Abstract
Background: According to modern concepts of burn disease development, one of the main burn complications is inflammation occurrence. Despite the large number of drugs used to treat the effects of chemical burns of the esophagus, we find conflicting information in the literature about their effectiveness. Moreover the problem of burn wound healing process quickening, as well as the prevention measures against possible post burn complications are really relevant if we aim to subjective portability of rehabilitation period facilitation.
Methods: Study the immune system cytokine levels in immature rat blood serum under the simulation of 1 and 2 degree esophageal chemical burns in case of melanin administration as a drug. Was estimated ELISA using sets of reagents and instructions from Biotrack ELISA System company «Healthcare».
Results: The significant increase of pro-inflammatory cytokines (PC) was shown mostly on day 7 of the experiment, which reflects the activity and severity of the disease process. In terms of further research the level of pro-inflammatory PC can be multidirectional. The significant decrease of anti-inflammatory cytokines amount was found under conditions of AEB at 15th and 21st days. At 1st and 2nd degree ABE the observed changes were multidirectional. Melanin administration resulted in the fast decrease of blood levels of all cytokines in our experiment to the values close to normal in conditions of esophagus chemical burn progress.
Conclusion: Therefore, it was shown that some factors have the ability of changing the levels of pro inflammatory and anti-inflammatory cytokines in the models 1st and 2nd degree ABE and AEB in immature rats. The obtained data showed, that 1st degree ABE progressed without septic complications. In the models of 2nd degree ABE and AEB, the risk of poly organic failure remains. Melanin administration resulted in the fast decrease of blood levels of all cytokines in our experiment to the values close to normal in conditions of esophagus chemical burn progress.
Introduction
According to modern concepts of burn disease development, one of the main burn complications is occurrence of inflammation. The main factor responsible for development of inflammation is weakening of the immune system weakening as well as compromise of skin barrier function. The significant role of the inflammatory response development is executed by immune system humoral factors of the specific and non-specific immunity, and by local actions of immune cells close to the source of antigenic information. These cells locally produce cytokines, as well as distinguish and kill other cells of the surrounding tissues Kazakov VNPaunel-Görgülü et al., 2012. The important function in the development of inflammation is performed by the pro-inflammatory cytokines, including interleukin (IL)-1β, IL-12, interferon (IFN)-gamma, IL-6, and tumor necrosis factor (TNF)-alpha) Zmushko EI, 2001. These cytokines are involved in the implementation of both specific and non-specific immunity Paunel-Görgülü et al., 2012Polikarpov A.V., 2011. The next processes (of burn wound healing) include subsiding inflammatory responses and increasing collagen synthesis.
Anti-inflammatory interleukins, such as IL-10 and IL-4, can reduce inflammatory manifestations Polikarpov A.V., 2011Ward P.A., 1999Zhou et al., 2012. Despite the large number of drugs used to treat the effects of chemical burns of the esophagus, we find conflicting information in the literature about their effectiveness. Indeed, accelerating the burn wound healing process and preventing possible post-burn complications are necessary for facilitating an effective rehabilitation Fistal E.Y 2004Hetyuhaylo, 2007Klimenko M.O, 2009. Thus, search of agents for chemical burn treatment is one of the main focuses of theoretical and practical medicine.
The use of non-toxic natural antioxidants as cyto-protectants are relevant Seniuk O. F., 2014. The protective effect of antioxidants has been widely studied (as well as any associated adverse factors) in various metabolic disorders. Analysis of recent data suggests that substances of natural origin based on polyphenolic compounds can be used as possible perspective treatment agents in normalization of biochemical parameters of 1st and 2nd degree EB. These substances include melanin, the eccrisis product of yeast-like fungi strain called Nadsoniella nigra strain X-1 Chyzhanska et al., 2007. These substances also have antioxidant Brenner and Hearing, 2008Keypour et al., 2008Romanovskaia et al., 2010 immune-modulating Chornenka N.M., 2016Racca et al., 2005 , anti-carcinogenic Golyshkin et al., 2015Seniuk et al., 2009, and stress-protective Chyzhanska et al., 2007Golyshkin et al., 2015 properties, which are beneficial for use in medicine.
In the study herein, the aim of the work was to evaluate immune system cytokine levels in immature rat blood serum under the simulation of 1st and 2nd degree EBs and using melanin as a protective drug.
Materials - Methods
Animal burn model
All experiments were performed following the general ethical principles of animal experiments approved by the first National Congress on Bioethics in Ukraine (and other national and international agreements/legislations of the area). We used nonlinear immature white female rats (~1 month of age) weighing 90-110 g (corresponding to 1-4 years of age in children) Gelashvili, 2008Raetska Ya.B., 2013. The animals were subjected to experimental AlEB simulation by administration with 10% (grade 1) and 20% (grade 2) sodium hydroxide (NaOH) solution. Some animals were subjected to AcEB (grade 2) using 30% trichloroacetic acid (TCA; CCl3СООН solution) Raetska Ya.B., 2013.
Experimental groups
The first part of our study was to separate the animals into the following groups:
(1) intact rats (n=8), (2) rats bearing 1st degree AlEB (n=28), (3) rats bearing 2nd degree AlEB (n=28), and (4) rats bearing 2nd degree AcEB (n=28).
In the second part of our study, animals are separated into 6 groups; Groups 1, 2 and 3 consisted of rats bearing 1st degree AcEB which were injected with melanin (starting from day 2 of the experiment) at a dose of 0.1 mg/kg, 0.5 mg/ kg, or 1 mg/kg, respectively, for 14 days (n=28 per group). Additionally, Groups 4, 5 and 6 consisted of rats bearing 2nd degree AcEB which were injected with melanin (starting from day 2 of the experiment) at a dose of 0.1 mg/kg, 0.5 mg/ kg, or 1 mg/kg, respectively, for 14 days (n=28 per group).
Source of Melanin
For our experiments we used melanin produced by the yeast-like fungi Nadsoniella nigra strain X1 from cliffs of Galindez Island Chyzhanska et al., 2007.
Serum preparation
Rats were evaluated on days 7, 15 and 21, according to the stage of burn disease Fistal E.Y 2004. The method of sacrificing the animals was cervical dislocation. Biochemical parameters were measured in serum of blood, following centrifugation of blood at 2000 g for 40 min.
Determination of cytokine level
Blood serum cytokine levels (IL-1beta, IL-12, IF-gamma, IL-6, TNF-alpha, IL-4 and IL-10) were evaluated by ELISA using reagents and instructions from the Biotrak ELISA System (GE Healthcare Life Sciences, Pittsburgh, PA). Determination of cytokine concentration was carried out on days 7, 15 and 21 days after chemical burn infliction. Immunoassay analysis was performed in accordance to standard protocols with certain modifications Chornenka NM, 2016. The 100 μl samples of blood serum were incubated in wells of 96-well plates overnight at 4°C. To block non-specific binding sites after washing, the plates were subsequently incubated (at 37°C) with 5% skimmed milk for 1 hour. After that, probes were washed with 0.05 M Tris-HCl buffer solution containing 0.1% Tween-20 and again with 0.05 M Tris-HCl buffer solution. After that, the highly specific primary antibodies were added and samples were incubated for 1 hour at 37°C. Next, samples were incubated with appropriate secondary antibodies. Between all these steps, the probes were washed twice with 0.05 M Tris-HCl buffer solution with 0.1% Tween-20. The substrate for the peroxidase reaction was o-phenylenediamine/hydrogen peroxide (Sigma-Aldrich, St. Louis, MO). The spectral measurements were set at 492 nm.
Statistical analysis
Statistical analysis of the results was performed using variation statistics with the aid of ANOVA. To determine the reliability of differences between two given samples we used the student t-test (t). Differences were considered significant if p < 0.05.
Results
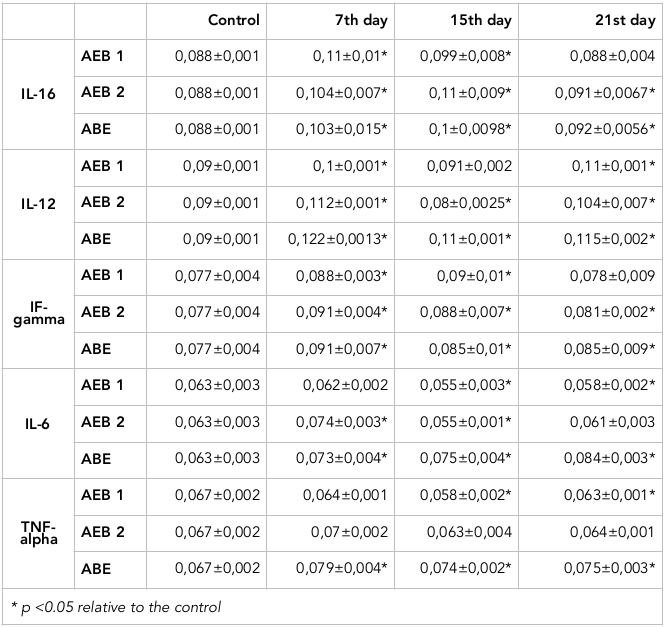
We have evaluated the levels of pro-inflammatory cytokines, including IL-1beta, IL-12, IF-gamma, IL-6, and TNF-alpha ( Table 1 ). The significant increase of pro-inflammatory cytokines was seen around day 7 of the experiment, which reflects the rapid severity of the burn and the associated inflammatory activity.
From our models, the level of IL-1β after 1st and 2nd degree AlEB and 2nd degree AcEB increased on days 7, 15 and 21- by 18.2%, 17% and 25% and 13.6% respectively, when compared to the control. The most significant change was observed after 2nd degree AcEB and 2nd degree AlEB on days 7 and 15. After 1st degree AcEB the most prominent increase occurred at days 7 and 15 by 25% and 12.5%, respectively. However, at day 21 the IL-1β level approached the reference value.
Regarding IL-12, after 1st degree AlEB induction the IL-12 concentration increased by 11% and 22% at days 7 and 21, respectively, when compared to the control. However, after 2nd degree AcEB and 2 nd degree AlEB, the level of IL-12 on days 7 and 21 increased by 24.4%, and 35.6%, and 15.6% and 27.8%, respectively. At day 15 following 2nd degree AcEB the level of IL-12 decreased by 11% in comparison to the control.
From the models of 1st and 2 nd degree AcEB and 2 nd degree AlEB, there was significant increase of IFN-gamma on day 7. The highest values were observed in the models of 2nd degree AcEB and AlEB on the 7th day; the level increased by 18% and 18.2%, respectively, in comparison to the control. For 1st degree AcEB the most prominent IFN-gamma increase (17%) occurred at the 15th day of the experiment.
The levels of TNF-alpha in the 2nd degree AlEB model were 18%, 10.5% and 12% higher on days 7, 15 and 21, respectively, in comparison to the control values. In the case of 1st and 2nd degree AcEB, the decrease of TNF-α level was clearly evident.
As for the levels of IL-6 in our burn models, we found that after 2nd degree AcEB and AlEB induction there was an increase of IL-6 levels on days 7, 15 and 21. The most significant increase of occurred in the 2nd degree AlEB model on days 7, 15 and 21- by 16%, 19% and 33%, respectively, when compared to the control values.
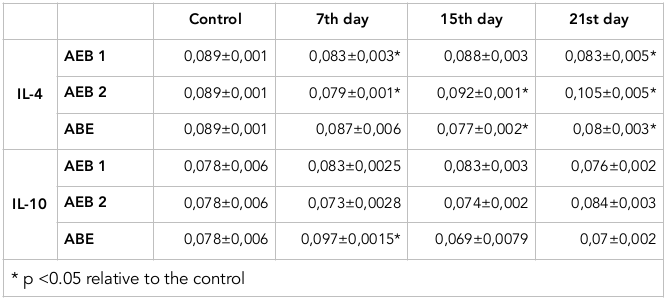
Furthermore, we investigated the levels of anti-inflammatory cytokines, including IL-4 and IL-10. These cytokines regulate specific immune responses and limit the development of inflammatory processes ( Table 2 . The significant decrease of levels of these cytokines were found after AlEB induction at days 15 and 21.
Regarding IL-4, after 1st degree AcEB induction the IL-4 concentration decreased by 7% at day 7, compared to the control values. After 2nd degree AcEB, the level of IL-4 decreased by 11.2% on day 7. On the 15th and 21th days, IL-4 increased by 4% and 18%, respectively. Additionally, after 2nd degree AlEB induction, the level of IL-4 decreased by 13.5% and 10% on days 15 and 21, respectively.
The level of IL-10 in the blood serum of immature rats also showed significant changes, but only in the 2nd degree AlEB model. It increased by 24.4% on the 7th day, but demonstrated diminished values on the 15 th and 21 st day, with a decrease of 11.5% and 10.3%, respectively, in comparison to the control values.
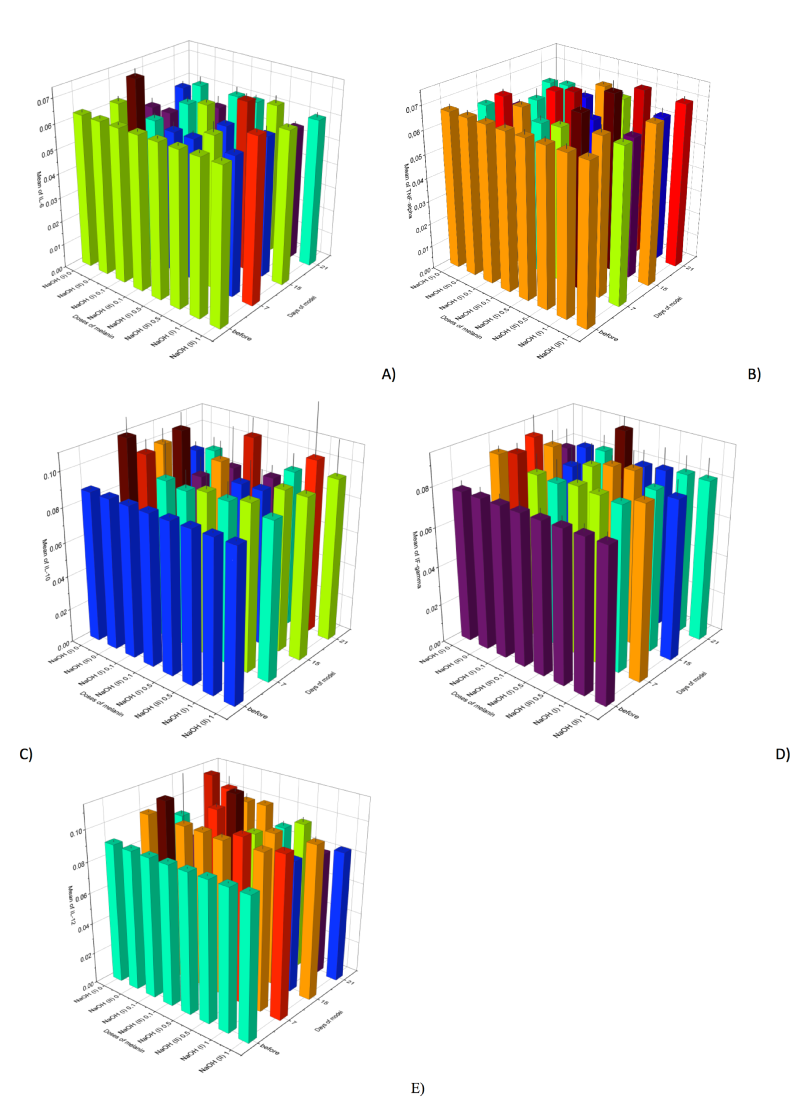
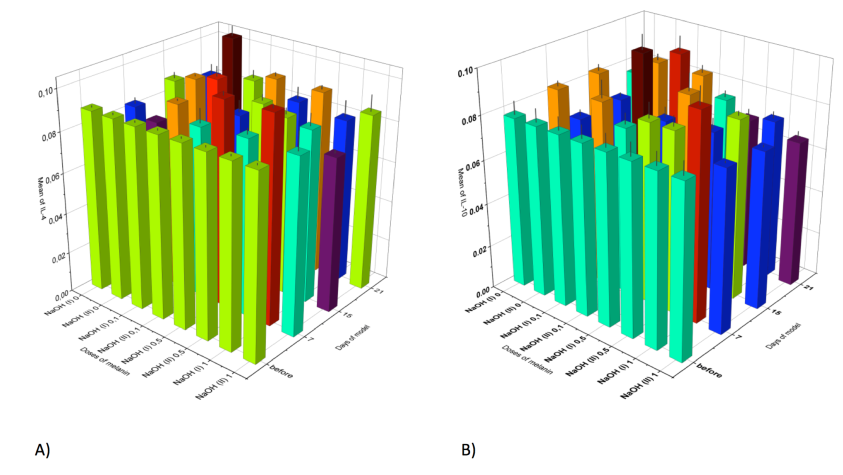
The second stage of our work was to estimate the cytokine profile in blood serum of rats in the model of esophagus chemical burns under the influence of an anti-oxidant drug (melanin) administered at different doses ( Figure 1 , Figure 2 ).
It has already been established that melanin is able to decrease levels of pro-inflammatory cytokines in treated animals, when compared to those that did not receive the drug. This decrease is observed mainly on the 7th day of the experiment, and all values still remain above that of the control. As for the pro-inflammatory cytokines, their level changes were diverse.
For instance, under conditions of melanin administration the level of IL-1beta in the model of 1st and 2 nd degree AcEB was lower than that of the group of the animals not receiving the drug but still remained above the control values. The significant changes of IL-1beta levels were observed after melanin administration at a dose of 1 mg/kg after 1st and 2nd degree AcEB induction. IL-1beta decrease was most evident on days 7 and 15- by 12.7% and 5%, and 12.5% and 14.6%, respectively, compared to animals with 1st and 2nd degree burns which did not receive melanin.
Evaluation of IFN-gamma levels after melanin administration showed lower values of IFN-gamma, compared to the group of animals that did not receive the drug on days 7 and 15 of the experiment. However, the level still remained above the control values (for those animals with 1st and 2nd degree AcEB that did not receive melanin).
The level of TNF-alpha after the melanin administration varied. In the case of 1st degree AcEB, the TNF-alpha concentration was reduced by 7.5% on the 15th day (using a melanin dose of 0.5 mg/kg) and by 10.5% (using a dose of 1 mg/kg), compared to the control. As for 2nd degree AcEB, from administration of 0.5 mg/kg melanin, TNF-alpha level increased by 9% on day 7 and by 12% on day 15, compared to intact (control) rats. The comparison of animals with 1st and 2nd degree burns showed no significant change of TNF-alpha.
Moreover, the level of IL-6 after melanin administration also varied. After 1st degree AcEB, and administration of melanin at a dose of 0.5 mg/kg, IL-6 was significantly reduced at days 7 and 15 by 6.5% and 8.1%, respectively. At a melanin dose of 1 mg/kg, IL-6 was reduced by 8.1%, 6 and 5%, respectively, in comparison to animals with 1st degree ACEB but without melanin administration. The level of IL-6 in the 2nd degree AcEB model in the presence of 0.5 mg/kg melanin was seen to decrease on day 7 by 7%; in the presence of 1 mg/kg melanin, it decreased on day 7 by 9.5%, in comparison to the animals with 2nd degree burns.
The IL-4 levels after administration of melanin were also varied. In the 1st degree AcEB model the level of IL-5 at day 7 was significantly increased in animals receiving melanin at doses of 0.5 mg/kg and 1 mg/kg; the increase was 20% and 21.7% higher, respectively, in comparison to the animals with 1st degree ACEB (but without melanin administration). As for the 2nd degree ACEB model the level of IL-4 was found to significantly increase at day 7 and 21- by 9% and 15.2%, respectively, at the 0.5 mg/kg melanin dose. At the 1 mg/kg melanin dose, at day 7 and 21 the levels increased by 9% and 10.1%, respectively, in comparison to the animals with 2nd degree ACEB (but without melanin administration).
Melanin administration also had an ambiguous effect on the level of IL-10. After 1st degree AcEB induction, IL-10 significantly increased by 5.2% and 10.3%, on 7th and 15th days, respectively, following melanin administration at a dose of 0.5 mg/kg, compared to intact animals. No significant changes in the 1st degree AcEB animals were observed. For the 2nd degree ACEB rats, the level of IL-10 was increased by 12.3% on day 7 in the presence of melanin at administration).
Discussion
It has already been shown that under burn conditions, organism are capable of eliciting non-specific inflammatory reactions, leading to cytokine synthesis Fistal E.Y 2004Paunel-Görgülü et al., 2012Zhou et al., 2012. The severity of these reactions depends on the area and depth of tissue damage, as well as the characteristic of the infectious agent and resulting immune responses activity. For the periods of early and late toxemic reactions, a decrease in oxygen transport activity occurs which can lead to other issues. The process of prolonged tissue hypoxia stimulates endothelial cells to release various inflammatory mediators and mitogens. These mediators of inflammation mediators are produced during neutrophil/granulocyte adhesion, and initiate non-specific proliferation of smooth muscle cells. During adhesion, leukocytes are activated which can lead to the release of free radicals and proteases (which are able to impair important biological molecules). Active proliferation of smooth cells can induce their phenotypic changes with subsequent loss of the physiological contractile ability. In addition, venous stasis causes cerebral ischemia and cytotoxic swelling with resulting metabolic acidosis Fistal E.Y 2004Zmushko EI, 2001.
Cytokines exert their effects on almost all cells and play key roles in progression of inflammation by modulating granulocytes, macrophages, fibroblasts, endothelial cells, epithelial cells, T-lymphocytes, and B- lymphocytes Belardelli, 1995. Their role in progression of inflammatory trauma both at the local and systemic levels is nearly impossible to overestimate. In strong inflammatory conditions cytokines can enter bloodstream, eliciting acute responses from various tissue organs. The most important role in the inflammation process belongs to pro-inflammatory cytokines.
These days, the treatment of chemically-induced inflammation for esophageal conditions presents some difficulties. Immune deficiency lessens the effect of therapeutic approaches, leading to conditional activation of pathogenic microflora; as a consequence, this impedes regeneration and promotes severe complications to patients. Thus, the recovery of functional immunity is a very important step in complex therapy of chemical-induced esophageal burns. The possibility of regulation of immunocompetent cell interactions, as well as correction of early cytokine level shifts, are the main priorities for EB therapeutic approaches Kovalenko et al., 2014.
The use of non-toxic natural antioxidants as cytoprotectors is relevant these days Seniuk O. F., 2014. The protective effect of antioxidants is widely studied, as are the influence of adverse factors, in several metabolic disorders. Analysis of recent data from the literature suggests that substances of natural origins based on polyphenolic compounds are possible as remedy for the normalization of biochemical parameters after 1st and 2nd degree esophageal burns. In this regard, there is huge interest in exploring the possible use of the drug melanin.
This substance appears to be one of the most powerful antioxidants; it prevents collagen degradation and promotes microcirculation Beregova T.V. , 2014Falalyeyeva T.M. , 2009. As well, melanin can be regarded as a gastro-protector and a powerful anti-stress agent Holyshkin D.V 2014.
Our main interest of our work was to evaluate pro-inflammatory cytokines (such as IL-1beta, IL-12, IFN-gamma, IL-6, TNF-alpha) which enhance the proliferation of T- and B- lymphocytes, antibody synthesis, production of adhesion molecules, and synthesis of acute phase proteins. Additionally, they are involved in processes of specific and non-specific immunity.
IL-1beta is one of the crucial cytokines for wound defense and regulation. The data obtained for IL-1beta levels in blood serum show that IL-1beta increases compared to intact animals ( Table 1 ). Our data are consistent with the literature, which also show increases in IL-1beta concentration in rats with inflammatory wounds [4]. It is also known that IL-1beta plays a critical obligatory role in connective tissue metabolism; namely, it stimulates fibroblast proliferation and prostaglandin production as well as increases the activity of growth factors and synthesis of cytokines. IL-1beta in the connective tissue cells also stimulates collagen production and synthesis of some enzymes. However, wound healing can result in hypertrophic or keloid scars with granulated tissue appearance Matevosyan J. R., 2013Reva I.V., 2013. Our experiments with melanin administration at various doses showed that there was a decrease of IL-1beta in animals with inflammatory wounds. This can be promising in regards to diminishing the amount of scar tissue formation during wound healing.
IFN-gamma and TNF-alpha are known to express immunomodulatory effects; they are considered as cell immunity inductors which are functionally connected to other crucial pro-inflammatory cytokines. Herein, we showed that in inflammatory conditions the amount of IFN-gamma increases on the 7th day of experiments. Use of melanin did not elicit any significant decrease IFN-gamma, when compared to the group of animals not receiving the drug. At the same time, the comparison to the control group showed that there was an increase of IFN-gamma. It is possible that one of the mechanisms of IFN-gamma increase in blood serum may be due to the restoration of a number of functionally active T-
lymphocytes, including CD4+ cells, which are involved in various cytokine productions. However, the role of other factors also cannot be excluded, such as the level of endogenic intoxication changes, endotheliocyte functional activity and so forth Reva I.V., 2013.
In the progress of inflammation a critical role is played by TNF-alpha. This cytokine stimulates T- and B- lymphocyte proliferation, antibody production, adhesion molecule synthesis, and acute phase protein activation. It is also involved in specific and non-specific immunity Gorbach O., 2013Zmushko EI, 2001. Our study showed that in conditions of AlEB, TNF-alpha concentration was increased at all time points. This also occurred in conditions of 1st and 2nd degree AcEB. The administration of melanin led to various (multidirectional) changes to TNF-alpha levels.
The basic producers of IL-12 are monocytes, macrophages, neutrophils and active lymphocytes. Microbial components and products are the main inductors of IL-12 synthesis. It has already been shown, that this cytokine is crucial for immune response amplification as well as initiation of protective mechanisms against infections. The basic target cells for IL-12 are natural killers and T-lymphocytes. This cytokine activates T-lymphocyte differentiation and increases their cytotoxic activity Zhou et al., 2012. The increase of IL-12 levels in our models of AlEB and AcEB was evident. The decrease of IL-12 level seen on day15 in the 2nd degree AcEB model can be a sign of poly-organic insufficiency Köller et al., 1997Wick et al., 2000. The addition of melanin at a dose of 1 mg/ kg, in the 1st and 2nd degree inflammation conditions, led to IL-12 decrease on day 21 of the experiments.
Some researchers have focused their attention on increased IL-6 levels during sepsis Pileri et al., 2008Zhou et al., 2012. In our study, we showed that there was an increase of IL-6 under ALEB and ACEB conditions. After melanin administration, we observed a decrease of this cytokine level, as compared to control animals not receiving melanin. This demonstrates the role of melanin as a possible guard against sepsis complications Chornenka N.M., 2016. It was established that one of the factors promoting the IL-6 increase in the inflammation trauma may very well be the increase of IL-4 (negative correlation). Therefore, changes in IL-4 levels can be regarded as a prognostic factor for complications.
Moreover, the aim of our work was to evaluate the levels of anti-inflammatory interleukins (such as IL-4 and IL-10). These cytokines are produced by TH2 and TH3 cells, and are antagonistic to pro-inflammatory cytokines. Thus, these molecules are able to inhibit pro-inflammatory cytokine activity, suppress T-lymphocyte proliferation, and participate in immune responses to various antigens. They also down-regulate IL-1, TNF-alpha, nitric oxide and prostaglandins, which can lead to inflammatory manifestations.
From our study, it was shown that on day 7 in the 2nd degree AcEB model the level of IL-4 decreased, compared to the control values. The level of this cytokine on days 15 and 21 in the 2nd degree AlEB model continued to decrease. Some research studies have shown that there is an increase of IL-4 during sepsis Messingham et al., 2001Polikarpov A.V., 2011. In the case of melanin administration, we observed an increase of IL-4 concentration which occurred mainly on day 7. Therefore, it can be assumed that melanin administration, in fact, can increase the risk of septic complications.
The levels of certain cytokines in rat blood under inflammatory or traumatic conditions can be regarded as a pathophysiological step of damage accompanied by polyorganic insufficiency of sepsis Agay, 2008. A group of researchers from China have already demonstrated the prognostic role of IL-10 levels in toxemia Zhou et al., 2012. High levels of IL-10 can be related to high risk of septic complications provoked by inflammation Lyons et al., 1997Napolitano and Campbell, 1994Raetska, 2016. Studies of IL-10 levels in immature rat blood serum showed an increase of this cytokine mostly on day 7 in the 2nd degree AlEB model, in comparison to intact rats. In the models of 1st and 2nd degree AcEB, this level had no significant changes at any time point; melanin administration also elicited no changes to this.
Conclusion
Overall, it was shown that some factors have the ability to modulate the levels of pro-inflammatory and anti-inflammatory cytokines in our models of 1st and 2nd degree AcEB and AlEB in immature rats. Our data show that 1st degree AcEB progressed without septic complications. In the models of 2nd degree ACEB and ALEB, the risk of polyorganic failure remains. Melanin administration resulted in a rapid decrease of chemical-mediated esophageal burns.
Abbreviations
ABE: acid burns the esophagus
AEB: alkali esophageal burn
IF: interferon
IL: interleukins
TNF: tumor necrosis factor
Author Contribution
Ya. B. Raetska - the experiment planning, article planning and writing; N.M. Chornenka measurement of the indicators presented in the article; T.V Koval - measurement of the indicators presented in the article; O.M Savchuk - the experiment planning, article planning and writing; T. V Beregova - the experiment planning, melanin preparation; L.I. Ostapchenko - the experiment planning, melanin preparation. All authors reviewed and commented on final draft.
References
-
D.
Agay,
M.
Andriollo-Sanchez,
R.
Claeyssen,
L.
Touvard,
J.
Denis,
A. M.
Roussel,
Y.
& Chancerelle.
Interleukin-6, TNF-alpha and interleukin-1 beta levels in blood and tissue in severely burned rats. European Cytokine.
2008;
Network
:
19(1), 1-7
.
PubMed Google Scholar -
F.
Belardelli.
Role of interferons and other cytokines in the regulation of the immune response . APMIS.
1995;
103(3)
:
161-179
.
PubMed View Article Google Scholar -
T.V. S.A.A.
Beregova,
K.S
Neporada.
The impact on the activity of melanin no-ergic system in the tissues of the salivary glands under the conditions hiperhastrynemiyi. World medicine and biology.
2014;
1
.
-
M.
Brenner,
V. J.
Hearing.
The protective role of melanin against UV damage in human skin . Photochemistry and Photobiology.
2008;
84(3)
:
539-549
.
PubMed View Article Google Scholar -
N.M.
Chornenka,
Y.B.R.,
Savchuk
O.M.,
Torgalo
E.O.,
Beregova
T.V.,
Ostapchenko.
L.I..
Correction Parameters of Endogenous Intoxication in Experimental Burn Disease at the Stage of Toxemia. RJPBCS.
2016;
7
:
1042
.
-
N. M. R.Y.B.
Chornenka,
OM
Savchuk,
EO
Torgalo,
TV
Beregova,
LI.
Ostapchenko.
Correction Parameters of Endogenous Intoxication in Experimental Burn Disease at the Stage of Toxemia. Research Journal of Pharmaceutical, Biological and Chemical Sciences.
2016;
7
:
1042-1047
.
-
N.
Chyzhanska,
O.
Tsyryuk,
T.
Beregova.
The level of cortisol in the blood of rats before and after stress action against the background of melanin. Visnik of problems of biology and medicine.
2007;
1
:
40-44
.
-
T.M. T.O.I.
Falalyeyeva,
N.V.
Chyzhanskaya,
V.P.
Zharova.
The influence of melanin on cortisol blood level in the rats in conditions of stress action. Ukrainian Antarctic Journal.
2009;
:
391-394
.
-
E. Y. G. P. K.
Fistal.
G.E Samoilenko et al. Kharkov: Combustiology.
2004
.
-
Gelashvili.
Option of periodization of biologically similar stages of human and rats ontogenesis. Saratov scientific-medical journal.
2008;
4
.
-
D.
Golyshkin,
T.
Falalyeyeva,
N.
Chyzhanska,
T.
Beregova.
LI Ostapchenko White blood count of rats under stress-induced stomach lesions and the prophylactic administration of melanin. Ukrainian Antarctic Journal.
2015;
114
.
-
O. K.N.
Gorbach,
O.
Skachkova.
Immunological biomarkers as surrogates for clinical response for dendritic cells vaccine therapy for non-small cell lung cancer and renal cell carcinoma. Paper presented at: Materials of Joint European Cancer Congress (Amsterdam, Netherlands).
2013
.
-
Hetyuhaylo.
Mechanisms of burn disease and justification of the drug "Kriohor" for its treatment. In Pathologic Physiology (Kharkiv: Kharkiv).
2007
.
-
D. V. F.T.N.
Holyshkin,
K.S
Neporada,
T.V.
Beregova.
Influence of melanin on the state of the gastric mucosa and the reaction of the hypothalamic-pituitary-adrenal axis under conditions of acute stress action. The Journal of Physiology.
2014;
61
:
65-72
.
-
VN M.B.
Kazakov,
AG
Bullfinch.
Ways of interaction between the nervous, endocrine and immune systems in the body's regulation of funktsiy. Archive for Clinical and Experimental Medicine.
;
13
:
3-10
.
-
S.
Keypour,
H.
Riahi,
M.-F.
Moradali,
H.
Rafati.
Investigation of the antibacterial activity of a chloroform extract of Ling Zhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst.(Aphyllophoromycetideae), from Iran. International Journal of Medicinal Mushrooms.
2008;
10
.
-
M.O N.L.G.
Klimenko.
Burn disease (pathogenesis and treatment). 2009
.
-
M.
Köller,
A.
David,
M.
Hahn,
G.
Muhr.
Liberation of interleukin 12 (IL12) after trauma and polytrauma. Paper presented at: Langenbecks Archiv fur Chirurgie Supplement Kongressband Deutsche Gesellschaft fur Chirurgie Kongress.
1997
.
-
O.M.
Kovalenko,
D.V.
Mal'tsev,
V.
Kazmirchuk,
A.O.
Kovalenko.
[Studying of cytokine dynamics in injured persons with severe burns for estimation of severity and prognosis]. Klinichna khirurhiia.
2014;
:
49-53
.
-
A.
Lyons,
J. L.
Kelly,
M. L.
Rodrick,
J. A.
Mannick,
J. A.
Lederer.
Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection . Annals of Surgery.
1997;
226(4)
:
450-458
.
PubMed View Article Google Scholar -
J. R. M.H.A.
Matevosyan.
The influence of interleukin-1β and its receptor antagonist on healing of pyloroduodenal ulcers. Medicine science Armenia.
2013;
53(3)
:
130-133
.
-
K. A.
Messingham,
S. A.
Heinrich,
E. J.
& Kovacs.
Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. Journal of Leukocyte.
2001;
Biology
:
70(6), 887-895
.
PubMed Google Scholar -
L. M.
Napolitano,
C.
Campbell.
Nitric oxide inhibition normalizes splenocyte interleukin-10 synthesis in murine thermal injury . Archives of Surgery (Chicago, Ill.).
1994;
129(12)
:
1276-1282
.
PubMed View Article Google Scholar -
A.
Paunel-Görgülü,
T.
Kirichevska,
T.
Lögters,
J.
Windolf,
S.
& Flohé.
Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis . Molecular Medicine (Cambridge, Mass.).
2012;
18(3)
:
325-335
.
PubMed View Article Google Scholar -
D.
Pileri,
A.
Accardo Palombo,
L.
D’Amelio,
N.
D’Arpa,
G.
Amato,
A.
Masellis,
C.
Conte.
Concentrations of cytokines IL-6 and IL-10 in plasma of burn patients: Their relationship to sepsis and outcome. Annals of Burns and Fire.
2008;
Disasters
:
21(4), 182-185
.
PubMed Google Scholar -
A.V. P.E.E.
Polikarpov.
Comparative study of the dynamics of pro-inflammatory and anti-inflammatory cytokine levels in skin burns razlichnoyprirody. Bulletin of the University of Kharkiv Series: Biology..
2011;
971
:
27-32
.
-
S.
Racca,
A.
Spaccamiglio,
P.
Esculapio,
G.
Abbadessa,
L.
Cangemi,
F.
DiCarlo,
P.
Portaleone.
Effects of swim stress and α-MSH acute pre-treatment on brain 5-HT transporter and corticosterone receptor . Pharmacology, Biochemistry, and Behavior.
2005;
81(4)
:
894-900
.
PubMed View Article Google Scholar -
Ya.B.
Raetska,
T.V.I.,
Savchuk
O.M.,
Ostapchenko.
L.I..
Experimental modeling of first-degree chemically-induced esophageal burns in rats. Medicinal Chemistry (Shariqah , United Arab Emirates).
2013;
15
:
30-34
.
-
Y. B.
Raetska.
Proinflammatory and anti-inflammatory cytokines level in rat serum under modelinf of esophagus alkali burns of 1st and 2nd degrees. Scientific Journal Visnik Biology..
2016;
70
:
67-70
.
-
I.V.
Reva,
R.G.V.,
T.
Yamamoto.
Interaction of immunocites in the reparative regeneration of the skin. Basic research.
2013;
:
453-459
.
-
V.
Romanovskaia,
A.
Tashirev,
S.
Shilin,
N.
Chernaia.
Resistance to UV radiation of microorganisms isolated from the rock biotopes of the Antarctic region. Mikrobiolohichnyi zhurnal (Kiev, Ukraine: 1993).
2010;
72
:
8
.
-
O. F.
Seniuk,
G.L.F.,
L. A.
Palamar,
N. I.
Krul.
Effects of melanin-glucan complex, isolated from polypore fungi, on the lifespan of female icr mice. Probl aging and longevity.
2014;
23
:
11-27
.
-
O.F.
Seniuk,
L.F.
Gorovoj,
V.
Kovalev,
L.A.
Palamar,
N.I.
Krul,
V.P.
Kurchenko.
Anticarcinogenic Properties of Melanin-Glucan Complex from Higher Fungi. Paper presented at: Proceedings of the 5~(th) International Medicinal Mushroom Conference.
2009
.
-
P. A.
Ward,
A. B.
& Lentsch.
The acute inflammatory response and its regulation . Archives of Surgery (Chicago, Ill.).
1999;
134(6)
:
666-669
.
PubMed View Article Google Scholar -
M.
Wick,
E.
Kollig,
M.
Walz,
G.
Muhr,
M.
Köller.
Does liberation of interleukin-12 correlate with the clinical course of polytraumatized patients?. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen.
2000;
71
:
1126-1131
.
-
H.
Zhou,
J.
Tu,
Y.
Huang,
X.
Chen,
Y.
Deng.
Changes in serum contents of interleukin-6 and interleukin-10 and their relation with occurrence of sepsis and prognosis of severely burned patients. Zhonghua shao shang za zhi= Zhonghua shaoshang zazhi= Chinese journal of burns.
2012;
28
:
111-115
.
-
EI B.E.
Zmushko,
Yu
Mitin.
Clinical immunology. Guidelines for doctors. (CПб).
2001
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 9 (2017)
Page No.: 1591-1606
Published on: 2017-09-20
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8554 times
- Download PDF downloaded - 2432 times
- View Article downloaded - 8 times
 Biomedpress
Biomedpress