and activation of diphtheria toxi n. Journal of Biological Chemistry.
1993;
268
:
26461-264615
.
-
A.
Vedi,
D.S.
Ziegler.
Antibody therapy for Pediatric Leukemia. Frontiers in Oncology.
2014;
4
:
1-10
.
-
F.
Winau,
O.
Westphal,
R.
Winau.
Paul Ehrlich-in search of the magic bullet. Microbes and Infection.
2004;
6
:
786-789
.
-
M.
Wu.
Are immunoconjugates useful for therapy with autoimmune diseases?. International Journal of Immunopharmacology.
1997;
19
:
83-93
.
-
M.
Yamaizumi,
E.
Mekada,
T.
Uchida,
Y.
Okada.
One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell.
1978;
15
:
245-250
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 1 (2015)
Page No.: 169-183
Published on: 2015-01-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 9386 times
- Download PDF downloaded - 1958 times
- View Article downloaded - 9 times
 Biomedpress
Biomedpress