Abstract
Background: Multiple-organ failure is the main cause of disability and death in diabetes mellitus. Imbalance in oxidative status is responsible for major diabetic complications and multiple-organ failure including blindness. Recently, plants with potential antioxidant properties have been used to manage hyperglycemia in diabetes. This study was undertaken to evaluate the beneficial effects of saffron aqueous extract (SAE) administration in streptozotocin (STZ)-induced diabetic rats by evaluation of oxidative stress parameters in the eye lens.
Methods: Thirty-two adult rats were randomly divided into four groups: normal control (I), saffron control (II), diabetic control (III), and saffron treated (IV). Diabetes was induced in animals by a single intraperitoneal (i.p.) injection of STZ (60 mg/kg body weight). Three days after STZ administration, diabetes was confirmed by measuring fasting blood glucose. At this time, groups II and IV were treated with SAE (200 mg/kg body weight) for a total of 5 doses, and then weekly from the 7th day after STZ injection. At the end of study, fasting blood glucose levels, activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), as well as malondialdehyde (MDA) content were determined in the lens tissues.
Results: In addition to the effects of SAE on blood glucose, SAE was effective on the enzymatic antioxidant defense system. The level of antioxidant enzymes (SOD, CAT, and GPx) decreased while there was an increase in the levels of MDA in the lens tissues of diabetic control rats, as compared to normal control rats. SAE could be modulating the changes in these parameters that are induced by diabetes.
Conclusion: The results of the present study demonstrate that SAE is able to control blood glucose and thus may be useful in delaying the complicated effects of diabetes, such as clouding of the eyes’ lens due to the antioxidant effect of SAE.
Introduction
Diabetes is characterized by the reduction in the glucose uptake into muscle and adipose tissue, which leads to chronic extracellular hyperglycemia and many other complications. This life- threatening disease is mostly associated with high risk of atherosclerosis, retinopathy, cataract formation, nephropathy, peripheral nerve damage, and liver apoptosis [1] [2] [3] [4]. Oxidative stress is a well-recognized factor which involves in pathogenesis of diabetes and its complications. Moreover, progression of microvascular and cardiovascular complications of diabetes are well associate with oxidative stress and reactive oxygen species (ROS) [5]. In other words; increase in ROS is a proven mechanism during hyperglycemia which is followed by activation of pathways resulting in several damages in different organs [6]. Oxidation may also be associated with cataract induced by hyperglycemia [7]. In hyperglycemia; cell death is the consequence of NFκB activation with impairment in the regulation of ROS. Based on the type of cell involved, diabetic patients will face complications in various organs such as liver, kidneys, lung and eyes [8] [9].
There are a number of medications used to treat people with diabetes, but synthetic drugs have
always shown adverse reactions and other undesirable side effects. On the other hand, common treatments for diabetes are mostly designed to control the blood glucose and do not show desirable effects on the complications [10]. In this regard, replacing effective natural therapies instead of chemical and pharmaceutical treatments are useful to control the development of diabetes. Recently, herbal medicines with high antioxidant activities have attracted considerable attention as a potential option to prevent and protect oxidative damage caused by free radical species [11]. Crocus sativus L. commonly known as saffron form Iridaceae family is a popular specie in Iran [12]. Saffron has been recognized for having interesting clinical properties such as being anticonvulsant, antidepressant, anti-inflammatory, and antitumor [13] [14] [15] [16]. The pharmacological activities of saffron are attributed to many of its active constituents including safranal, picrocrocin, crocetin, and crocins [17] [18]. Accumulating evidence has suggested the possibility of saffron being used as a candidate for the treatment of diabetes. Although, hypoglycemic effects of this plant have been studied in diabetic rats, the effect of saffron on different organs remains obscure [19].
Therefore, the present research was undertaken to evaluate the effects of saffron on alteration of oxidative parameters including superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and malondialdehyde (MDA) on the lens of diabetic rats.
Material and Methods
Experimental animals
Thirty-two adult healthy albino Wistar rats (male; 7-8 weeks) with the mean weigh of 215 g were obtained from Shiraz Institute for Stem Cell and Regenerative Medicine (Shiraz, Iran). All of the animals were maintained in standard cages at the room temperature (23±1 ◦C) and natural light- dark cycle. They also had free access to water and balanced diet (ad libitum). For adaptation all animals were kept in this condition one week before the study.
Animal ethics
All the experiments on animals were approved by the State Committee on Animal Ethics, Shiraz University, Shiraz, Iran (IACUC no: 4687/63). The recommendations of European Council Directive (86/609/EC) of November 24, 1986, regarding the standards in the protection of animals used for experimental purposes, were also followed.
Preparation of plant extract 3
In order to prepare saffron aqueous extract (SAE), 25 ml of distilled water was added to one gram of dried saffron threads powder and they were mixed well. The mixture was placed into an orbital shaker in a dark room for three days. The extract was than filtrated with Whatman No. one filter paper [20] [15] [21]. At the final step; freeze-drying was used to powder the filtrated extract and kept at -20 ºC for further studies.
STZ- diabetic Induction
Diabetes was induced as described in our previously work (Ashrafi et al., 2017). For induction of diabetes; a single intraperitoneal (i.p.) injection of freshly prepared solution of STZ (Sigma, St. Louis, MO, USA) (60 mg/kg body weight) in 0.1 M cold citrate buffer (pH 4.5) were used.
Rats in the normal control group and saffron control group were injected with the same volume of citrate buffer as the diabetic groups. 72 h after STZ injection, the development of diabetes was confirmed by measuring the level of fasting blood sugar (FBS) through glucometer (AccuChek, Germany). Animals with the FBS above 250 mg/dl were included in the experiment.
Experimental design
Thirty-two rats were randomly divided into four groups as follows:
Group I: Served as the normal control group, injected with normal saline once a week for 5 weeks.
Group II: Served as the saffron control group, injected once a week with SAE (200 mg/kg body weight, i.p. for 5 weeks).
Group III: Served as the diabetic control group, injected with normal saline once a week for 5 weeks.
Group IV: Served as the saffron treated group, injected once a week with SAE (200 mg/kg body weight, i.p. for 5 weeks).
Body weights of animals were measured weekly during the experiment. After 5 week treatment, the animals were sacrificed under anesthesia, and the lens tissues of rats were harvested.
For each animal the harvested tissue (lens) was weighed and a solution of phosphate buffer (0.1 M, pH 7.4) was added and homogenized by sonication at 4 ◦C using a Cole Farmer 4710 series ultrasonic homogenizer (Cole Farmer). Soft tissues homogenate of each animal were divided in
aliquots and kept at –80◦C until analysis.
Biochemical analysis
Antioxidant enzyme activities
The SOD and GPx activity were measured by commercial kits (RANSOD kit, Randox Com, UK). Also, the activity of Catalase (CAT) was measured using the commercial Catalase assay kit (Oxford Biomedical Research, Inc., USA), based on the colorimetric method.
Lipid peroxidation of tissue
Measurement of malondialdehyde (MDA) MDA level in lens tissues was measured by using thiobarbituric acid (TBA) reagent and absorbance was read in 535 nm according to the method of Peeri et al. (2012) [22]. Final concentration of MDA was calculated using its molar extinction coefficient (1.56×105 cm/mmol) in equation A=ECL.
Total protein measurement Total protein of the homogenate tissues was estimated by Lowry method and specific activity of mentioned enzymes was reported.
Statistical analysis
The values are reported as mean ± SD. Data were analyzed by One-Way ANOVA using SPSS version 17 software. The significance level was considered (P <0.05) and included in the study.
Results
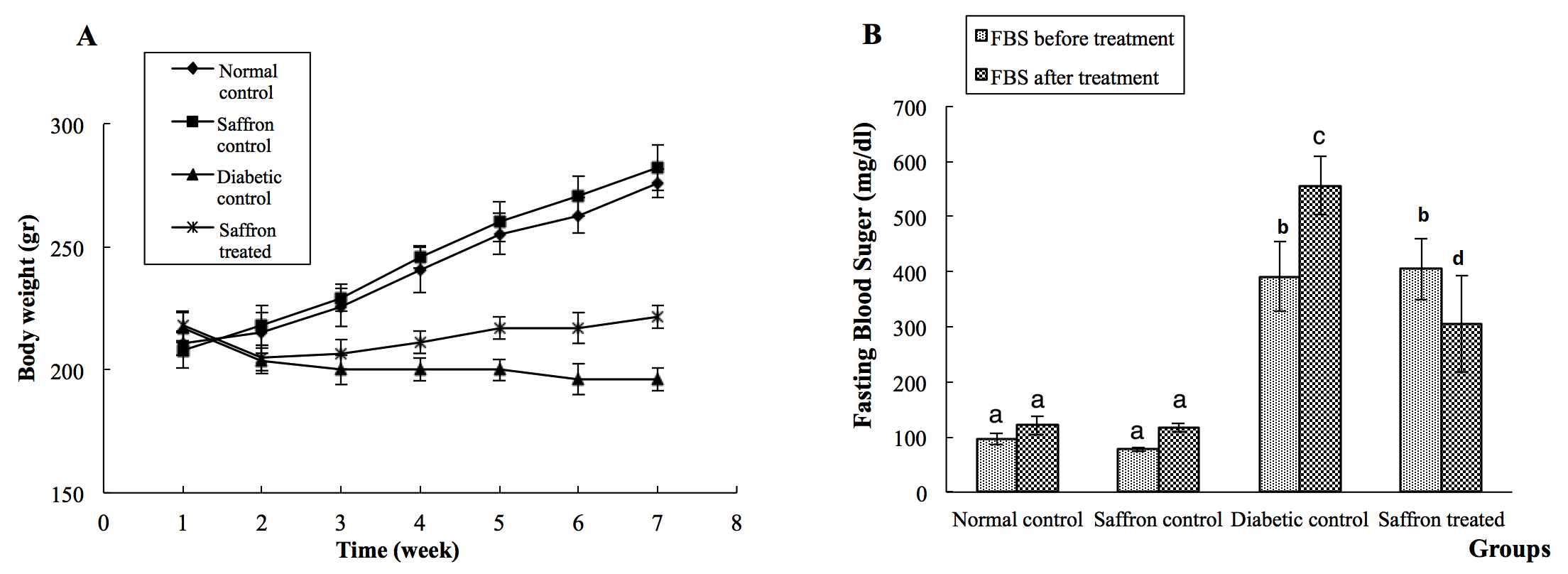
Body weight and FBS of rats are shown in Figure 1 . The baseline weight of the rats at the beginning of the study was similar in all groups. The results showed that at the end of the study, the mean body weight of diabetic adult rats had a 10% reduction compared to the beginning of the study. On the other hand, SAE not only prevented weight loss in diabetic rats but also caused a significant increase (2%) in the body weight of diabetic rats Figure 1A .
Measurement of FBS levels at the end of the study showed a significant (P<0.05) increase in diabetic rats. However, diabetic rats treated with SAE showed a significant (P<0.05) decrease in the level of FBS compared to the diabetic control group Figure 1B .
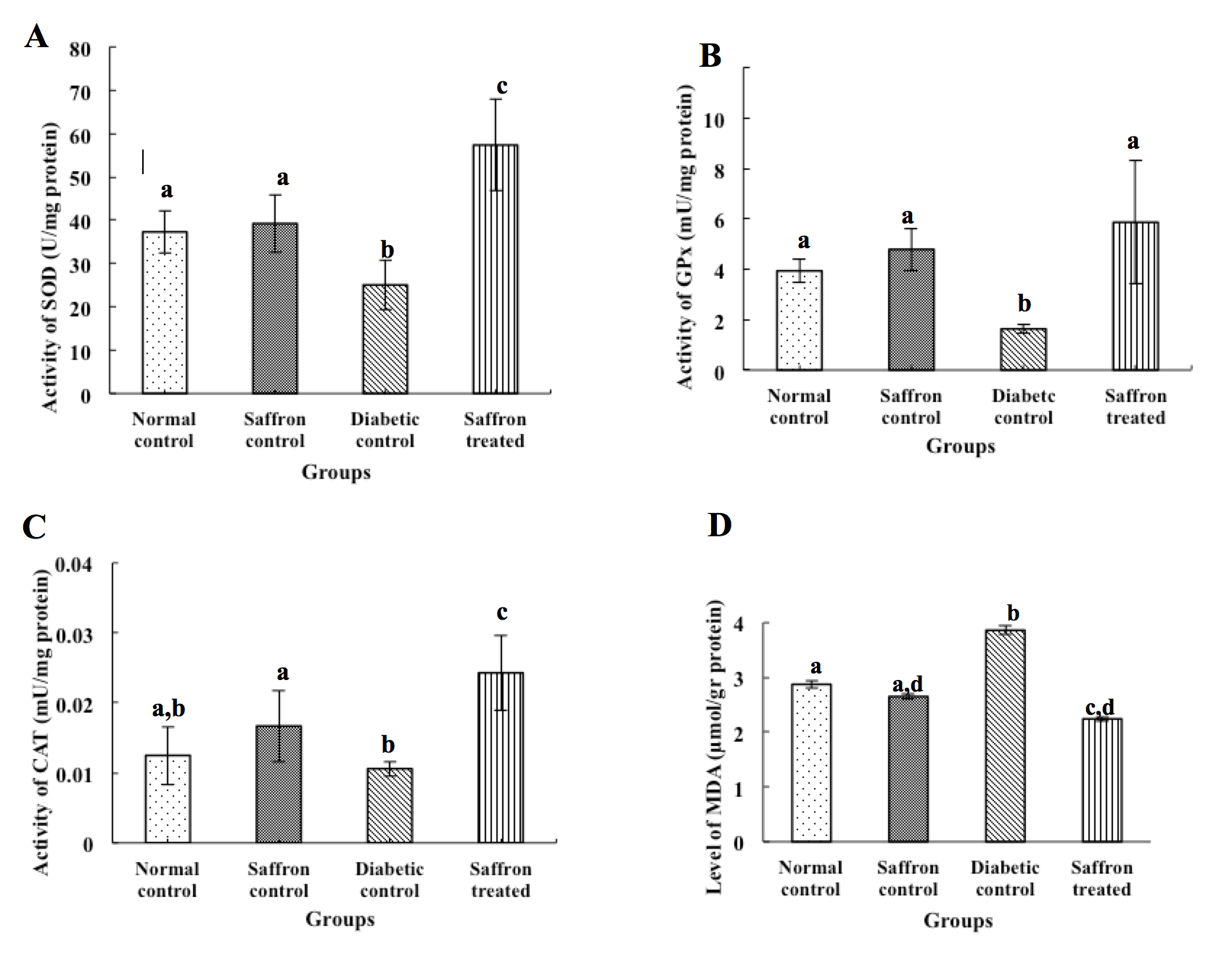
As shown in Figure 2A and Figure 2B , the activity of SOD and GPx were significantly (P<0.05) reduced in the lens tissues of diabetic rats. However, when treated with SAE the diabetic rats showed a significant increase (P<0.05) in the level of antioxidant enzymes (SOD and CAT), as compared to the diabetic control rats.
Figure 2C indicates that STZ causes a reduction in CAT activity. Although the reduction in CAT activity was not significant, SAE administration was able to increase the activity of this enzyme in the lens of diabetic rats, in comparison to other groups (P<0.05).
Figure 2D shows the measured MDA level in the lens of experimental animals. In diabetic control rats, the level of MDA showed a significant increase (P<0.05) compared to the normal control group. However, the level of MDA in saffron treated rats significantly decreased, in comparison with the diabetic control group.
Discussion
Diabetes is a serious and growing disorder which is associated with severe acute and chronic complications. Different sources of evidence indicate that oxidative stress has been implicated in diabetic patients [23] [24] [25]. In other words, ROS have been defined as an autocatalytic mechanism that can lead to apoptosis [26]. Therefore, regulation of oxidative stress could be a promising therapeutic strategy used to prevent or delay diabetes complications.
After STZ induction, body weight of diabetic rats begins to decrease [26] [27] [28]. In diabetes, both muscle atrophy and loss of weight are the consequence of the body impairment to use the excessive glucose produced during gluconeogenesis [29]. Results of this study demonstrated that SAE is able to prevent weight loss. Moreover, the hypoglycemic effects of saffron which reverses gluconeogenesis is another underlying mechanism involved in the prevention of weight loss in diabetes [30]. As Figure Figure 2 shows, in the diabetic control group, STZ causes a constant high blood glucose which proves that beta cells of pancreas are destroyed. However, diabetic rats treated with SAE showed a significant reduction in the level of blood glucose. Previous studies have also reported the hypoglycemic effects of saffron along with reversing weight loss. In other words; Saffron ethanolic and aqueous extract have the potential to regulates of insulin, improve blood glucose and weight loss in diabetic rats [31] [10] [32] [19]. In addition, in a study done by Samarghandian et al. (2014), saffron was able to decrease hyperglycemia and ameliorate the status of oxidative stress in rats with diabetic encephalopathy [33]. In this regard, the suggested hypoglycemic mechanism of saffron includes a reduction in insulin resistance, prevention of intestinal glucose absorption, and stimulation of glucose uptake in peripheral tissues [34] [35] [36]. Over-production of ROS, reduction in the level of GSH and vitamin E, and the defects in the activity of antioxidant enzymes, such as SOD and GPx, has a major contribution in the pathogenies of diabetes. There are numerous recent publications which describe the effect of oxidative stress on the damage to the lens fibers in diabetes and cataract is a major cause of blindness in this life-threatening disease. Results of the present experiment indicates that the level of SOD and GPx are significantly (P<0.05) decreased in the lens of diabetic control group in comparison with the normal control group. However, the reduction of CAT in the diabetic control group was not significant compared to the normal control group. Moreover, the level of MDA showed significant (P<0.05) increase in the lens of diabetic rats in comparison with the normal control group. In addition to the results obtained from the present study, there are several reports indicating the alteration of antioxidant enzymes and lipid peroxidation in diabetes. Fecondo et al. (1983) measured the activities of the protective enzymes, SOD, CAT, and GPx in the cortical and nuclear sections of the human lens as well as in calf, rabbit, and rat lens. They have reported a quick decrease in both SOD and GPx in the nuclear region of the lens at the onset of nuclear cataract; however, no changes were reported in the activity of CAT. Based on the findings of Fecondo et al. (1983) inactivation of antioxidant enzymes results in an increase in the level of hydrogen peroxide and superoxide anion and these are the molecules causing oxidative modification in lens proteins observed in nuclear cataracts. In another study, Cekic et al. (1999) reported that in the lens of alloxan-induced diabetic rats the activities of SOD and GPx decreased and the level of CAT and xanthine oxidase increased. Moreover, Balog et al.’s (2001) research showed that the lens of alloxan-induced diabetic rats had lower GPx activity than normal rats. They also represented that lipid peroxidation increased in alloxan-induced diabetic rats. Another study published in 2013 mentioned that 4 weeks after the induction of diabetes with STZ, the level of MDA in the lens tissue of diabetic rats increased while the activity of SOD and GPX decreased [37]. Thiraphatthanavong et al. (2014) studied the effect of combined extract of purple waxy corn and ginger on cataractogenesis and retinopathy in STZ diabetic rats [38]. Findings of their study showed an elevated MDA level and a reduction of SOD and GPx in the lens of diabetic rats. Since oxidative stress is an important factor in the pathogenesis of diabetic complications, agents with high antioxidant capacity are considered as an alternative therapeutic method in this life-treating disease. Saffron is a plant which exhibited significant radical scavenging and antioxidant activity [39]. In the present investigation, SAE administration significantly (P<0.05) increased the level of antioxidant enzymes CAT, GPx, and SOD compared to the diabetic control group. The saffron treated group also showed a significant decrease in the level of MDA in comparison with the diabetic control group. Consistent with the present study, different agents with antioxidant activity have been examined to see if they could prevent cataractogenesis in diabetes. A study on Venoruton, a flavonoid with high antioxidant capacity, showed its protective effect on rat lens with diabetic cataract [40]. Quercetin a flavonoid, have shown potential prevention in cataractogenesis in the rat lens organ [41]. Moreover, the protective effect of traditional antioxidants such as vitamin C, vitamin E, and superoxide mimics has shown promising effect against cataract, possibly through protection of membrane lipids against peroxidation [42].
Conclusion
The results of the present study indicate that the protective effects of saffron on diabetes complications due to its effects on antioxidant enzymes but also due to its inhibition of lipid peroxidation. This study suggests that saffron aqueous extract might be a promising candidate for therapy of chemically induced diabetes and its associated complications.
Abbreviations
CAT: Catalase; FBG: Fasting Blood Glucose; GPx: Glutathione peroxidase; MDA: Malondialdehyde; NFκB: Nuclear Factor κB; SAE: Saffron Aqueous Extract; D: Superoxide dismutase; STZ: Streptozotocin
Author Contribution
Envisaged and designed the experiments: M Ashrafi, N Kazemipour and S Nazifi; Extract preparation, animal study and measurement of biochemical parameters: M Ashrafi, S Talebanzadeh; Data analysis: M Ashrafi, H Erjaee; Manuscript writing: S Nazifi, H Erjaee. All authors reviewed, commented and approved the final manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Competing interests
The authors declare that no competing interests exist.
References
-
M
Brownlee.
Biochemistry and molecular cell biology of diabetic complications. Nature.
2001;
414
:
813-20
.
View Article PubMed Google Scholar -
T
Hayashi,
K
Ina,
M
Kuzuya.
Effect of the education in hospitalization of diabetic individuals for diabetes control and prevention of complications as a tool of hospital and clinic cooperation. Atherosclerosis.
2016;
252
:
e139
.
View Article Google Scholar -
F
Paneni,
JA
Beckman,
MA
Creager,
F
Cosentino.
Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. European Heart Journal.
2013;
34
:
2436-43
.
View Article PubMed PMC Google Scholar -
MN
Karimi,
R
Abbasalipourkabir,
ZA
Sadeghabadi,
N
Ziamajidi.
The level of gene expression of Bax and Bcl-2 and the activity of caspase 3 in the liver tissues of normal, type 1 and type 2 diabetic rats before and after treatment with aqueous extract of garlic. SSU_Journals.
2017;
25
:
547-555
.
-
F
Giacco,
M
Brownlee.
Oxidative stress and diabetic complications. Circulation Research.
2010;
107
:
1058-70
.
View Article PubMed PMC Google Scholar -
IC
West.
Radicals and oxidative stress in diabetes. Diabetic Medicine.
2000;
17
:
171-80
.
View Article PubMed Google Scholar -
J
Zhang,
H
Yan,
MF
Lou.
Does oxidative stress play any role in diabetic cataract formation? —-Re-evaluation using a thioltransferase gene knockout mouse model. Experimental Eye Research.
2017;
161
:
36-42
.
View Article PubMed Google Scholar -
MA
Haidara,
DP
Mikhailidis,
MA
Rateb,
ZA
Ahmed,
HZ
Yassin,
IM
Ibrahim.
Evaluation of the effect of oxidative stress and vitamin E supplementation on renal function in rats with streptozotocin-induced Type 1 diabetes. Journal of Diabetes and Its Complications.
2009;
23
:
130-6
.
View Article PubMed Google Scholar -
K
Stadler,
V
Jenei,
G
von Bölcsházy,
A
Somogyi,
J
Jakus.
Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Radical Biology & Medicine.
2003;
35
:
1240-51
.
View Article Google Scholar -
AF
Elgazar,
AA
Rezq,
HM
Bukhari.
Anti-hyperglycemic effect of saffron extract in alloxan-induced diabetic rats. Eur J Biol Sci.
2013;
5
:
14-22
.
-
H
Erjaee,
H
Rajaian,
S
Nazifi,
M
Chahardahcherik.
The effect of caraway (Carumcarvi L.) on the blood antioxidant enzymes and lipid peroxidation in streptozotocin-induced diabetic rats. Comparative Clinical Pathology.
2015;
24
:
1197-203
.
-
FI
Abdullaev.
Biological effects of saffron. BioFactors (Oxford, England).
1993;
4
:
83-6
.
-
FI
Abdullaev,
JJ
Espinosa-Aguirre.
Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detection and Prevention.
2004;
28
:
426-32
.
View Article PubMed Google Scholar -
H
Hosseinzadeh,
V
Khosravan.
Anticonvulsant effects of aqueous and ethanolic extracts of crocus sativus L stigmas in mice. Archives of Iranian Medicine.
2002;
5
:
44-7
.
-
H
Hosseinzadeh,
HR
Sadeghnia,
T
Ziaee,
A
Danaee.
Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion- induced oxidative damage in rats. Journal of Pharmacy & Pharmaceutical Sciences.
2005;
8
:
387-93
.
PubMed Google Scholar -
H
Hosseinzadeh,
HM
Younesi.
Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacology.
2002;
2
:
7
.
-
F
Taheri,
SZ
Bathaie,
M
Ashrafi,
E
Ghasemi.
Assessment of Crocin Toxicity on the Rat Liver. Modares Journal of Medical Sciences. Pathobiology.
2014;
17
:
67-79
.
-
J
Rios,
M
Recio,
R
Giner,
S
Manez.
An update review of saffron and its active constituents. Phytotherapy Research.
1996;
10
:
189-93
.
View Article Google Scholar -
D
Mohajeri,
G
Mousavi,
Y
Doustar.
Antihyperglycemic and pancreas-protective effects of Crocus sativus L. (Saffron) stigma ethanolic extract on rats with alloxan-induced diabetes. The Journal of Biological Sciences.
2009;
9
:
302-10
.
-
SZ
Bathaie,
H
Miri,
MA
Mohagheghi,
M
Mokhtari-Dizaji,
AA
Shahbazfar,
H
Hasanzadeh.
Saffron Aqueous Extract Inhibits the Chemically-induced Gastric Cancer Progression in the Wistar Albino Rat. Iranian Journal of Basic Medical Sciences.
2013;
16
:
27-38
.
PubMed Google Scholar -
S
Shirali,
SZ
Bathaei,
M
Nakhjavani,
MR
Ashoori.
Effects of saffron (Crocus Sativus L.) aqueous extract on serum biochemical factors in streptozotocin-induced diabetic rats S. Tahqiqat-i Giyahan-i Daruyi va Muattar-i Iran.
2012;
28
:
293-308
.
-
M
Peeri,
MM
Haghigh,
MA
Azarbayjani,
S
Atashak,
G
Behrouzi.
Effect of aqueous extract of saffron and aerobic training on hepatic non enzymatic antioxidant levels in streptozotocin-diabetic rats. Archival Science.
2012;
65
:
525-32
.
-
M
Brownlee.
Biochemistry and molecular cell biology of diabetic complications. Nature.
2001;
414
:
813-20
.
View Article PubMed Google Scholar -
Y
Hinokio,
S
Suzuki,
M
Hirai,
M
Chiba,
A
Hirai,
T
Toyota.
Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia.
1999;
42
:
995-8
.
View Article Google Scholar -
PI
Ingaramo,
MT
Ronco,
DE
Francés,
JA
Monti,
GB
Pisani,
MP
Ceballos.
Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Molecular Immunology.
2011;
48
:
1397-407
.
View Article PubMed Google Scholar -
BE
Jones,
CR
Lo,
H
Liu,
A
Srinivasan,
K
Streetz,
KL
Valentino.
Hepatocytes sensitized to tumor necrosis factor-α cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. The Journal of Biological Chemistry.
2000;
275
:
705-12
.
View Article PubMed Google Scholar -
S
Muruganandan,
K
Srinivasan,
S
Gupta,
PK
Gupta,
J
Lal.
Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. Journal of Ethnopharmacology.
2005;
97
:
497-501
.
View Article PubMed Google Scholar -
P
Pushparaj,
CH
Tan,
BK
Tan.
Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. Journal of Ethnopharmacology.
2000;
72
:
69-76
.
View Article Google Scholar -
A
Shirwaikar,
K
Rajendran,
CD
Kumar,
R
Bodla.
Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. Journal of Ethnopharmacology.
2004;
91
:
171-5
.
View Article PubMed Google Scholar -
D
Mohajeri,
BA
Tabrizi,
G
Mousavi,
M
Mesgari.
Anti-diabetic activity of Crocus sativus L. (Saffron) stigma ethanolic extract in alloxan-induced diabetic rats. Research Journal of Biological Sciences.
2008;
3
:
1102-8
.
-
A
Arasteh,
A
Aliyev,
S
Khamnei,
A
Delazar,
M
Mesgari,
Y
Mehmannavaz.
Crocus sativus on serum glucose, insulin and cholesterol levels in healthy male rats. Journal of Medicinal Plants Research.
2010;
4
:
397-402
.
-
S
Kianbakht,
R
Hajiaghaee.
Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. Faslnamah-i Giyahan-i Daruyi.
2011;
3
:
82-9
.
-
S
Samarghandian,
M
Azimi-Nezhad,
F
Samini.
Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. BioMed research international 2014.
2014
.
-
L
Xi,
Z
Qian,
G
Xu,
C
Zhou,
S
Sun.
Crocetin attenuates palmitate-induced insulin insensitivity and disordered tumor necrosis factor-α and adiponectin expression in rat adipocytes. British Journal of Pharmacology.
2007;
151
:
610-7
.
View Article PubMed PMC Google Scholar -
YC
Yang,
HK
Hsu,
JH
Hwang,
SJ
Hong.
Enhancement of glucose uptake in 3T3-L1 adipocytes by Toona sinensis leaf extract. The Kaohsiung Journal of Medical Sciences.
2003;
19
:
327-33
.
View Article Google Scholar -
JY
Youn,
HY
Park,
KH
Cho.
Anti-hyperglycemic activity of Commelina communis L.: inhibition of α-glucosidase. Diabetes Research and Clinical Practice.
2004;
66
:
S149-55
.
-
X
Gong,
Q
Zhang,
S
Tan.
Inhibitory effect of r-hirudin variant III on streptozotocin- induced diabetic cataracts in rats. The Scientific World Journal.
2013;
2013
:
630651
.
View Article PubMed PMC Google Scholar -
P
Thiraphatthanavong,
J
Wattanathorn,
S
Muchimapura,
W
Thukham-mee,
K
Lertrat,
B
Suriharn.
The combined extract of purple waxy corn and ginger prevents cataractogenesis and retinopathy in streptozotocin-diabetic rats. Oxidative Medicine and Cellular Longevity.
2014;
2014
:
789406
.
View Article PubMed PMC Google Scholar -
AN
Assimopoulou,
Z
Sinakos,
VP
Papageorgiou.
Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytotherapy Research.
2005;
19
:
997-1000
.
-
F
Kilic,
R
Bhardwaj,
JR
Trevithick.
Modelling cortical cataractogenesis. XVIII. In vitro diabetic cataract reduction by venoruton. A flavonoid which prevents lens opacification. Acta Ophthalmologica Scandinavica.
1996;
74
:
372-8
.
-
J
Sanderson,
WR
McLauchlan,
G
Williamson.
Quercetin inhibits hydrogen peroxide- induced oxidation of the rat lens. Free Radical Biology & Medicine.
1999;
26
:
639-45
.
View Article Google Scholar -
SD
Varma.
Scientific basis for medical therapy of cataracts by antioxidants. The American Journal of Clinical Nutrition.
1991;
53
:
335S-45S
.
View Article PubMed Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 4 (2018)
Page No.: 2133-2141
Published on: 2018-04-16
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7920 times
- Download PDF downloaded - 2270 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress