Abstract
Introduction: Pathological animal models provide the foundation for developing new methods for disease treatment. This research aims to establish a rabbit model of femoral head necrosis.
Methods: Osteonecrosis of the femoral head (ONFH) was induced in rabbits by using methylprednisolone (MPS) combined with Complete Freund's Adjuvant (CFA). New Zealand White rabbits were divided into two groups. ONFH group (n=10) was given an intramuscular injection of 0.5 mg/kg CFA and 40 mg/kg methylprednisolone. Normal group (n=6) received normal saline at the same location and same volume as those in ONFH group. The efficiency of the ONFH rabbit model was assessed at week 7 after the last injection. Body weight was detected, and the histological structure of head femoral and bone were assessed by H&E staining. The empty lacunae were counted. Cartilage degeneration was evaluated using image analysis software. Blood vessel density was assessed after ink artery infusion. The cell cycle of bone marrow-derived mononuclear cells was analyzed by flow cytometry.
Results: The results showed that there was no difference in body weight changes of rabbits between the two groups. However, the bone morphology and cartilage surface of the femoral head showed abnormalities in the ONFH group. The percentage of empty osteocyte lacunae was significantly higher in ONFH group than normal group. Chondrocyte degeneration and fibrocartilage expression were observed in the ONFH group. Compared to the normal group, the ONFH group had less ink-stained blood vessels. However, the fraction of bone marrow-derived mononuclear cells in S phase and G2/M phase of the cell cycle was significantly increased in the ONFH group.
Conclusion: Thus, CFA combined with MPS for 7 weeks can be used to establish an early-stage femoral head necrosis model in rabbits.
Introduction
Osteonecrosis of the femoral head is defined as the death of bone and bone marrow cells. As a result, blood supply is disrupted and the femoral head is destroyed, leading to an increase in intraosseous pressure. The main causes include alcohol, trauma, steroid-based medicine for autoimmune diseases, and chronic inflammation Mont and Hungerford, 1995. There is a relationship between glucocorticoid and bone death, which appear due to blood stasis and ischemia in the trabecular bone, and in autoimmune diseases. Increase of osteocyte apoptosis owing to micro damage in the bone could have a role too Mendiratta et al., 2008. The most common risk factors for osteonecrosis are the use of corticosteroids, especially in high doses (independent variable). Studies suggest that long-term use of oral or intravenous (IV) corticosteroids is associated with non-traumatic osteonecrosis Yamamoto et al., 1995Yamamoto et al., 1997.
Although the disease can be treated by conservation or surgical methods, the results are not as expected. This causes great inconvenience to the patient. Early diagnosis of the disease and appropriate treatment are important to protect the integrity of the femoral head. It is important to establish an animal ONFH model with high reproducibility of necrosis. Furthermore, it is important to deepen the understanding of the prevention, diagnosis and treatment of steroid-induced ONFH.
Many studies have reported that the use of glucocorticoid alone or in combination with certain substances, such as LPS or horse serum, can induce early avascular necrosis of the femoral head Li et al., 2004Wen et al., 2008a. CFA is widely used in animal models of osteoarthritis or autoimmune diseases Hirose and Tanaka, 2011McCarson, 2015. Freund's supplement is an emulsified solution in mineral oil and used as an agent to stimulate immune responses and enhance the production of pro-inflammatory elements (e.g. cytokines IL-1β, TNF-α and IL-6).
The aim of this study was to establish an experimental model of ONFH in rabbits induced by a combination of MPS and CFA.
Materials - Methods
Experimental animals
All experimental rabbits were fed in a single cage and received a standard laboratory diet. Experimental protocols and animal care methods were approved by the Animal Experimentation Ethics Committee of the Stem Cell Institute, University of Science, Vietnam National University, Ho Chi Minh City, Vietnam.
Establishment of a rabbit model of ONFH
Male New Zealand white rabbits (28 weeks of age), weighing 2.5-3.5 kg, were separated into two groups. The ONFH group (n=10) was injected in the gluteus medius site with a dose of 0.5 mg/kg CFA (Santa Cruz, USA) at day 1 and 40 mg/ kg MPS (Pfizer, New York city, NY) at day 7. The normal group (n=6) received 0.9% saline at the same location and volume as those in the ONFH group. Rabbits were assessed at week 7 after the last injection. All rabbits were followed to record any body weight changes.
Morphological and histopathological assays
Seven weeks later, rabbits were sacrificed. Femoral head and thighbone were collected. The morphology was observed and recorded using a camera.
In the next step, both femoral head and thighbone were fixed in 10% paraformaldehyde for 3 days. After that they were soaked in 10% disodium ethylene diamine triacetic acid (Na2-EDTA) to decalcify. Na2-EDTA solution was refreshed every 2 days until sufficient demineralization was achieved. Demineralization was detected by stabbing a needle to the bone and observing the surface color change of samples. The samples were then dehydrated in 30% sucrose solution. After 2 days, the samples were cut into 7-10 µm-thick sections and stained with hematoxylin-eosin and safranin O. The changes in the histological structure of the periosteum, trabeculae and cartilage were observed using a microscope. Ten random fields were chosen to count the number of empty osteocyte lacunae. Bone damage level was detected by the rate of empty osteocyte lacunae.
Ink artery infusion angiography
Ink artery infusion angiography was conducted according to the method of Wen Qian et al. Wen et al., 2008b. Briefly, the rabbit was anesthetized and injected with heparin (5,000 IU/mL) into an ear vein. The abdomen was operated to expose the abdominal aorta and the abdominal vein was cut. A 12-gauge syringe was inserted in the opposite direction of the heart. About 2,000 mL of physiological saline solution was pumped to wash away the blood from the body. Then, about 100 mL of gelatin and ink mixture (at a rate of 7:3 in phosphate-buffered saline) were injected continuously until the skin and nails became uniformly black. Viscera from the rabbit was removed and kept at 4°C overnight. Over the next day, the femur was collected and fixed in 10% paraformaldehyde for 3 days. Subsequently, samples were decalcified as described above and cut into 30 µm-thick sections and blood vessel density was observed using a microscope.
Cell Cycle
To assess the effect of CFA and MPS on the proliferation and apoptosis of nuclear cells in bone marrow, rabbits received epidural anesthesia using 3% Novocain (Hanvet, Ho Chi Minh city, Vietnam). The bone marrow was harvested through a hole in the top of the ilium with a 18-gauge biopsy needle. Bone marrow-derived nuclear cells were enriched by incubation in hemolysis solution. Nuclear cells were fixed in 70% ethanol for 24 hours and stained with propidium iodide (Sigma-Aldrich, Louis St, MO) for 10 minutes. Cell cycle was analyzed by flow cytometry.
Statistical Analysis
All data were analyzed using Graphpad Prism 6.0 software. Images were analyzed using ImageJ software.
Results
Morphological changes of the cartilage and bone
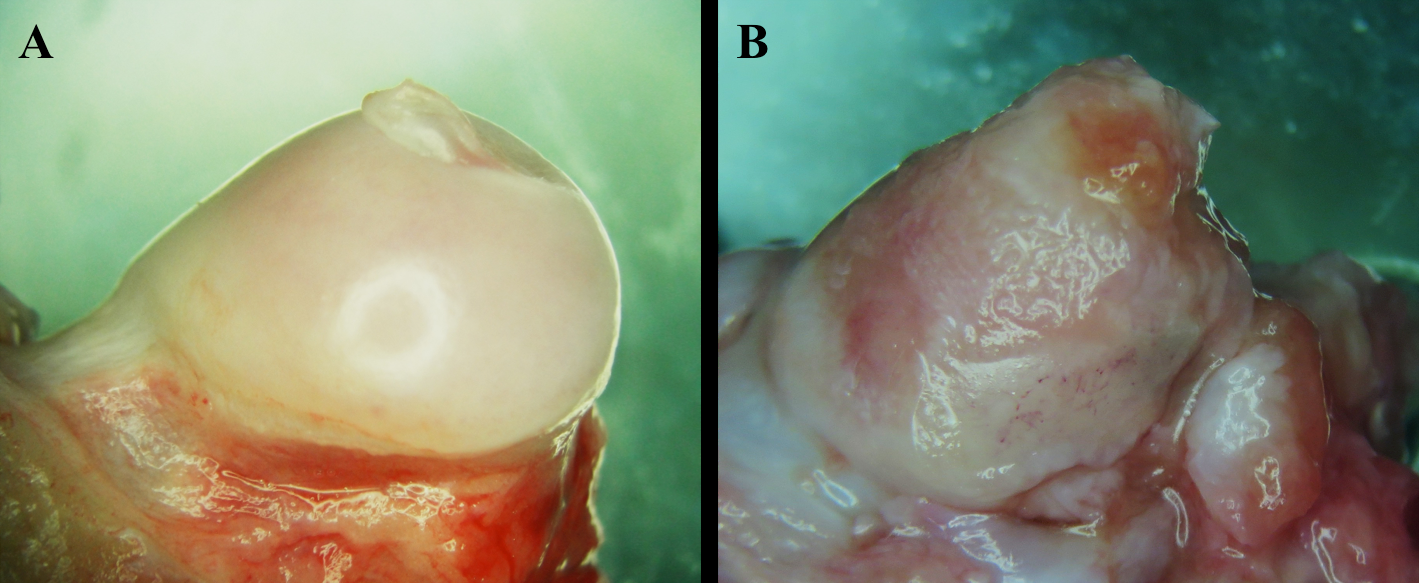
After 7 weeks of drug administration, bone morphology had significant changes in normal rabbits compared to treated rabbits ( Figure 1 ). The femoral head was shiny, smooth and opalescent in the normal group. In contrast, in the treatment group, the cartilage surface of the femoral head was slightly rough, uneven and pink.
Osteocyte damage
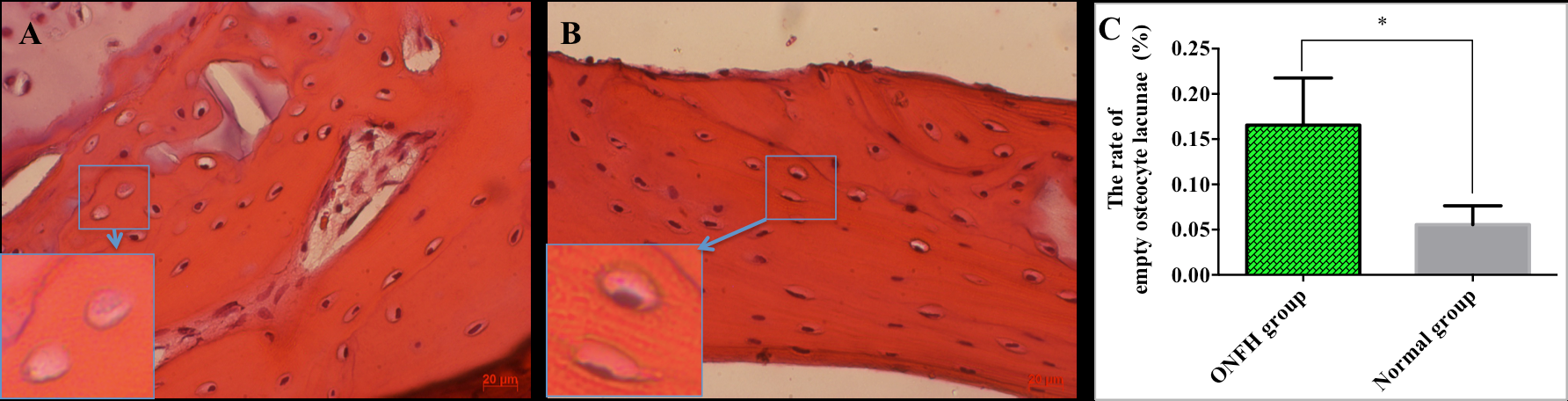
In group B, almost all osteocyte lacunae were filled with cells that was identified by the presence of oval-shaped nuclei inside ( Figure 2B ). In contrast, the number of empty lacunae was displayed at many bone sites in the ONFH group. The percentage of empty osteocyte lacunae was significantly higher in ONFH group than normal group (p <0.05). The percentage of empty bone lacunae defects in the normal group was 0.06 ± 0.01%, while for the ONFH group it was 0.17 ± 0.02% ( Figure 2 ).
Proteoglycan content
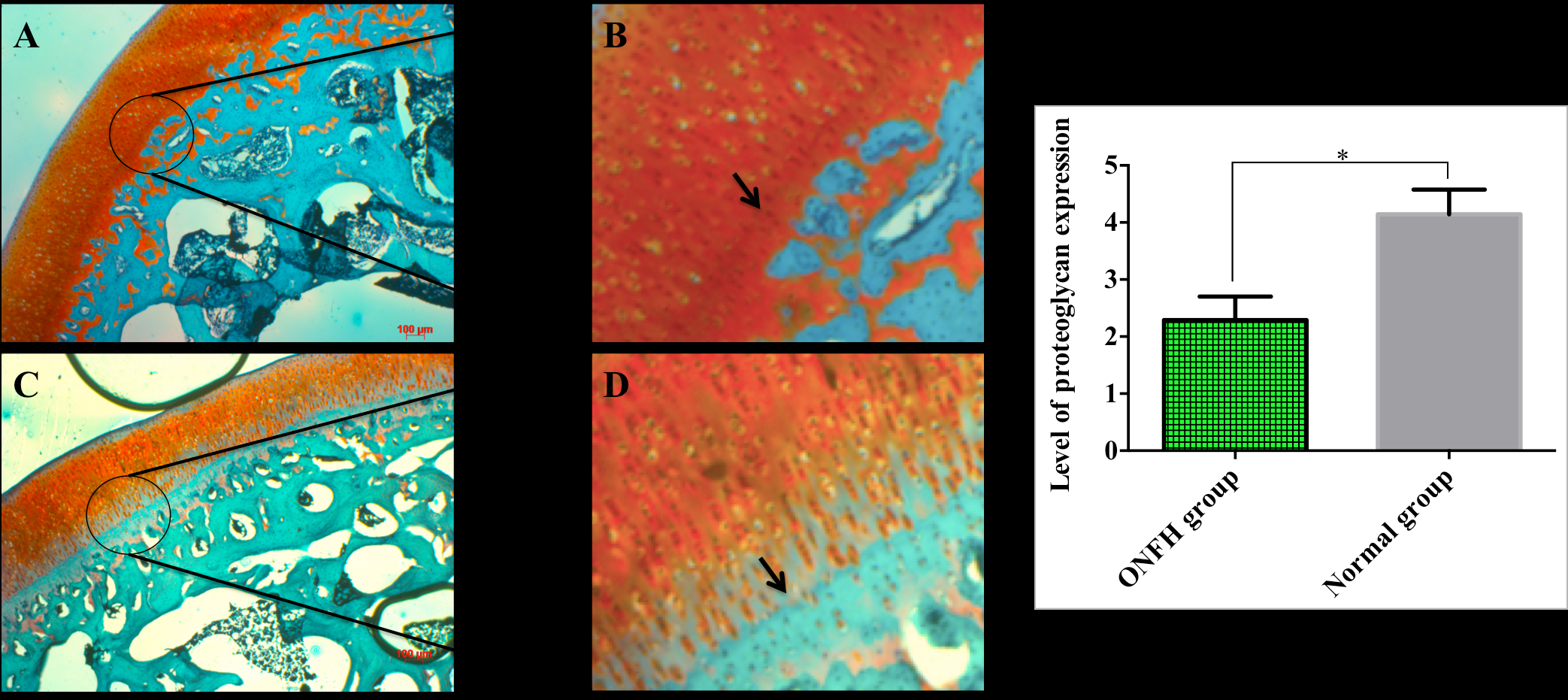
The concentration of proteoglycans that displayed by orange color was significant higher in the normal group ( Figure 2A,B ), compared to the ONFH group ( Figure 3A,D ) at 7 weeks. Statistical data showed that the proteoglycan level of the control group was 4.14 ± 0.29 μm while for the ONFH group the values were 2.29 ± 0.17 μm (p <0.05).
At the zone of calcified and hypertrophic cartilage, the fibrocartilage (characterized by the presentation of collagen via green color) was strongly expressed in the ONFH group after staining with safranin O and fast green (narrow; Figure 3D ), as opposed to the control group (narrow, Figure 3B ).
Ink artery infusion angiography
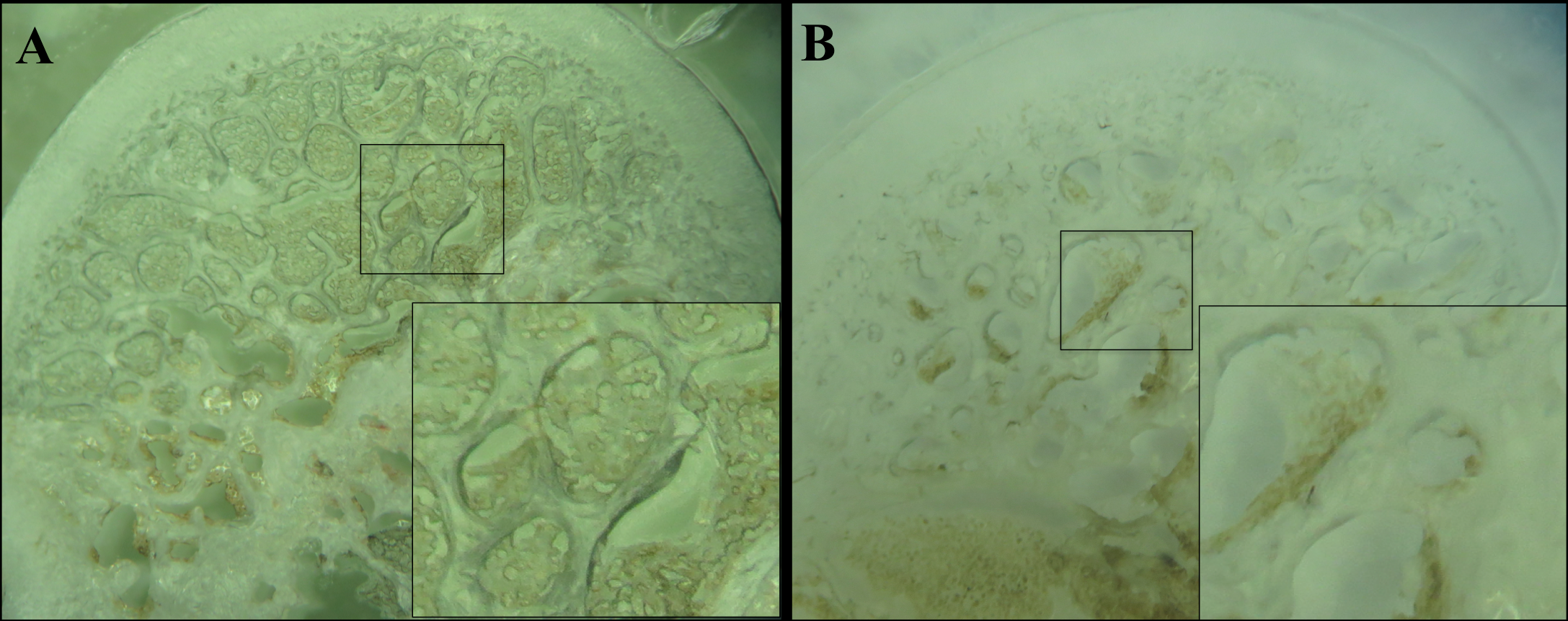
The capillaries network in the medullary cavity was high at femoral heads of the normal group, as displayed by the presentation of black color after ink injection into the abdominal aorta ( Figure 4A ). In contrast, in the ONFH model group, the density of black medullary cavity was less, indicating that the capillary network was sparse and that there might have been some injuries within the femoral head ( Figure 4B ).
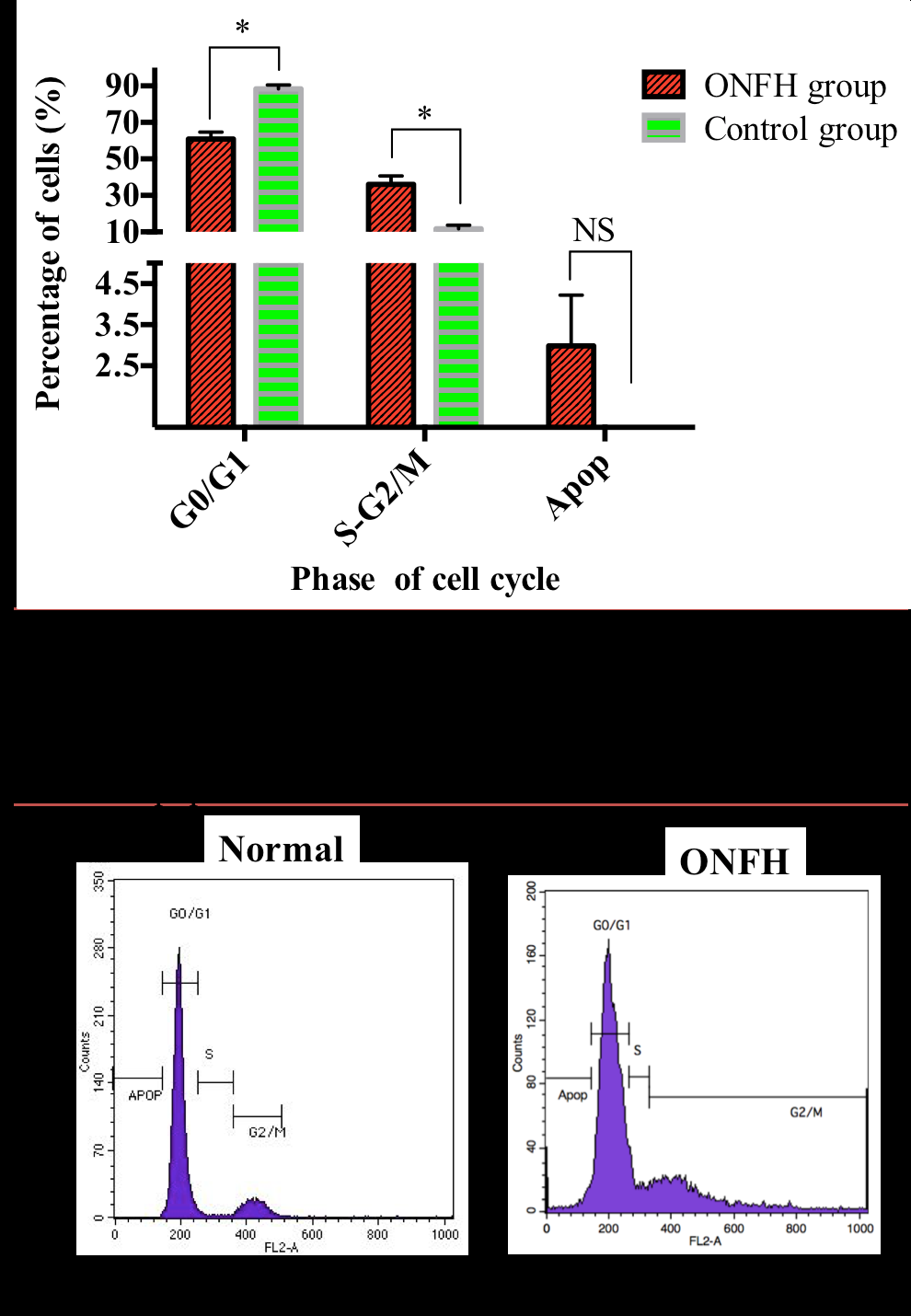
Cell cycle
Compared to the control group, the proportion of bone marrow derived nuclear cells in the ONFH group at G0/G1 phase was significant increased (p<0.05) and S and G2/M significantly decreased after week 7. The rate of apoptotic cells in the ONFH group was higher than for the control group at week 7; however, the difference was not significant ( Figure 5 ).
Discussion
Models of femoral head necrosis have been established in a variety of animal species, such as ostriches Conzemius et al., 2002, dogs Nakamura et al., 1997 rats Norman et al., 1998, and rabbits Yamamoto et al., 1995. Each species has its own strengths and weaknesses in establishing a successful disease model. The success depends, in part, on the laboratory conditions. Both traumatic and non-traumatic osteonecrosis models have been reported in previous studies Hwang et al., 2011Le et al., 2016Mont and Hungerford, 1995. Using drugs to create an animal disease model is a non-traumatic method. This method intends to limit invasiveness and reduce pain in animals. In our study, a combination of endotoxin and corticosteroid was used to enhance the immune response of animals, thereby increasing the effectiveness of establishing a successful animal model.
Damage of osteocytes may lead to their apoptosis or death. As a consequence, the bones get weaker Heino et al., 2009. A study showed that osteocyte apoptosis was promoted by glucocorticoids Bonewald, 2011. Cartilage injuries can cause tissue structural deformation. MPS and CFA have been demonstrated to induce changes in cartilage structure. Many glycosaminoglycans attach to a core protein to form a proteoglycan, which are secreted into the cartilage matrix by chondrocytes. The interaction between chondrocytes and the cartilage matrix play an important role in proliferation and cartilage remodeling Esko et al., 2009.
In our study, the results from safranin O/fast green staining and ink artery perfusion angiography showed that proteoglycans and blood vessels at the femoral head were damaged after methylprednisolone and CFA injection. However, cell cycle analysis showed that at the same time, the percentage of cells at S/G2M phase was higher in the ONFH rabbit group than in the normal rabbits (p<0.05). The rate of increase in cell division may play a role in repairing damaged tissues due to drug administration at an early stage. On the other hand, the percentage of apoptotic cells and empty bone lacunae was greater in the ONFH group than normal group, indicating that MPS combined with CFA had a severe impact on bone.
Conclusion
MPS combined with CFA can be used in rabbits to induce a model of early osteonecrosis of femoral head. However, further studies should be performed to provide more experimental data to support its preclinical application.
Abbreviations
CFA: Complete Freund’s Adjuvant
IV: Intravenous
LPS: Lipopolysaccharide
MPS: Methylprenisolone
ONFH: Osteonecrosis of the femoral head
References
-
L. F.
Bonewald.
The Amazing Osteocyte. Journal of Bone and Mineral Research.
2011;
26(2)
:
229-238
.
View Article Google Scholar -
M.G.
Conzemius,
T.D.
Brown,
Y.
Zhang,
R.A.
Robinson.
A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. Journal of orthopaedic research: official publication of the Orthopaedic Research Society.
2002;
20
:
303-309
.
View Article Google Scholar -
J. D.
Esko,
K.
Kimata,
U.
Lindahl.
Proteoglycans and Sulfated Glycosaminoglycans. In A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi,Etzler, M. E. (Eds.). Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press The Consortium of Glycobiology Editors, La Jolla, California.
2009
.
-
T.J.
Heino,
K.
Kurata,
H.
Higaki,
H.K.
Vaananen.
Evidence for the role of osteocytes in the initiation of targeted remodeling. Technology and health care: official journal of the European Society for Engineering and Medicine.
2009;
17
:
49-56
.
-
J.
Hirose,
S.
Tanaka.
Animal models for bone and joint disease. CIA, CAIA model. Clinical Calcium.
2011;
21
:
253-259
.
-
Y.
Hwang,
J.
Park,
S. H.
Choi,
G.
Kim.
Traumatic and Non-traumatic Osteonecrosis in the Femoral Head of a Rabbit Model. Laboratory Animal Research.
2011;
27(2)
:
127-131
.
View Article Google Scholar -
H. T.-N.
Le,
L. T.
Phi,
T. T.-T.
Dao,
N. K.
Phan,
P.
Van Pham,
N. B.
Vu.
A mouse model of osteonecrotic femoral head induced by methylprednisolone and liposaccharide. Biomedical Research and Therapy.
2016;
3(3)
:
12
.
View Article Google Scholar -
Y.
Li,
J.
Chen,
Z.
Zhang,
K.
Wang,
Z.
Tong,
H.
Yan.
The experimental study on treatment of glucocorticoid-induced ischemic necrosis of femoral head by gu fu sheng capsule. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan.
2004;
24
:
303-307
.
-
K.E.
McCarson.
Models of Inflammation: Carrageenan- or Complete Freund's Adjuvant (CFA)-Induced Edema and Hypersensitivity in the Rat. Current protocols in pharmacology.
2015;
70
:
5.4.1-9
.
-
V.
Mendiratta,
A.
Khan,
R. S.
Solanki.
Avascular necrosis: A rare complication of steroid therapy for pemphigus. Indian Journal of Dermatology.
2008;
53(1)
:
28-30
.
View Article Google Scholar -
M. A.
Mont,
D. S.
Hungerford.
Non-traumatic avascular necrosis of the femoral head. The Journal of Bone and Joint Surgery. American Volume.
1995;
77(3)
:
459-474
.
-
T.
Nakamura,
T.
Matsumoto,
M.
Nishino,
K.
Tomita,
M.
Kadoya.
Early magnetic resonance imaging and histologic findings in a model of femoral head necrosis. Clinical Orthopaedics and Related Research.
1997;
:
68-72
.
-
D.
Norman,
D.
Reis,
C.
Zinman,
I.
Misselevich,
J. H.
Boss.
Vascular deprivation-induced necrosis of the femoral head of the rat. An experimental model of avascular osteonecrosis in the skeletally immature individual or Legg-Perthes disease. International Journal of Experimental Pathology.
1998;
79(03)
:
173-181
.
-
Q.
Wen,
L.
Ma,
Y. P.
Chen,
L.
Yang,
W.
Luo,
X. N.
Wang.
A rabbit model of hormone-induced early avascular necrosis of the femoral head. Biomedical and environmental sciences. BES.
2008a;
21
:
398-403
.
-
Q.
Wen,
L.
Ma,
Y. P.
Chen,
L.
Yang,
W.
Luo,
X. N.
Wang.
Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells. Gene Therapy.
2008b;
15(23)
:
1523-1535
.
View Article Google Scholar -
T.
Yamamoto,
K.
Hirano,
H.
Tsutsui,
Y.
Sugioka,
K.
Sueishi.
Corticosteroid Enhances the Experimental Induction of Osteonecrosis in Rabbits With Shwartzman Reaction. Clinical Orthopaedics and Related Research.
1995;
(316)
:
235-243
.
-
T.
Yamamoto,
T.
Irisa,
Y.
Sugioka,
K.
Sueishi,
T.
Yamamoto,
T.
Irisa,
K.
Sueishi.
Effects of pulse methylprednisolone on bone and marrow tissues: Corticosteroid-induced osteonecrosis in rabbits. Arthritis and Rheumatism.
1997;
40(11)
:
2055-2064
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 11 (2017)
Page No.: 1749-1759
Published on: 2017-11-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 7869 times
- Download PDF downloaded - 1756 times
- View Article downloaded - 9 times
 Biomedpress
Biomedpress