Abstract
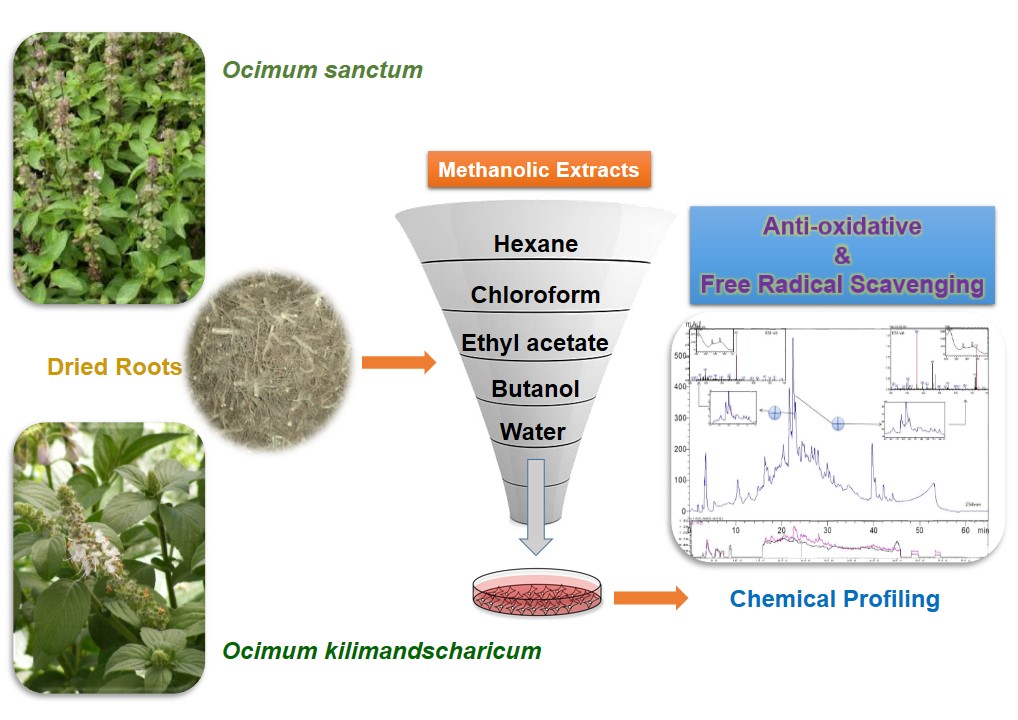
Background: Ocimum sanctum and Ocimum kilimandscharicum are cultivated in Indian subcontinent both for the religious and medicinal properties. Traditionally, the leaves have been reported for their enormous therapeutic potentials but the roots which are otherwise considered as a waste part have not been explored for their pharmacological activity.
Methods: Total phenolic content, free radical scavenging and ferric reducing antioxidant potential of various extracts from Ocimum sanctum and Ocimum kilimandscharicum were assessed and compared. In vitro antioxidant activity was estimated spectrophotometrically and the most potent ethyl acetate extract was chemically characterized by developing the chemical fingerprint and quantifying the probable constituents with the help of HPLC/LC-MS techniques.
Results: The ethyl acetate extract of both the species exhibited significant free radical scavenging potential and also reduced the ferric ions. It was observed that ethyl acetate extract have superior ferric reducing potential than other tested extracts, which were evidenced by high ferrous sulphate equivalent value of 77.05 ± 1.54 and 80.98 ± 0.80 at 100 µg/ml for O. sanctum and O. kilimandscharium respectively. The ferric reducing capacity of ethyl acetate extract for both the species was also evidenced by an elevated optical density of 1.64 ± 0.12 and 2.14 ± 0.08. Ocimum sanctum exhibited better antioxidant capacity (11.31 ± 0.20 AScE) as compared to Ocimum kilimandscharium (9.08 ± 0.27 AScE). The total phenolic and flavonoid content were estimated by spectrophotometric method and tentatively characterized by HPLC/LC-MS profiling which revealed the presence of rosmarinic acid, caffeic acid along with its derivatives such as caffeoyl-dihydroxyphenyllactoyl-tartaric acid.
Conclusion: The ethyl acetate extract of both the species being rich in phenolic and flavonoid contents exhibited potent antioxidant activity. The presence of flavonoid in ethyl acetate extracts further co-relates the antioxidative properties of roots extracts. To the best of our knowledge and understanding this is the first report of comparative chemical profiling by RP-HPLC/LC-MS and antioxidant potential of roots of two Ocimum species.
Introduction
Traditionally, medicinal plants are known to possess enormous therapeutic potentials and have been used for treating various ailments and diseases. In recent years, phytochemicals with antioxidant properties have received great consideration mainly due to their role in preventing diseases which are caused by oxidative stress Uyoh et al., 2013. Damage to the cells caused by free radicals is believed to play a central role in aging and in progression of many diseases and disorders. Antioxidants are believed to be the first line of defense against free radical damage and are critical for maintaining optimum health and well-being Bozin et al., 2006. Due to the presence of many active molecules, various parts of plants, such as leaves, stem, bark and roots are now being used in formulations as natural antioxidants for prevention and treatment of various complex conditions in humans Katalinic et al., 2006. Many phytochemicals have revealed to support in maintaining the balance between oxidants and antioxidants in humans and natural antioxidants are gaining significant attention amongst food dieticians, pharmacists, manufacturers and consumers because of their enormous therapeutic potential and safety Ito et al., 1986. Several findings have proven that many medicinal plants and food crops are a rich source of many phytochemicals which have excellent antioxidant potentials Pizzale et al., 2002Vieira et al., 2014.
Ocimum, belonging to the family Lamiaceae, also known as the queen of herbs, has been used for centuries in the different traditional systems of medicines. The genus Ocimum consist of about more than 140 different species of aromatic plants and generally found in tropical and sub-tropical regions of the world Devi, 2001. The O. sanctum, O. basilicum, O. gratissimum, O. kilimandsachricum and O. americanum are some common examples of the various species being grown in various parts of world for numerous medicinal values. The essential oils from different Ocimum species have been reported to possess antimicrobial, anticonvulsant and antioxidant activities Akinmoladun et al., 2007Balaji et al., 2011Singh et al., 2010Vieira et al., 2014. A recent study by Ahmad et al., (2012) revealed the anti-diabetic activity of new constituents characterized as urs-12-en-3β,6β,20β-triol-28-oic acid, 1´´-menthyl-2-glucopyranosyloxybenzoate and n-decanoyl-β-D-glucopyranosyl-(2a→1b)-β-D-glucopyranosyl-(2b→1c)-β-D-glucopyranosyl-(2c→1d)-β-D-glucopyranosyl-2d-2-hydroxybenzoate, along with ursolic acid and palmityl glucoside Ahmad et al., 2012. In another study by Maity et al., (2000) the effect of methanolic root extract of Ocimum sanctum, on mouse swimming performance was reported Maity et al., 2000. The antimicrobial activity and HPLC fingerprinting of crude methanolic and aqueous extracts of O. sanctum and O. kilimandsacharicum have also been revealed Deo et al., 2011. The antioxidant properties of leaves extracts of O. onites, O. vulgaris and O. basilicum along with total phenolic and rosmarinic acid content have been also reported Lagouri and Nisteropoulou, 2009. There are very few reports related to potential bioactivities and pharmaceutical applications of Ocimum root. The roots of Ocimum species which are otherwise considered as a waste by product and are generally used as mulching, manure or as a fuel by the farmers after cultivation can thus be a potential source of many active phytomolecules possessing novel biological activities. Hence, in the present study, antioxidant potential and chemical characterization of Ocimum (Tulsi) species were carried out by using in vitro assay procedure and HPLC-UV/LC-MS analysis respectively. The roots of Ocimum sanctum and Ocimum kilimandsacharicum were subjected to extraction and further evaluated for their antioxidant prospective. The chemical profile of an active fraction by HPLC method was also developed to characterize the extracts.
Materials - Methods
Plant materials
The fresh roots of O. sanctum and O. kilimandsacharicum after harvesting were collected from the experimental fields of CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, Uttar Pradesh, India. The voucher specimen samples of both the species were authenticated and deposited in the herbarium unit under reference number 2013-12598 and 2014-12934 respectively in the Botany and Pharmacognosy Department of CSIR-CIMAP, Lucknow, Uttar Pradesh, India.
Preparation of extracts
The fresh roots of both the species of Ocimum were washed in warm water and dried under the controlled conditions in a hot air oven at 40-42°C.The roots were then segregated and grounded in a hammer mill into a coarse powder of 30-40 mesh size. 500 g each of the dried and powdered roots were extracted exhaustively with methanol in a soxhlet type apparatus. The methanolic extract was concentrated under controlled conditions to get a viscous mass. The extracts were re-dissolved in double distilled water and successively partitioned with hexane, chloroform, ethyl acetate and butanol. All the extracts were concentrated under low temperatures and vacuum to obtain the different fractions. The fractions were air dried at room temperature and stored at 4°C till further use.
Solvents and chemicals
Hexane, methanol, butanol, ethyl acetate and chloroform (all LR grade) were purchased from Molychem (India) Ltd. Gallic acid, ferrous sulphate, tris-[hydroxymethyl] aminomethane, sodium nitropruside dihydrate, N-(1-naphthyl) ethylenediamine dihydrochloride, sulphanilamide, sodium carbonate, potassium ferricyanide, ferric chloride, tripyridyl-s-triazine (TPTZ), hydrogen phosphate, and m-phosphoric acid aluminum chloride, potassium dihydrogen phosphate, potassium phosphate monobasic, phosphate buffer saline (PBS), ammoniummolybdate, 2, 2-diphenyl-1 picrylhydrazyl (DPPH), dimethyl sulfoxide (DMSO), Folin–Ciocalteureagent, sodium acetate quercetin, and ascorbic acid were bought from Sigma Aldrich, Bangalore, India. HPLC grade solvents were procured from Merck Pvt. Ltd, Mumbai, India, Ultrapure water was prepared using a Milli-Q System (Millipore, Milford, MA, USA).
Antioxidant Activity Evaluation
DPPH radical scavenging activity
DPPH radical scavenging assay is a widely used method for the in vitro estimation of antioxidant potential of extracts or phyto-molecules. DPPH radicals produces a dark purple colour in methanol and its characteristic deep purple colour bleaches on accepting hydrogen from a corresponding donor from active extracts Luciana et al., 2001. The antioxidant potential of the different extracts of Ocimum root were assessed according to the method described by Chung et al. (2002) with some variations as reported by Luqman et al. (2009) Chung et al., 2002Luqman et al., 2009. According to the described method, different concentrations of the samples (10, 25, 50, and 100 µg/ml) were added to 100µl of methanol followed by 500µl of Tris HCl buffer (pH 7.4) and 500µl of the DPPH reagent. The reaction mixture was then incubated at 37ºC for 20 min in the dark and the absorbance was measured at 517nm against a blank buffer and percent DPPH inhibition was calculated with respect to reagent control.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay is based on the principle of reduction of ferric tripyridyl triazine (Fe3+ TPTZ) complex to ferrous form Benzie and Strain, 1996. The change in absorption is directly proportional to the sum total of reducing capacity of the electron donating antioxidants present in the reaction mixture. A reagent mixture consisting of 20 mM ferric chloride 10 mM TPTZ, and 300 mM acetate buffer (pH 3.6) in ratio of 1:1:10 was prepared. The Ocimum extracts (10, 25, 50 and 10µg/ml) were taken and 50µl of water and 1.5ml of FRAP reagent were added to it. The reaction mixture was then incubated at 37ºC for 5 min and the absorbance was measured at 593nm. The results were expressed in terms of ferrous sulphate equivalents (FSE) which were obtained from the standard curve of ferrous sulphate Luqman et al., 2011.
Reducing power assay
Phytochemicals have the ability to reduce iron like radicals reacts with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which further retorts with ferric chloride to form ferric-ferrous complex. The reducing power potential was measured according to the method of Yen and Chen (1995) with some modifications Negi et al., 2011Yen and Chen, 1995. Various concentrations Ocimum root extracts (10, 25, 50 and 100 µg/ml) were pooled with phosphate buffer (200mM, pH6.6) and potassium ferricyanide. The mixture was heated on water bath at 50ºC for 20 min. The supernatant was then transferred to micro plate and to it distilled water and freshly prepared 0.10% FeCl3 was added and optical density was read at 700nm against reagent blank.
Total antioxidant capacity (TAC) estimation
The estimation of the total antioxidant capacity (TAC) is based on method reported by Prieto et al., (1999) where the Mo (VI) is reduced to Mo (V) in suitable acidic medium that lead formation of a greenish complex of phosphate and Mo (V) Prieto et al., 1999. For the experiment different concentration of the Ocimum root extracts (10, 25, 50 and 100 µg/ml) were mixed with 1ml of the TAC reagent (0.605 M ammonium molybdate, 25 mM phosphate mono-basic and 0.60 M concentrated sulphuric acid). The reaction mixture was heated on boiling water bath for 90 min. The optical density of the formed complex was noted at 695nm against a reagent blank and results were expressed as ascorbic acid equivalent.
Total flavonoids (TF) content
The estimation of the TF content in the extract was performed by colorimetric method as described by Meda et al., 2005 Meda et al., 2005. In this phytochemical estimation, various concentrations of the Ocimum roots extract (10, 25, 50 and 100 µg/ml) were dissolved in 50 L of double distilled water with the successive addition of 10 µl of aluminum chloride (10%), 150 µl of methanol, 1 M potassium acetate, and 280 µl of double distilled water. Optical density at 415 nm was measured and compared to blank reagent. The total flavonoid content in the different Ocimum root extracts were articulated as quercetin equivalent (QCE) as mentioned recently Ahmad et al., 2014.
Total phenolic (TP) estimation
Total phenolic compounds present in the plant extracts was estimated by the assay described by Singleton and Rossi, 1965 with some modifications Luqman et al., 2011Singleton and Rossi, 1965. Different concentrations of the Ocimum root extracts (10, 25, 50, and 100 µg/ml) were taken to which folins reagent was added followed with addition of 7.5 % sodium bicarbonate. The reaction cocktail was then incubated at 37ºC for 90 min and the optical density was measured at 765 nm against a reagent blank. The results were expressed as gallic acid equivalent (GAE) which was obtained from a standard curve of gallic acid.
HPLC-UV/PDA fingerprint analysis
As the ethyl acetate extract exhibited the most potent antioxidant activity, so it was taken for further analytical studies. The extraction was carried out in triplicate, filtered, collected and concentrated under vacuum. The uniformity in the three batches of extracts preparation was assured by their reproducible characteristic chemical chromatographic fingerprint. The phytochemical finger printing was done on a chromatographic system from Shimadzu (Shimadzu Corporation, Kyoto, Japan) which consist of model LC-20AD pumps, a rheodyne manual injector, a model CTO-20A oven and a model SDP-M20 diode array detector. For controlling the LC system, LC-MS Solution 3.21 software from Shimadzu was used on a computer system (3000H Series, Lenovo). A model of CBM-20 Shimadzu interface was used to send the signals from the detector to the computer. The separation was achieved by a gradient elution using X select C18 (4.6 × 250 mm, 5µm) at 35°C. The mobile phase consisting, acetic acid (0.5%, v/v) in water (solvent A) and in acetonitrile (solvent B) was used Kumar et al., 2015. Prior to the use, mobile phase was degassed for 15 min using ultrasonication technique (Oscar Ultrasonics, Mumbai, India). The samples and mobile phase was filtered through a 0.45 µm membrane filter in solvent filtration apparatus (Millipore, USA). The gradient elution was started with 5% B with a flow rate of 1.0 ml/min. Detector wavelength was set at 254nm and the injection volume was 20µl. The data acquisition was performed in the range of 200–400 nm to monitor any possible co-elution in plant sample solution. Therefore, considering maximum chromatographic signal response for fingerprint, wavelength 254 nm was selected.
Statistical analysis
All the data are expressed as mean± SD (n=3) which were computed with the help of MS Office 2007. Comparisons were made between test compounds and reference control for antioxidant activity by one-way analysis of variance (ANOVA) followed by post hoc Dunnet test, all vs control, and a p value of <0.05 was considered statistically significant.
Results - Discussion
Extraction and fractionation in different solvents
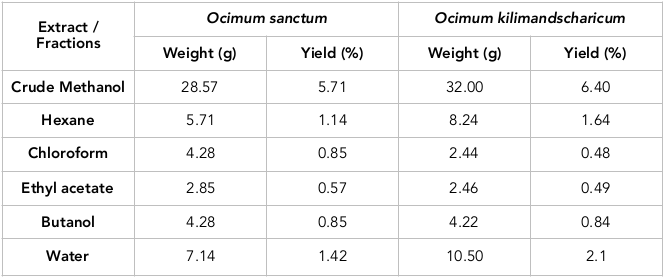
The crude methanolic extract was prepared and fractionated with the solvents of varying polarity to get different extracts containing mixture of compounds. Better yield was obtained in aqueous fraction for both the species of Ocimum which was followed by hexane fraction. The results of extraction procedure are presented in Table 1 .
Antioxidant activity evaluation
A concentration-dependent evaluation of anti-oxidative properties was studied for all the extracts of two Ocimum species. The potential of the extracts were tested using several well established in-vitro assays, such as reducing power, DPPH, FRAP, and total antioxidant capacity. Total flavonoid and total phenolic contents were also estimated by colorimetric procedure following established protocols.
DPPH radical scavenging assay
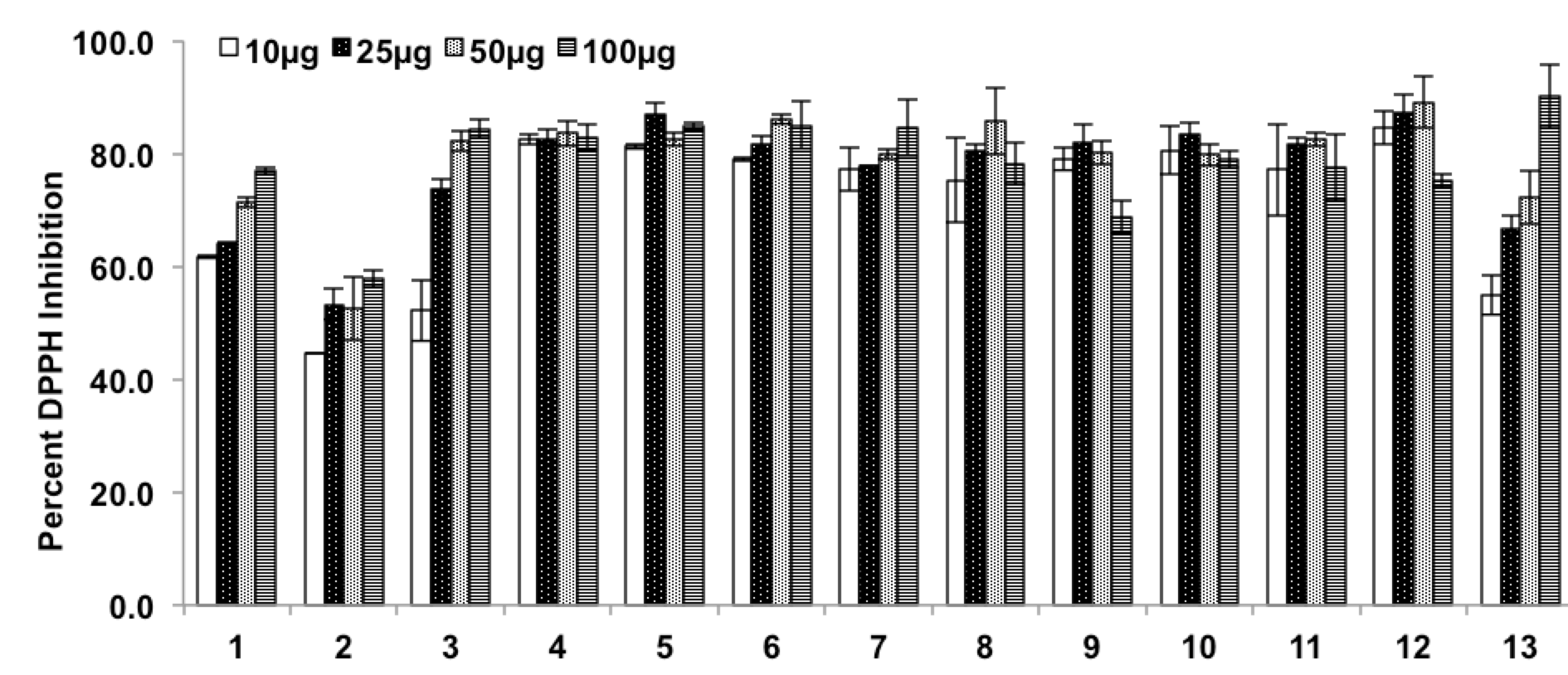
Various root extracts of two Ocimum species were evaluated for their free radical scavenging activity by DPPH assay. Our finding showed that methanolic root extract of O. kilimandscharicum has the better scavenging potential followed by the methanolic extract of O. sanctum, order of their DPPH radical scavenging potential was 6>5>3>4>1>2 (O. sanctum) and 12>10>8>7>11>9 (O. kilimandscharicum). Overall, it was also observed that methanolic extract of O. kilimandscharicum exhibited low IC50 value (7.02- 24.19 μg/ml) compared to O. sanctum (12.93- 35.46 μg/ml) for DPPH radical scavenging ( Figure 1 ).
FRAP assay
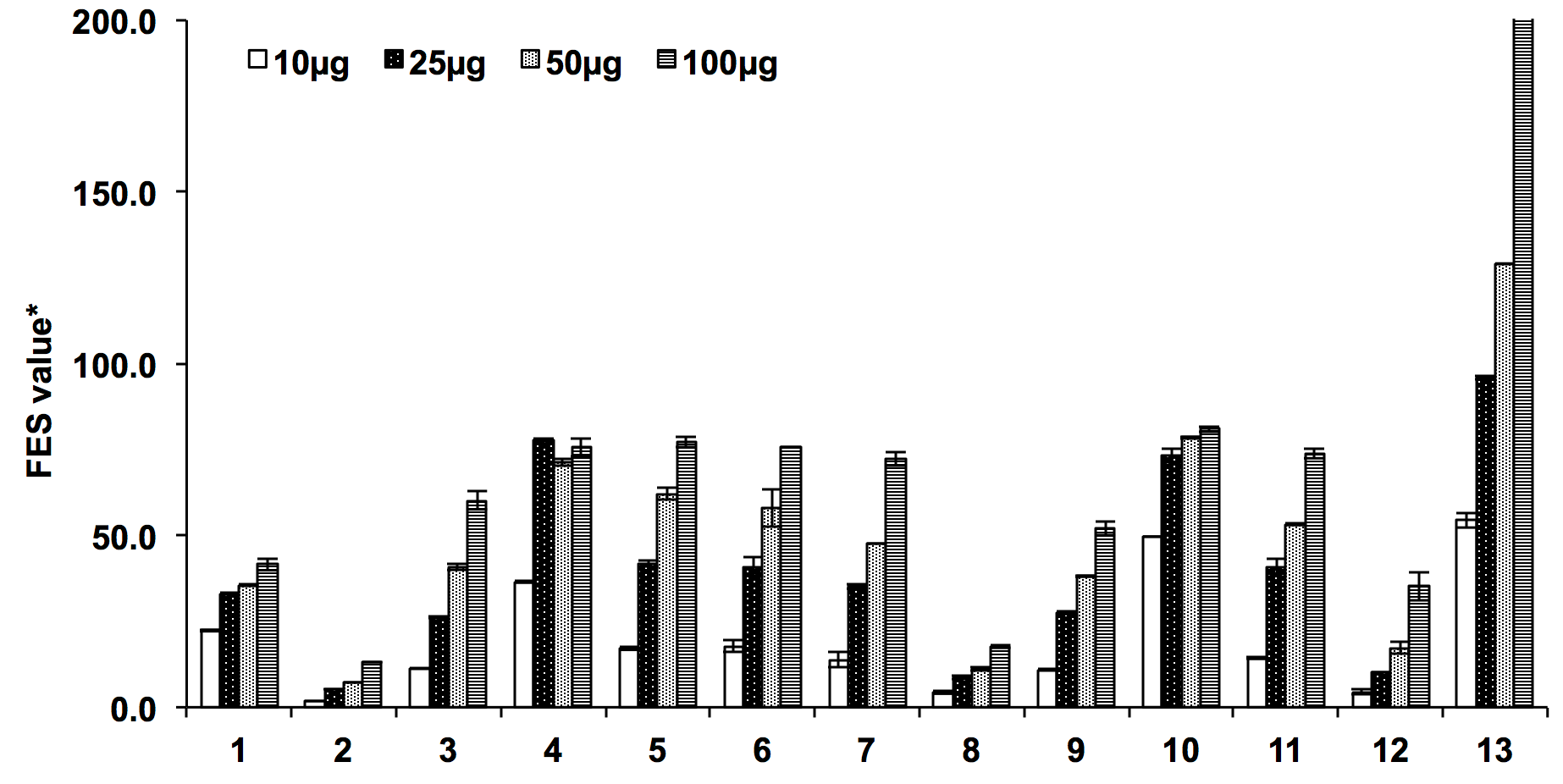
The ferric reducing antioxidant power of the Ocimum extracts was expressed as equivalence of ferrous sulphate and it was observed that reducing power of the extracts increases as the concentration increases. The ethyl acetate extract of O.sanctum and O. kilimandscharicum exhibited better ferric reducing activities among all the tested extract (77.05±2.67 and 80.98±3.50 FSE respectively) which was followed by butanolic extracts (75.84±4.85 and 73.91±4.52 FSE respectively). The ethyl acetate extracts of both the Ocimum species showed the maximum potency to donate electrons to the free radical thus terminating the reactive free radical chain reaction. Interestingly, it was also observed that methanolic and butanolic extracts of O. sanctum exhibited similar ferric reducing activity indicated by 75.84±4.85 and 75.61±3.25 FSE respectively. The order of their ferric reducing potential were found as 4>6>5>3>1>2 for O. sanctum and 10>11>7>9>12>8 for O. kilimandscharicum ( Figure 2 ).
Reducing power assay
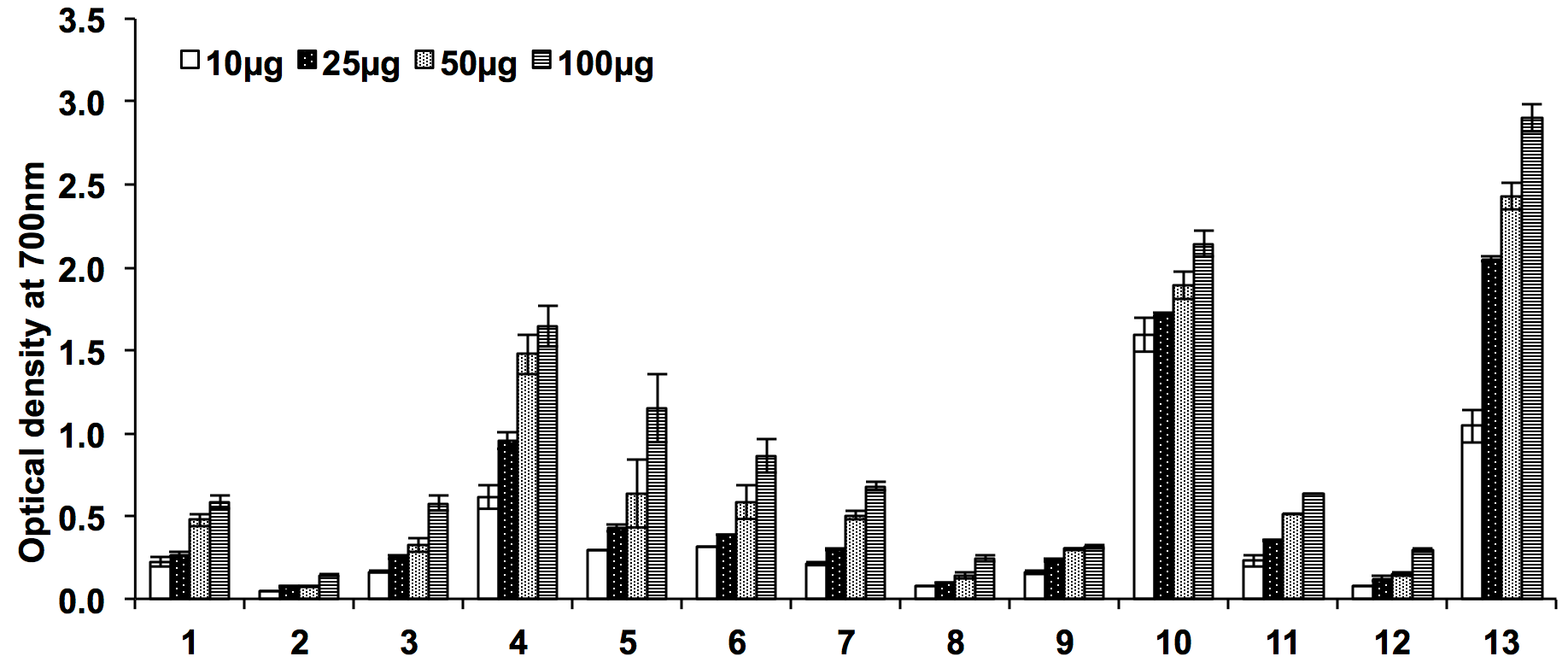
The ethyl acetate root extracts of both the species of Ocimum were found most active in iron reducing capacities as compared to other tested extracts, evidenced by a higher optical density recorded at 700 nm (1.64±0.23 and 2.14±0.36 respectively). The reducing power of these extracts rises as the concentration of the extracts increased and was found to follow the similar pattern as that of ferrous reducing power in FRAP assay. The results of iron reducing assays of various extracts are shown in Figure 3 . The optical density of Ocimum extracts ranged from 0.14±0.01 to 2.14±0.36 at 100µg/ml and the order of their iron reducing property was noted as 4>5>6>1~3>2 for O. sanctum and 10>7>11>9>12>8 for O. kilimandscharicum.
Total antioxidant capacity
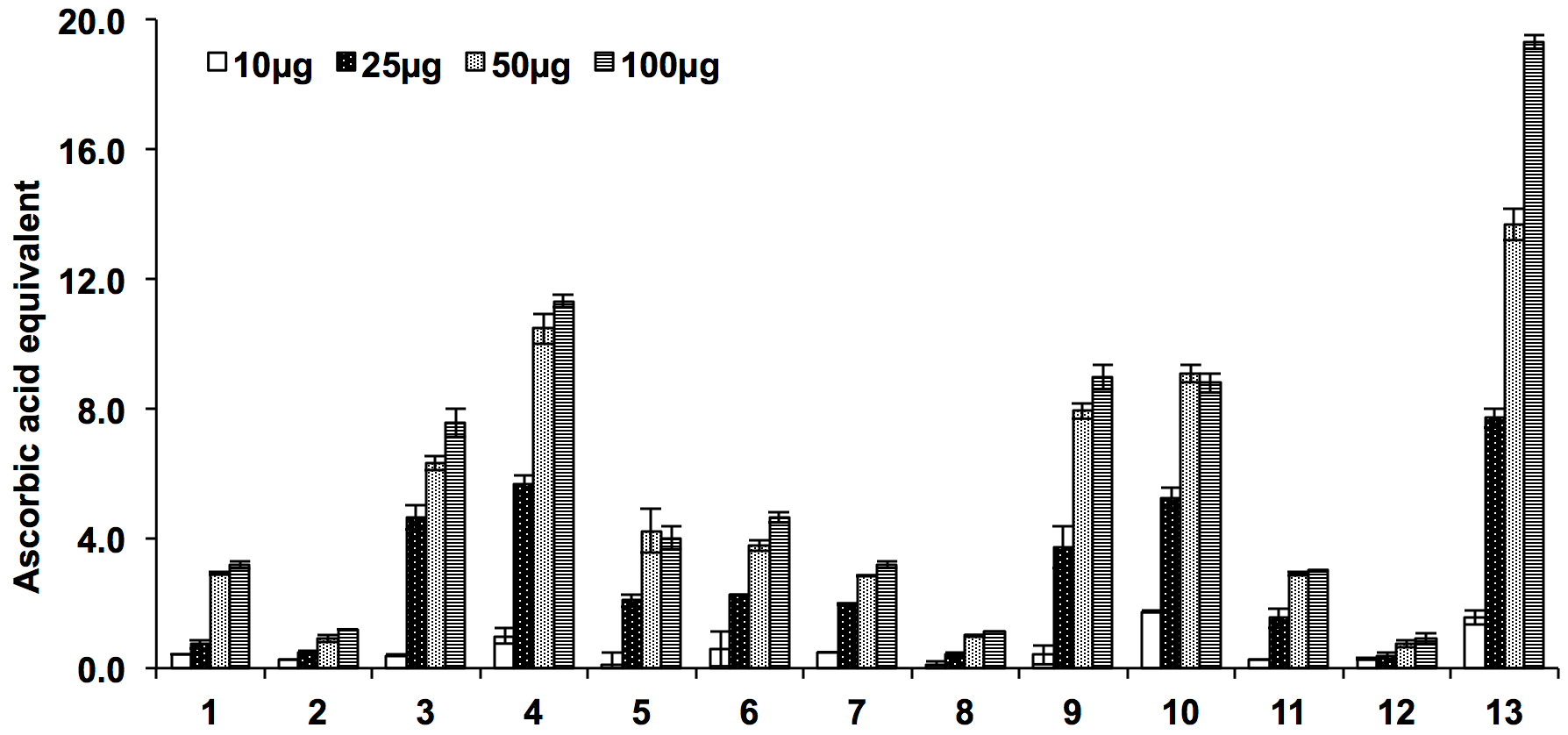
The total antioxidant capacity was determined in terms of ascorbic acid equivalent and amongst the tested species of Ocimum, ethyl acetate extract of O. sanctum showed maximum antioxidant capacity. The total antioxidant capacity of chloroform and ethyl acetate extracts of both the species exhibited better total antioxidant potential compared to other which ranges from 7.56±0.47 to 11.31±1.02 µg AsE/mg of dried extracts. The order of antioxidant capacities was observed to be 4>3>6>5>1>2 for O. sanctum 10>9>7>11>8>12 for O. kilimandscharicum ( Figure 4 ).
Total flavonoids estimation
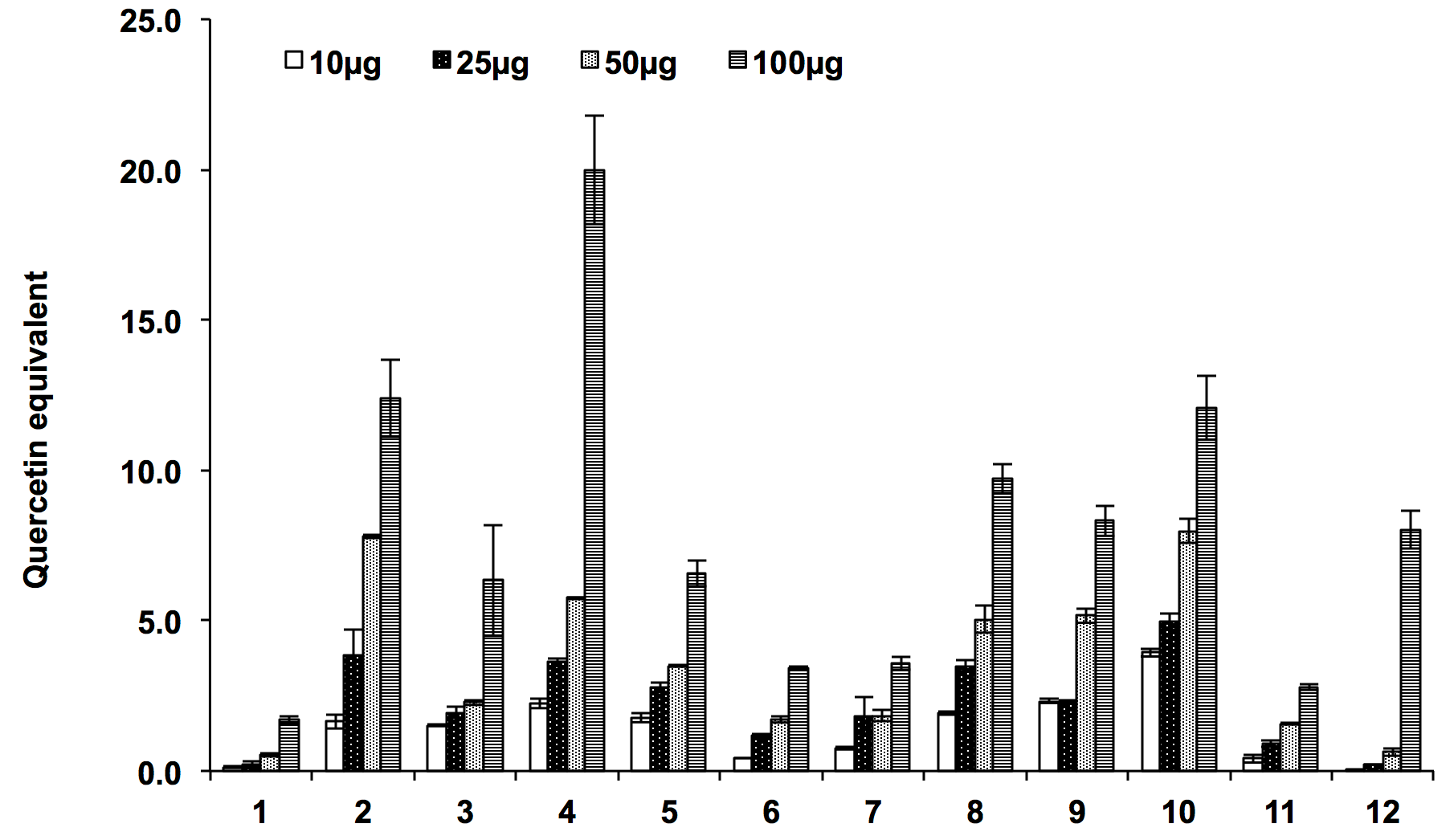
Flavonoids are the important secondary metabolites reported for various biological activities. Most of the plant extract reported elsewhere for their antioxidant activities are found to contain flavonoids, therefore we have also quantified the total flavonoids content in the extracts. High flavonoid content was reported in ethyl acetate extracts which was followed by hexane extracts of both the Ocimum species. The aqueous and methanolic extracts of O. sanctum and aqueous and butanolic extract of O. kilimandscharicum were found to possess poor amount of total flavonoids. The presence of high amount of flavonoids in ethyl acetate extracts was positively co-related with total antioxidant capacity and the order of flavonoid content were found to be as 4>2>5~3>6>1 for O. sanctumand 10>8>9>12>7>11 O. kilimandscharicum ( Figure 5 ).
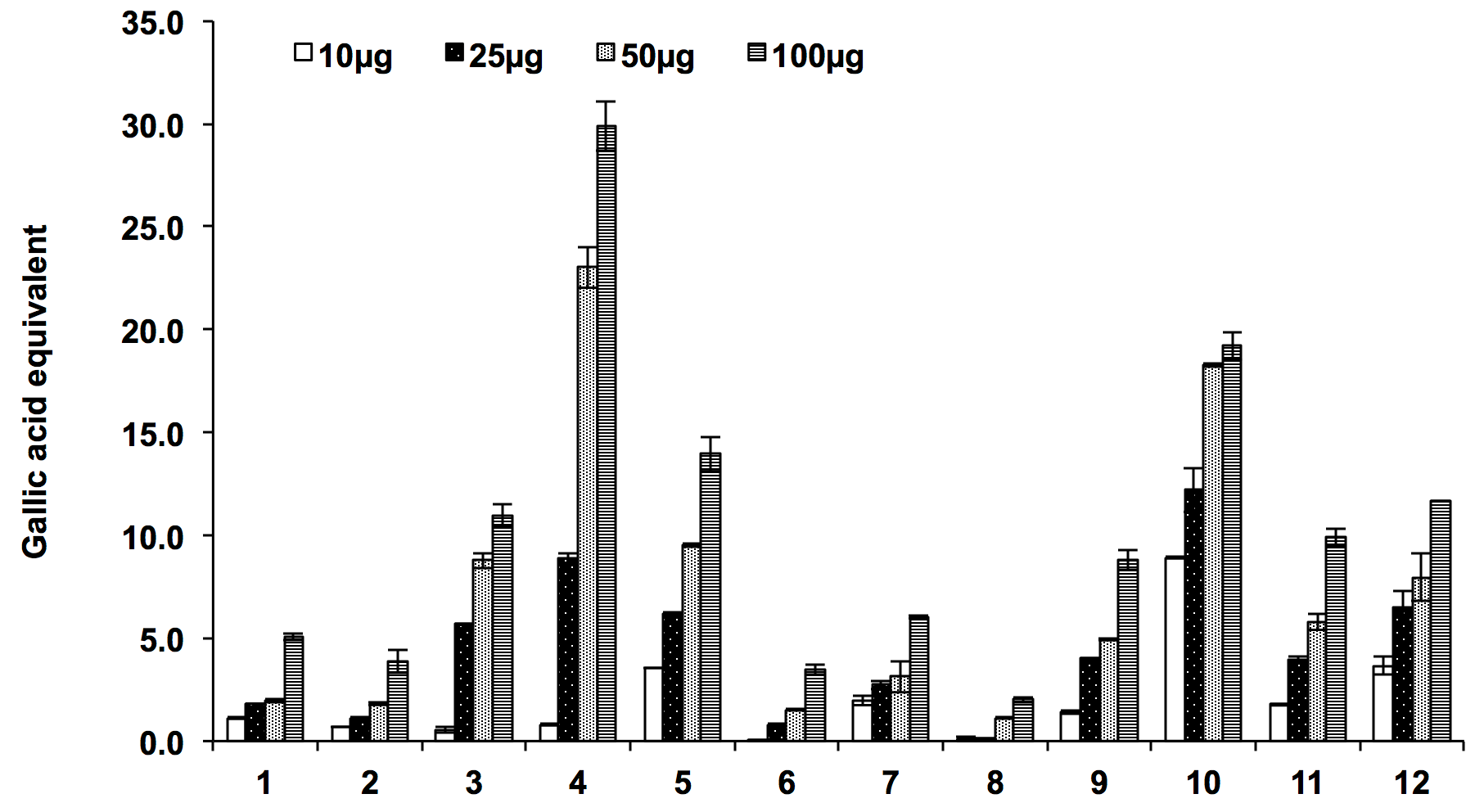
The presences of phenolic compound in plant extract also contribute to its antioxidant activity. The total phenolic content was estimated in terms of gallic acid equivalent. The total phenolic content of the two Ocimum species ranges from 3.46±0.49 to 29.88±2.89 µg GAE/mg of dried extracts and the ethyl acetate fraction of both the species showed better phenolic content with respect to other extracts which was positively co-related with the total antioxidant capacity. The order of the total phenolic content of different extracts was found to be as 4>5>3>1>2>6 for O. sanctum and 10>12>11>9>7>8 for O. kilimandscharicum ( Figure 6 ).
Phytochemical Profiling
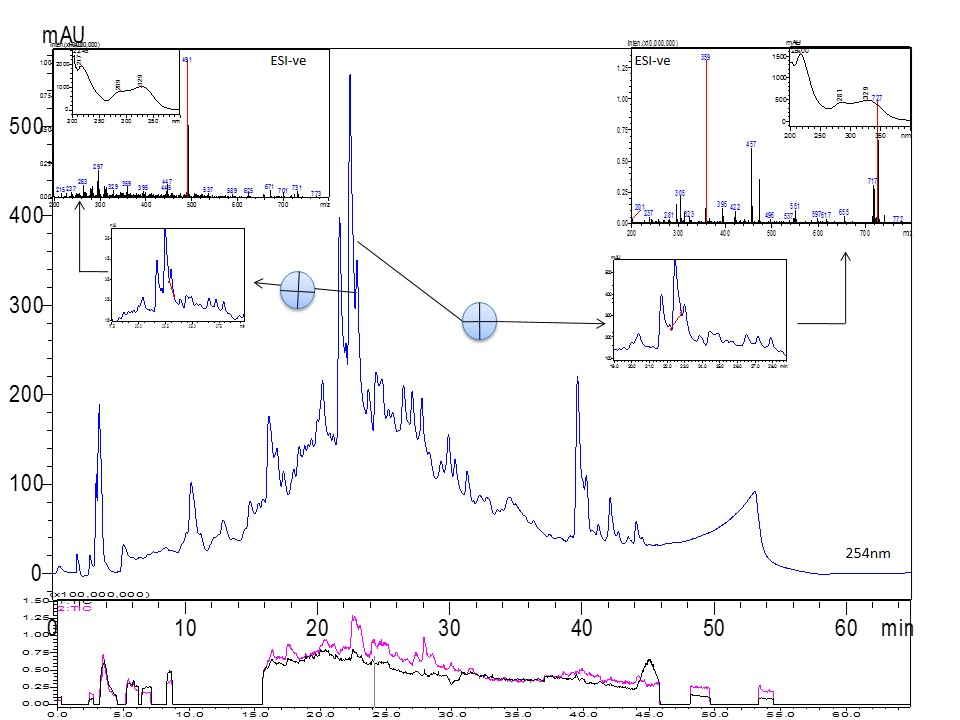
Since the ethyl acetate extract of both the species have shown the potent antioxidant activity, the phytochemical profiling of the ethyl acetate extracts with HPLC-PDA and LC-MS was carried out. The presence of various compounds and the hydroxyl cinnamic acid derivatives was observed. It has already been reported that other species of Ocimum contains many phenolics like rosmarinic acid, caffeic acid, etc. The RP-HPLC chromatogram of the ethyl acetate fraction of O. sanctum revealed eight major peaks which were characterized as flavonoids on the basis of UV-spectra matching ( Figure 7 ). The presences of UV active chromophore due to the presence of two aromatic rings provided great support to the flavonoids aglycones which consequently absorb UV absorption peaks. The first maxima found at 240-285 nm range due to the presence of the ring-A benzoyl system and second maximum at 300-400nm range, to the substitution pattern and conjugation of the B-ring cinnamoyl system Mabry et al., 1970Markham, 1982. Our previous studies on roots of O. sanctum have already revealed the presence of ocimol, galactose, arabinose, β-sitosterol, ocimic acid, ursolic acid, trihydroxy ursolic acid, palmityl glucoside, menthylsalicylic glucoside and capryl tetraglycosidic salicylate Ahmad et al., 2012. The UV spectra of eight major peaks showed two absorptions at the max range of 238-240 nm of band-II and another at the range of 307-354 nm of band-I indicated that these compounds tentatively belongs to 3-substituted flavonol or flavones. According to available characteristic, UV spectra of each peak can be used as an indicative tool for the characterization of C-ring, whereas the MS spectra could provide additional, significant information in prediction of active molecules ( Figure 7 ).
Conclusion
Our investigation on two species of Ocimum root extracts revealed that the ethyl acetate extracts of both the species revealed potent antioxidant potential and possess elevated phenolic and flavonoid content. The presence of polyphenolic compounds such as rosmarinic acid, caffeic acid and its derivatives were further tentatively characterized by HPLC/LC-MS profiling of the extract which strengthens the antioxidant potential and usefulness of the waste roots for tapping its prospective commercial utilization.
Abbreviations
DPPH: 2, 2-diphenyl-1-picrylhydrazyl.
FRAP: Ferric reducing antioxidant power
FSE: Ferrous sulphate equivalent.
GAE: Gallic acid equivalent.
LC/MS: Liquid chromatography–mass spectrometry.
QCE: Quercetin equivalent.
RP-HPLC: Reverse phase high performance liquid chromatography.
TAC: Total antioxidant capacity
UV: Ultraviolet
Author Contribution
Envisaged and designed the experiments: ST, KS, SL; Root collection and extract preparation: KA, AA; Antioxidant Test: DKS, SL; HPLC/LC-MS Characterization: J, KS; Data Analysis: KA, DKS, AA, KS, ST, SL; Manuscript writing: KA, DKS, J, KS, ST, SL. All authors reviewed and commented on final draft.
References
-
A.
Ahmad,
D. K.
Singh,
K.
Fatima,
S.
Tandon,
S.
Luqman.
New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity . Industrial Crops and Products.
2014;
58
:
125-132
.
View Article Google Scholar -
M. Z.
Ahmad,
M.
Ali,
S. R.
Mir.
Anti-diabetic activity of Ocimum sanctum L. roots and isolation of new phytoconstituents using two-dimensional nuclear magnetic resonance spectroscopy . Journal of Pharmacognosy and Phytotherapy.
2012;
4
:
75-85
.
View Article Google Scholar -
A. C.
Akinmoladun,
E. O.
Ibukun,
E.
Afor,
E. M.
Obuotor,
E. O.
Farombi.
Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Scientific Research and Essays.
2007;
2
:
163-166
.
-
R.
Balaji,
G.
Prakash,
P. D.
Suganya,
K. M.
Aravinthan.
Antioxidant activity of methanol extract of Ocimum tenuiflorum (dried leaf and stem). Int J Pharm Sci Rev Res.
2011;
3
:
20-7
.
-
I. F.
Benzie,
J. J.
Strain.
The ferric reducing ability of plasma (FRAP) as a measure of 'antioxidant power': the FRAP assay . Analytical biochemistry.
1996;
239
:
70-76
.
View Article Google Scholar -
B.
Bozin,
N.'Simin
Mimica-Dukic,
G.
Anackov.
Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils . Journal of agricultural and food chemistry.
2006;
54
:
1822-1828
.
View Article Google Scholar -
Y. C.
Chung,
C. T.
Chang,
W. W.
Chao,
C. F. Chou
Lin.
Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1 (https:// doi.org/10.1021/jf011369q). Journal of Agricultural and Food Chemistry.
2002;
50
:
2454-2458
.
-
S. S.
Deo,
F.'Mahashabde
Inam.
Antimicrobial activity and HPLC fingerprinting of crude ocimum extracts. Journal of Chemistry.
2011;
8
:
1430-1437
.
-
P. U.
Devi.
Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum. Tulasi.
2001
.
-
N.
Ito,
M.
Hirose,
S.
Fukushima,
H.
Tsuda,
T.
Shirai,
M.
Tatematsu.
Studies on antioxidants: their carcinogenic and modifying effects on chemical carcinogenesis . Food and Chemical Toxicology.
1986;
24
:
1071-1082
.
View Article Google Scholar -
V.
Katalinic,
M.
Milos,
T.
Kulisic,
M.
Jukic.
Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols . Food chemistry.
2006;
94
:
550-557
.
View Article Google Scholar -
A.
Kumar,
K.
Agarwal,
A. K.
Maurya,
K.
Shanker,
U.
Bushra,
S.
Tandon,
D. U.
Bawankule.
Pharmacological and phytochemical evaluation of Ocimum sanctum root extracts for its antiinflammatory, analgesic and antipyretic activities . Pharmacognosy magazine.
2015;
11
:
S217
.
View Article Google Scholar -
V.
Lagouri,
E.
Nisteropoulou.
Antioxidant properties of O. onites, T. vulgaris and O. basilicum species grown in Greece and their total phenol and rosmarinic acid content . Journal of food Lipids.
2009;
16
:
484-498
.
View Article Google Scholar -
M.
Luciana,
E. D.
Burgund,
M.
Berman,
K. L.
Hanson.
Effects of tryptophan loading on verbal, spatial and affective working memory functions in healthy adults . Journal of Psychopharmacology.
2001;
15
:
219-230
.
View Article Google Scholar -
S.
Luqman,
R.
Kumar,
S.
Kaushik,
S.
Srivastava,
M. P.
Darokar,
S. P.
Khanuja.
Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash. Indian J Biochem Biophys.
2009;
46
:
122-125
.
-
S.
Luqman,
S.
Srivastava,
R.
Kumar,
A. K.
Maurya,
D.
Chanda.
Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evidence-Based Complementary and Alternative Medicine Article ID.
2011;
519084
.
-
T. J.
Mabry,
K. R.'Thomas
Markham.
The ultraviolet spectra of flavones and flavonols. The systematic identification of flavonoids . Springer.
1970;
:
41-164
.
View Article Google Scholar -
T. K.
Maity,
S. C.
Mandal,
B. P.
Saha,
M.
Pal.
Effect of Ocimum sanctum roots extract on swimming performance in mice. Phytotherapy Research.
2000;
14
:
120-121
.
-
K. R.
Markham,
others.
Techniques of flavonoid identification. Academic press London.
1982;
31
.
-
A.
Meda,
C. E.'Romito
Lamien,
J.
Millogo,
O. G.
Nacoulma.
Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity . Food chemistry.
2005;
91
:
571-577
.
View Article Google Scholar -
A. S.
Negi,
S.
Luqman,
S.
Srivastava,
V.
Krishna,
N.
Gupta,
M. P.
Darokar.
Antiproliferative and antioxidant activities of Juglans regia fruit extracts . Pharmaceutical biology.
2011;
49
:
669-673
.
View Article Google Scholar -
L.
Pizzale,
R.
Bortolomeazzi,
S.
Vichi,
E.
Überegger,
L. S.
Conte.
Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content . Journal of the Science of Food and Agriculture.
2002;
82
:
1645-1651
.
View Article Google Scholar -
P.
Prieto,
M.
Pineda,
M.
Aguilar.
Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E . Analytical biochemistry.
1999;
269
:
337-341
.
View Article Google Scholar -
S. K.
Singh,
A. N.
Anand,
S. K.
Verma,
M. A.'Mathur
Siddiqui,
S. O.
Saklani.
Analysis of phytochemical and antioxidant potential of Ocimum kilimandscharicum Linn. Int J Curr Pharm Res.
2010;
3
:
40-46
.
-
V. L.
Singleton,
J. A.
Rossi.
Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture.
1965;
16
:
144-158
.
-
E. A.
Uyoh,
P. N.'David
Chukwurah,
A. C.
Bassey.
Evaluation of antioxidant capacity of two Ocimum species consumed locally as spices in Nigeria as a justification for increased domestication . American Journal of Plant Sciences.
2013;
4
:
222
.
View Article Google Scholar -
P. R.
Vieira,
S. M.
de Morais,
F. H.
Bezerra,
P. A. T.
Ferreira,
Í. R.
Oliveira,
M. G. V.
Silva.
Chemical composition and antifungal activity of essential oils from Ocimum species . Industrial Crops and Products.
2014;
55
:
267-271
.
View Article Google Scholar -
H. Y.
Yen G. C.Chen.
Antioxidant activity of various tea extracts in relation to their antimutagenicity . Journal of Agricultural and Food Chemistry.
1995;
43
:
27-32
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 9 (2017)
Page No.: 1574-1590
Published on: 2017-09-16
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 9559 times
- Download PDF downloaded - 2635 times
- View Article downloaded - 7 times
 Biomedpress
Biomedpress