Abstract
Introduction: Pulmonary Fibrosis is characterized by excessive matrix deposition which leads to airway remodeling and disruption of the typical architecture of the lung parenchyma. The disease progression is associated with a high mortality rate. The current treatment for pulmonary fibrosis includes drugs which either reduce progression of the disease or provide symptomatic relief. Multiple studies have examined the effect of cell-based therapy in pulmonary fibrosis. We investigated the effect of administration of pre-conditioned bone marrow-derived mesenchymal stem cells (BM-MSC) in a mouse model of pulmonary fibrosis.
Methods: Firstly, we examined the effect of pre-conditioning on the cells using cell-based assays. We found that pre-conditioning did not significantly alter cell proliferation or led to cellular inflammation. The cells continued to express MSC marker, CD105, and pluripotency marker Oct3/4. Next, we evaluated the proliferative and anti-inflammatory potential of BM-MSC administration using a series of assays in a mouse model of pulmonary fibrosis. Bleomycin was administered to induce pulmonary fibrosis in mice on Day 0. MSCs were administered on day 1 and day 3; the mice were sacrificed on day 22, and their tissues were collected for analysis.
Results: We found that similar to untreated cells, administration of pre-conditioned cells resulted in an increase in the proliferative potential and reduction in inflammation in the lung tissue, bronchoalveolar lavage, bone marrow, and blood. We observed reduction in the number of granulocytes in peripheral blood upon MSC administration. However, we did not observe any structural changes in the lung upon MSC administration. We found a small reduction in collagen content in the lung which was also seen upon staining with Masson's trichrome.
Conclusion: These results demonstrate that pre-conditioned BM-MSC lead to improvement in the disease state through paracrine effects but pre-conditioning of cells for 24 hours does not significantly improve the beneficial effect of MSC administration.
Background
Pulmonary fibrosis is characterized by excess deposition of extracellular matrix and fibroblasts in the lung parenchyma which leads to stiffness in the lung and difficulty in breathing [1]. The treatment options include drugs such as pirfenidone and ninetedanib which reduce progression of the disease but do not facilitate regeneration of damaged lung tissue [2]. Therefore, ongoing research has been focusing on the development of novel therapeutic options to provide relief to the patients.
In the last decade, cell-based therapy has emerged as a viable treatment option. Mesenchymal stem cells (MSC) are multipotent stem cells which may be derived from various tissues including bone marrow, adipose and umbilical cord [3]. MSC treatment has been successfully used for amelioration of pulmonary fibrosis [4]. The MSCs are known to act through engraftment in the damaged tissue or through their paracrine effects. Previous studies have reported their role in ameliorating the disease by immunomodulation, inhibition of pro-fibrotic cytokines, reducing oxidative stress and release of microvesicles [5]–[7]. MSC administration leads to the replacement of fibrotic lesion with lung cells, decrease in pro-inflammatory and angiogenic cytokines and reduction in collagen content in the lung [6]-[8]. MSCs have also progressed to human trials where they have not demonstrated any toxicity upon administration [9]-[11].
Pre-conditioning of MSC has been adopted recently as a strategy to ensure better survival of these cells upon transplantation in a diseased organ. Along with improved survival, the pre-conditioned cells also demonstrated better paracrine effects, migration and homing to the diseased organ, increased regenerative ability and enhanced immunomodulation [12]. The different types of pre-conditioning include exposure to hypoxia, hydrogen sulfide, anoxia, hydrogen peroxide along with exposure to factors such as erythropoietin, insulin growth factor- 1, and heat shock proteins. Transplantation of hypoxia-treated MSC leads to amelioration of pulmonary fibrosis [13]. Exposure to hydrogen peroxide was used to induce cardiac lineage differentiation of stem cells [14]. However, the effect of oxidative stress pre-conditioned cells in lung fibrosis has not been examined. We hypothesize that exposure of mesenchymal stem cells to oxidative stress will alter signals necessary for their differentiation to lung lineages. In the present study, we used oxidative stress pre-conditioned cells in a mouse model of bleomycin-induced pulmonary fibrosis.
Methods
Animal housing and maintenance
BALB/c mice acquired from National Institute of Nutrition, Hyderabad were used in this study. All experiments were performed according to rules laid down by the Institutional and departmental animal ethics committee and the animals housed under specific pathogen-free conditions at the animal housing vivarium of the Department of Zoology, University of Calcutta. The mice were regularly monitored for general well-being.
Isolation and Harvesting of Bone Marrow-Derived Mesenchymal Stem Cells
6-8 weeks old BALB/c mice were euthanized by cervical dislocation. The skin and muscles were carefully removed from the hind limbs, and the femur bone was dissected and stored in Dulbecco’s Modified Eagle Medium (DMEM) culture media (HiMedia Laboratories Pty Ltd, Nasik, India). In a tissue culture hood, the bone marrow was harvested by flushing the bone with excess DMEM supplemented with 15% serum (HiMedia Laboratories Pty Ltd, Nasik, India) and 100U penicillin/0.05 mg streptomycin antibiotics (HiMedia Laboratories Pty Ltd, Nasik, India). These cells were seeded in a tissue culture plate and fed with fresh media for the next three days.
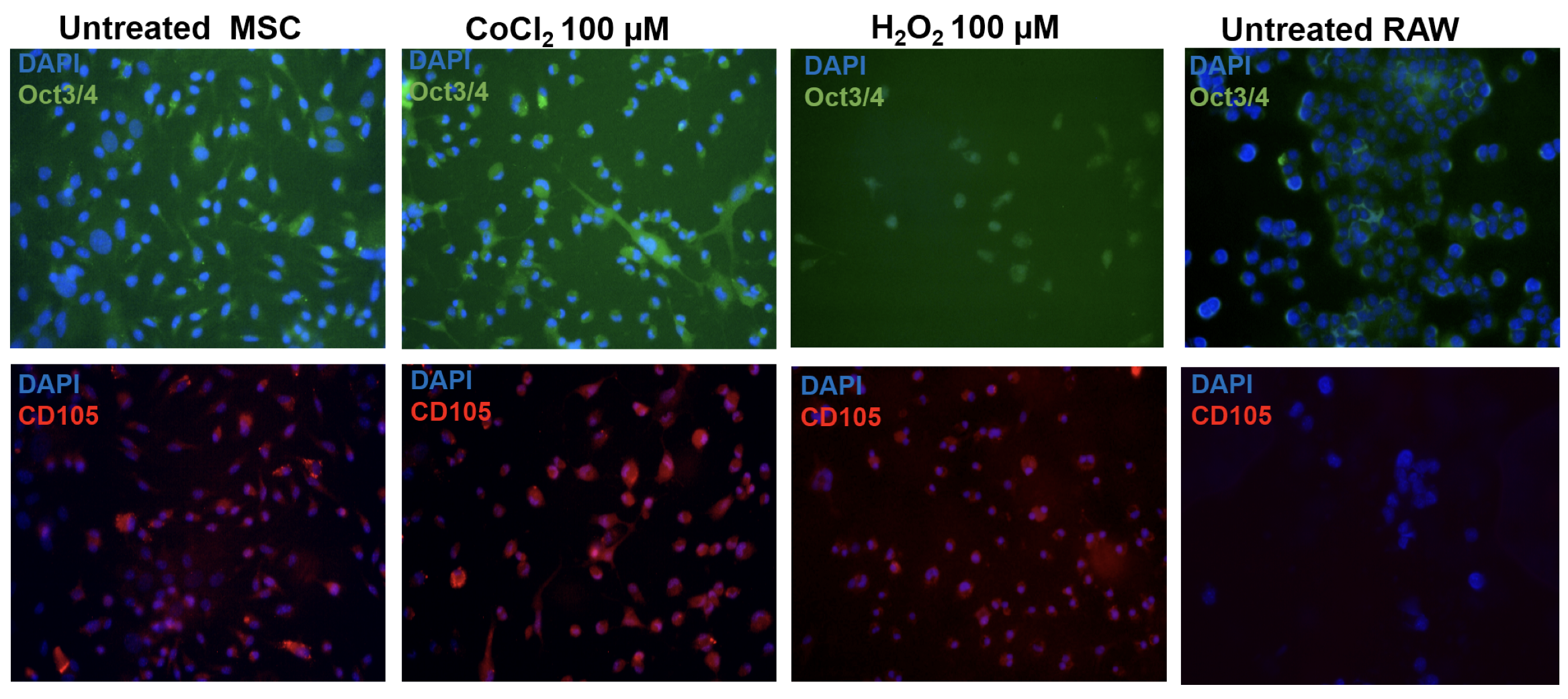
Following this, they were grown to 70-80% confluency before passaging. The cells were subjected to immunolabelling Figure 1 (Left panel) and fluorescence activated cell analysis (data not shown) to confirm expression of MSC marker, CD105. Cells from third, fourth or fifth passages were used for all the experiments.
Pre-conditioning cells using abiotic conditions
Equal number of cells were seeded in a 6-well or a 96-well tissue culture plate. On the following day, the cells were treated with 100 µM of CoCl2 (Merck, Delhi, India) or H2O2 (B.D. Pharmaceutical works, Pvt. Ltd., Howrah, India) to induce hypoxia and oxidative stress, respectively for 24 hours in a 5% CO2 incubator (37◦C). Untreated cells were used as negative control. The cells were then used for assays or transplantation.
Cell-based Assays
The cell-based assays were performed after 24 hours of the treatment. Only culture media was used as negative control. To estimate cell viability MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5- Diphenyltetrazolium bromide assay for performed. The MTT dye (Sisco Research Laboratory, Mumbai, India) was added to the cells for 3 hours and solubilized using acidic isopropanol (0.04M hydrochloric acid in absolute isopropanol). The OD was measured at 550 nm using a microplate reader (Thermo Fisher Scientific, USA).
Nitric oxide production was analyzed to estimate inflammation. Sulfanilamide solution (1% Sulfanilamide in 5% orthophosphoric acid; Sisco Research Laboratory, Mumbai, India) was then added at room temperature for 5 minutes followed by addition of N-1-naphthyl ethylenediamine- dihydrochloride (NED) solution (0.1% NED in distilled water) (Sisco Research Laboratory, Mumbai, India) at room temperature for 5 minutes. Absorbance was measured in a plate reader at 540 nm.
Marker expression analysis
For marker expression analysis, the cells were grown on coverslips and subjected to hypoxia or oxidative stress. The pre-conditioned cells were fixed using 4% paraformaldehyde, permeabilised using Triton X-100, blocked using bovine serum albumin and then hybridized with Anti- Oct3/4 conjugated with Alexa Fluor 488 (1:100, BD Biosciences, Kolkata, India) and Anti-CD105 conjugated with Per-CP 5.5 (1:100, BD Biosciences, Kolkata, India) antibodies. The cells were then mounted using buffered glycerol and imaged using 40X magnification on Olympus BX41 fluorescence microscope.
Bleomycin-induced fibrosis
The mice were divided into five groups Control (n=2), Bleomycin treated (n=2), Bleomycin treated with MSC administration (n=3), Bleomycin with hypoxia pre-conditioned MSC administration (n=3) and Bleomycin with oxidative stress pre-conditioned MSC administration (n=3). BALB/c mice received a single dose of bleomycin (Miracalus Pharma Pvt Ltd, Mumbai, India; 0.075 U/ml bleomycin dissolved in 1 ml of 0.09% sterile saline water) both intratracheally and intranasally. All mice received a single dose of bleomycin at day 0. Twenty microliters were administered intranasally, and 40 µl was administered intratracheally. After treatment for 24 hours, 2.5 × 104 and 6.5 × 104 cells were administered through tail vein injection in mice at Day 1 and
Day 3, respectively. The mice were sacrificed at the end of the experiment on Day 22 and their lung tissues collected for cell-based assays and histological assessment. Bone marrow, blood and bronchial alveolar lavage fluid (BALF) were obtained from all the treatment groups for analysis.
Cell-based assays
The cells were harvested from lung tissue by mincing and debris was removed using a mesh. Equal number of cells were seeded in a 96-well plate in DMEM supplemented with 10% fetal calf serum and antibiotics. The bone marrow were harvested by flushing the femur bone with excess media, and the cells were used for analysis. Cells from peripheral blood and BALF were sieved through a mesh and seeded in 96-well plates for experiments. The MTT and NO assays were performed on the following day as described previously in section 2.4. Nitro Blue Tetrazolium (NBT) assay was performed as described elsewhere [15].
Flow cytometry Analysis
The cells were harvested from lung tissue by digestion using Collagenase/Hyaluronidase cocktail (STEMCELL Technologies, Chennai, India) at 37◦C overnight. These cells were incubated with anti-CD45-PerCP (1:100, BioLegend, San Diego, USA) , anti-B220-FITC (1:100, BD Biosciences, Kolkata, India) and anti-CD3-PE (1:100, BD Biosciences, Kolkata, India) antibodies to examine the distribution of T- and B-cells. They were hybridized with CD3-PE (1:100, BD Biosciences, Kolkata, India), CD4-V450 (1:100, BD Biosciences, Kolkata, India) and CD8-Alexa Fluor 488 (1:100, BD Biosciences, Kolkata, India) antibodies to analyze the T-helper and cytotoxic T-cell population. The cells were hybridized with anti-CD45-PerCP, anti-GR1-FITC (1:100, BD Biosciences, Kolkata, India) and anti-F4/80-R-PE (1:100, Thermo Fischer Scientific, USA) antibodies to differentiate between the granulocyte and macrophage population.
Hydroxyproline estimation
The lung tissue was used to estimate hydroxyproline content as described previously [16].
Tissue sectioning and staining
The tissues were fixed in 10% formalin and paraffin embedded. Five micron thick sections were cut and stained using hematoxylin and eosin stains to examine changes in tissue structure. The collagen content was examined using Masson’s trichrome staining. They were imaged using the microscope (Dewinter Technologies, New Delhi, India) equipped with ToupView software (ToupTek Photonics, Zhejiang, China) at 10X or 40X magnification.
Results
In the present study, we examined the effect of oxidative stress pre-conditioned MSC in a mouse model of pulmonary fibrosis. We found that similar to hypoxia pre-conditioning, oxidative stress pre-conditioning reduced inflammation and increased proliferative ability of the cells.
Effect of pre-conditioning on cells
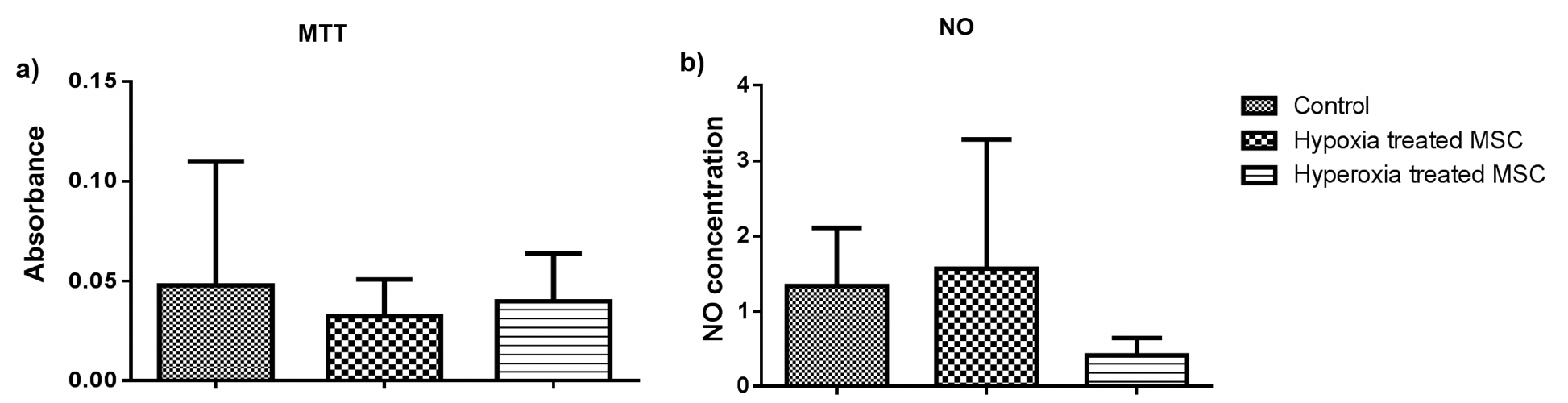
Firstly, we examined the effect of pre-conditioning on cells. All the assays were performed at least twice with appropriate controls. The pre-conditioned cells were subjected to cell-based assays and marker expression analysis. To examine if pre-conditioning altered the pluripotency or nature of the cells the expression of pluripotency marker Oct3/4 and MSC marker CD105 was examined. We found that the pre-conditioning did not alter marker expression and both untreated and pre-conditioned cells expressed both the markers. Further, the effect on their proliferation and induction of oxidative stress was estimated using MTT and NO assays. We found that pre- conditioning did not have a significant effect on the proliferative ability of cells estimated by MTT assay ( Figure 2a ) or induced inflammation as estimated by NO assay Figure 2 . On the contrary, oxidative stress pre-conditioning of cells led to a three-fold reduction in inflammation as compared to the untreated and hypoxia pre-conditioned cells. However, these changes were not statistically significant. Therefore pre-conditioning of cells did not affect the viability or induce oxidative stress in BM-MSCs. Hence, these cells were used for transplantation in a mouse model of bleomycin-induced pulmonary fibrosis.
Effect of cell-based therapy in IPF
The untreated and pre-conditioned cells were administered in mice, and their tissues were harvested at the end of the experiment for analysis. The cells were subjected to MTT, NO, NBT and flow cytometry analysis to examine the effect of MSC administration of cell proliferation and inflammation. All the assays were performed in triplicates on tissues obtained from at least two mice in each group. The lung tissue was subjected to histological analysis to examine structural changes upon MSC administration.
Cell-based assays
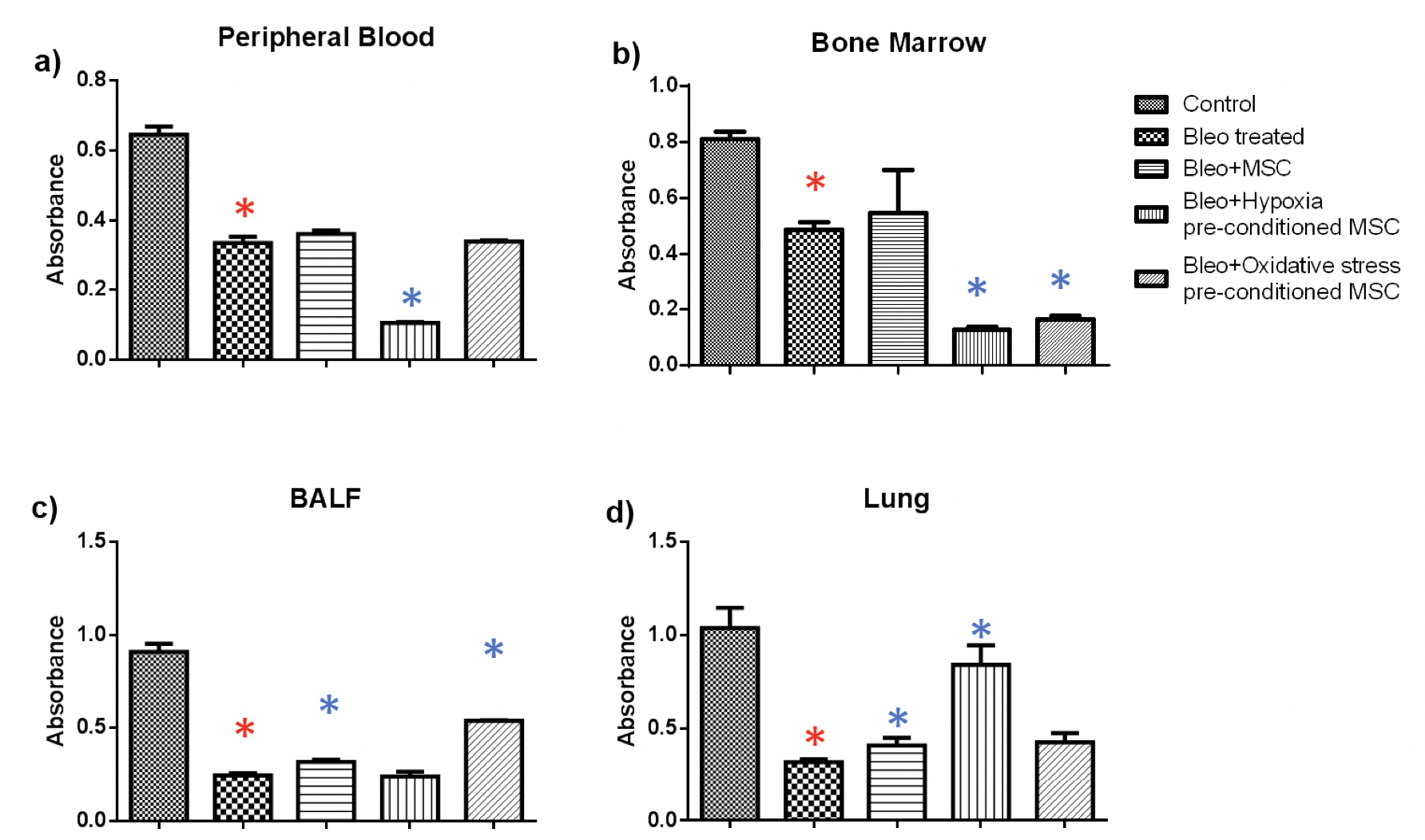
MTT: MTT assay was used to examine the cell viability in peripheral blood, bone marrow, BALF and lungs from mice with pulmonary fibrosis treated with of hypoxia or oxidative stress pre-conditioned BM-MSC. We found out that the proliferative ability of cells decreased upon bleomycin administration which increased upon administration of untreated or pre-conditioned MSC Figure 3 .
In blood, we found a statistically significant reduction of 2-fold in the proliferative ability upon bleomycin treatment as compared to the control group ( Figure 3a ). There was a small increase in proliferation upon administration of untreated or oxidative stress pre-conditioned MSC, however, administration of hypoxia pre-conditioned MSC led to a significant reduction of 3-fold in proliferation. In bone marrow, bleomycin administration led to a significant reduction of 1.6 fold as compared to the control which increased upon MSC administration ( Figure 3b ). Similar to peripheral blood, administration of pre-conditioned MSC led to a significant reduction in cell proliferation.
Next, in BALF tissue, a significant reduction of 3.6 fold was observed due to bleomycin administration which increased significantly by 1.3 and two-fold upon administration of untreated and oxidative stress pre-conditioned MSC ( Figure 3c ). Administration of hypoxia pre- conditioned MSC did not bring about a change in cell proliferation. In lung tissue, administration of untreated, hypoxia pre-conditioned and oxidative stress pre-conditioned MSC led to a significant increase in cell proliferation by 1.2, 2.6 and 1.3 fold, respectively, as compared to the bleomycin-treated samples ( Figure 3 ).
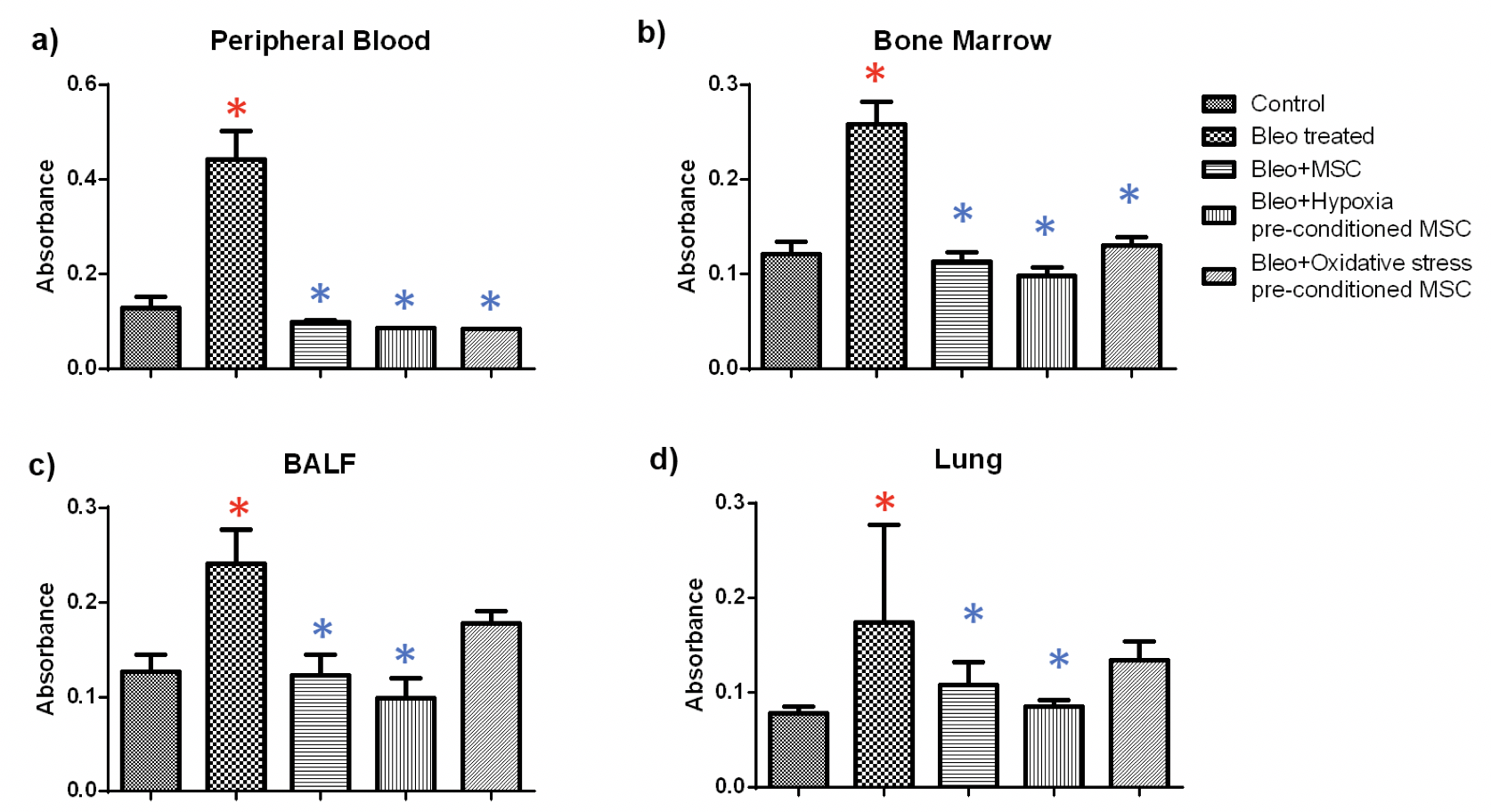
NBT: The NBT assay was used to examine superoxide generation, an indicator of inflammation, in the tissues of treated and untreated mice. We found that bleomycin administration led to a significant increase in inflammation in all the tissues and MSC treatment resulted in significant reduction of inflammation in all the tissues Figure 4 .
In blood, we found a 3.4 fold increase in inflammation in bleomycin-treated samples as compared to the control ( Figure 4a ). Administration of untreated and hypoxia or oxidative stress pre-conditioned MSC led to a reduction of 4.5, 5 and five fold, respectively as compared to bleomycin-treated samples. Similarly, in bone marrow, a 2.1 fold increase was observed which reduced by 2.3, 2.6 and two-fold upon administration of untreated, hypoxia and oxidative stress pre-conditioned MSC administration ( Figure 4b ). In BALF, administration of MSC and hypoxia pre-conditioned MSC led to a significant reduction of 1.9 and 2.4 fold, respectively ( Figure 4c ). Likewise, in lung we found a 1.6 and 2 fold reduction upon treatment with untreated and hypoxia pre-conditioned MSC ( Figure 4d ). Treatment with oxidative stress pre-conditioned MSC reduced inflammation both in BALF and lung, but the difference was not statistically significant.
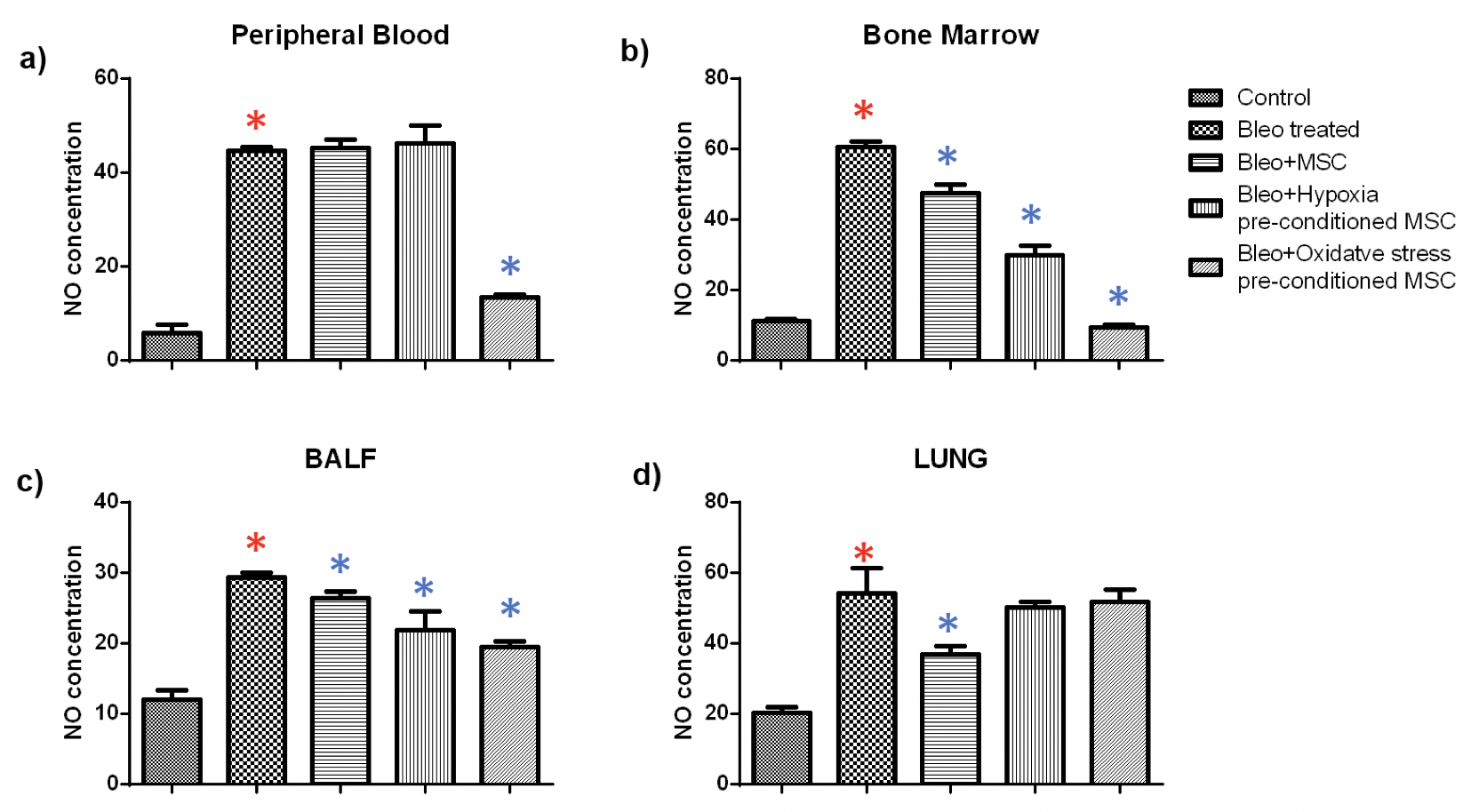
NO: Further, we examined nitric oxide generation in tissues from untreated, and MSC treated mice. Nitric oxide is produced by macrophages as part of the non-specific immune response and has been shown to be toxic to invasive microorganisms. Overproduction of NO has been observed in infectious or inflammatory states of the colon, bile duct, and liver. As expected we found that bleomycin treatment led to a significant increase in NO production in all the tissues. Administration of either untreated or pre-conditioned MSC resulted in a significant reduction in NO production.
In blood, we found that treatment with oxidative stress pre-conditioned MSC reduced the NO production significantly by 3.3 fold. Administration of untreated or hypoxia pre-conditioned MSC did not alter the NO production. In bone marrow, bleomycin treatment led to a significant fivefold increase in NO production; administration of untreated and hypoxia or oxidative stress pre-conditioned MSC resulted in a considerable reduction of 1.2, 2 and 6.5 fold. Similarly, in BALF, a 2.2 fold increase in NO production due to bleomycin administration was reduced significantly by 1.1, 1.3 and 1.5 fold, respectively, by treatment with untreated, hypoxia pre-conditioned and oxidative stress pre-conditioned MSC. In the lung, administration of untreated MSC led to a significant reduction in NO production by 1.5 fold while pre-conditioned MSC did not alter the NO production significantly Figure 5 .
These results suggest that MSC administration led to a reduction in inflammation as compared to the diseased mice. It led to an increase in cell proliferation in lung tissue but did not have a systemic effect in the blood and the bone marrow.
Flow cytometry analysis
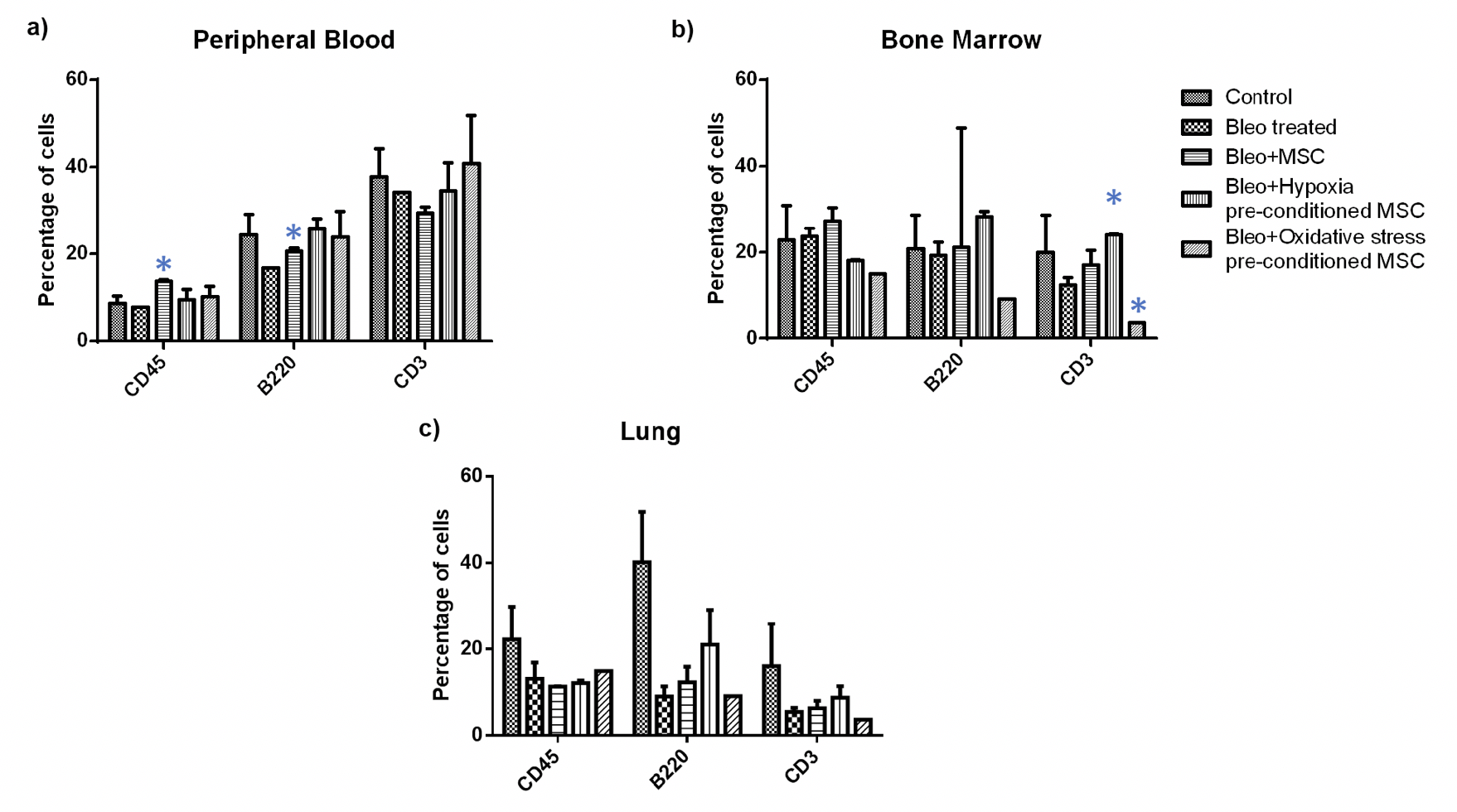
To further analyze if any specific inflammatory cell groups were altered, we performed flow cytometry analysis in blood, bone marrow, and lung tissue. Firstly, we examined if there was a change in the proportion of T-cell and B-cells as a result of the treatment ( Figure 6 ). We found a significant increase in the percentage of B-cells upon MSC administration in peripheral blood; no difference was observed between other treatment groups. Further, in bone marrow, we observed a significant increase in the number of T- cells upon administration of hypoxia pre-conditioned MSC and a significant reduction in the number of T- cells upon administration of oxidative stress pre-conditioned MSC; no difference was observed in other treatment groups. In the lung, none of the treatment groups showed any difference in the number of T- and B- cells.
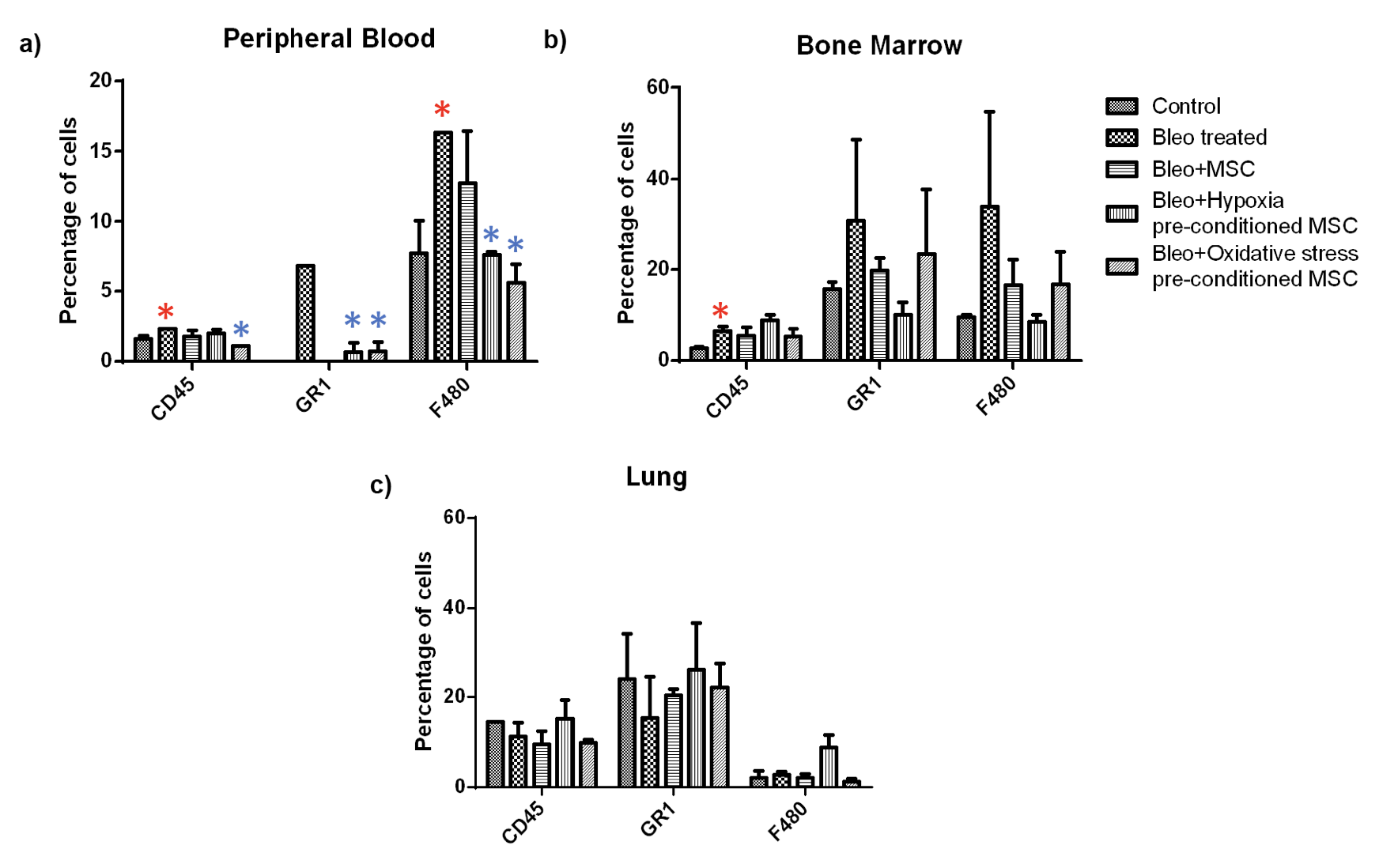
Next, we examined the percentage of granulocytes and macrophage in these tissues Figure 7 . We found a significant increase in the granulocyte and macrophage population in peripheral blood upon bleomycin treatment which reduced significantly upon treatment of pre-conditioned MSC. A similar trend was observed in the bone marrow. However, these changes were not statistically significant. In the lung, we did not observe any specific trend, and none of the changes were statistically significant.
Hydroxyproline analysis
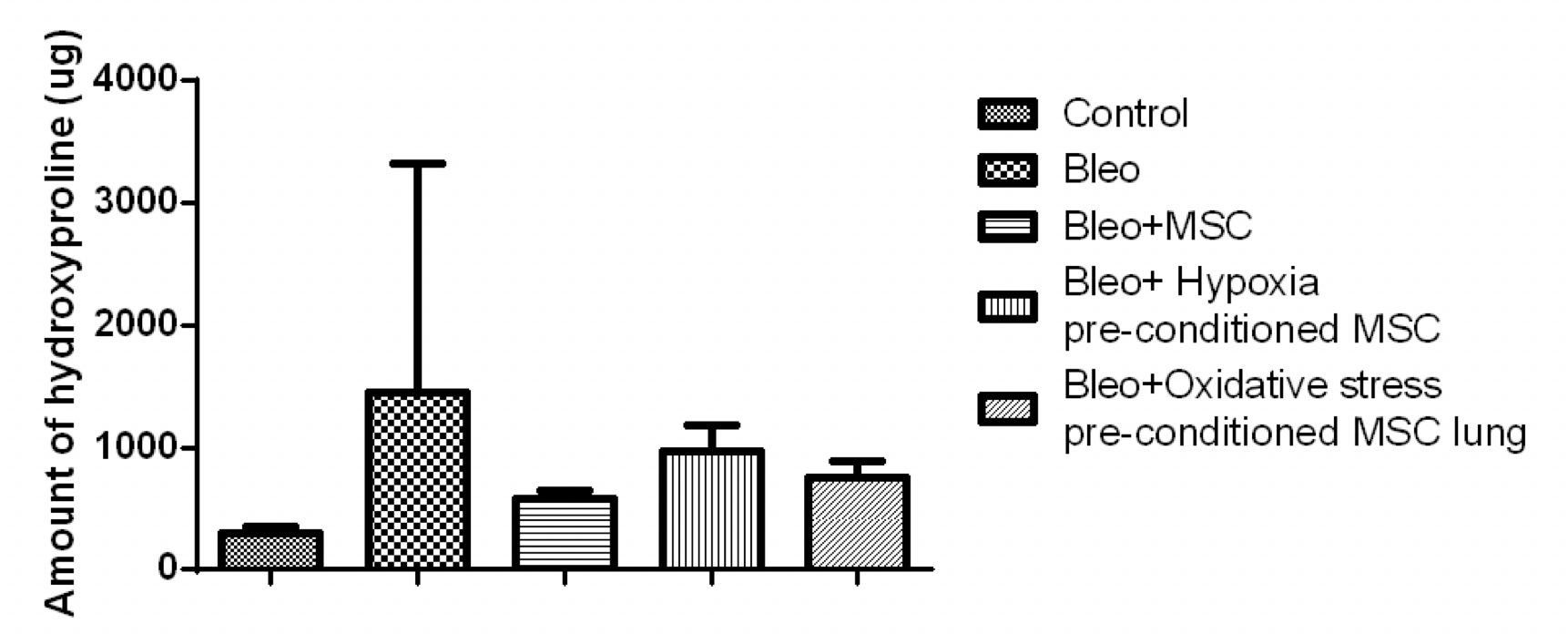
Next, we examined the hydroxyproline content in the lung. We found that the amount of hydroxyproline increased by five fold in the bleomycin-treated mice as compared to the control. This reduced by 2.4 fold upon treatment with MSC and by 1.5 and 1.9 fold upon treatment with hypoxia or oxidative stress pre-conditioned MSC, respectively ( Figure 8 ).
Histological analysis
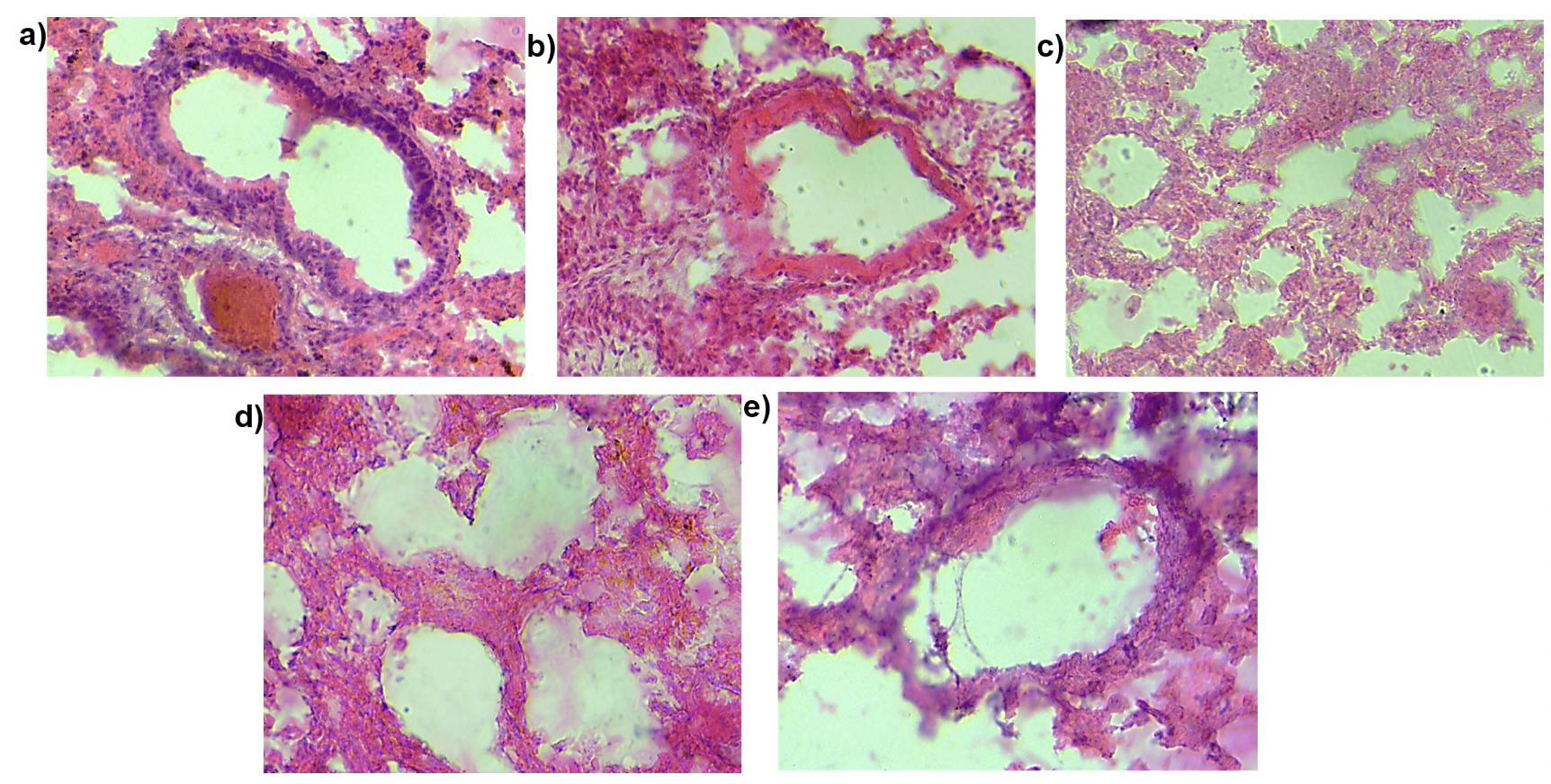
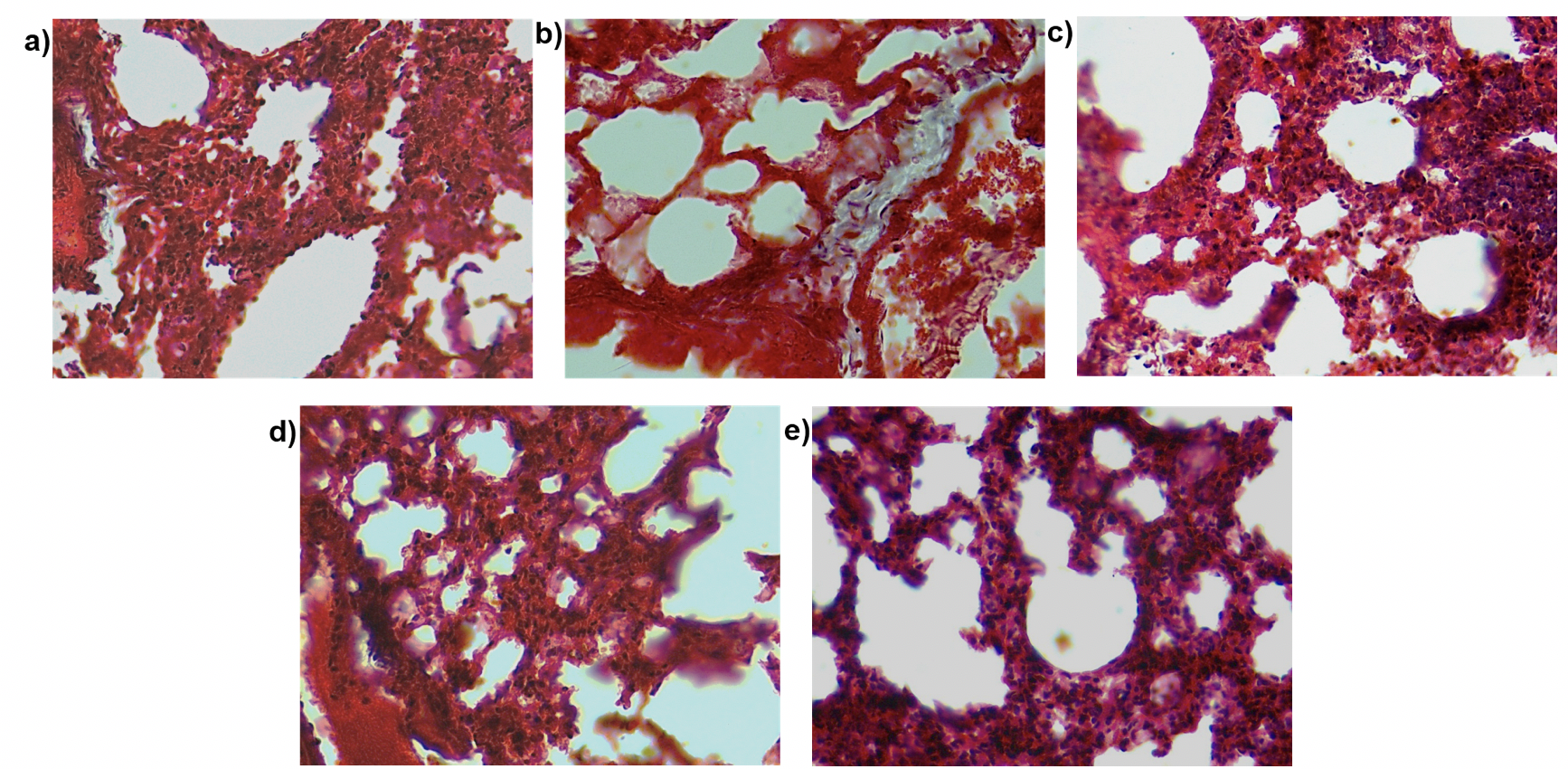
Pulmonary fibrosis as the name suggests leads to increased fibrosis in the lung. To examine the effect of MSC transplantation on lung histology and fibrosis we stained lung sections with hematoxylin and eosin stain ( Figure 9 ) and performed Masson’s trichrome staining ( Figure 10 ). This staining helps visualize the collagen content in the lung tissue. The purpose of Masson’s Trichome staining is to differentiate between collagen and smooth muscle in tumors and the increase of collagen content in diseases such as cirrhosis and fibrosis. In this experiment, the mice were treated with bleomycin which causes pulmonary fibrosis which in turn leads to increased collagen content of the lung tissue. Upon staining with hematoxylin and eosin, we observed a thickening of the alveolar walls in the bleomycin-treated mice as compared to the control mice. Additionally, bleomycin-treated lungs showed increased cell infiltration as compared to the control. There was a small decrease in the thickness of alveolar wall upon MSC transplantation. We did not observe any other structural changes in the lungs transplanted with MSC. Similarly, on Masson’s trichrome staining we found a few areas of the fibrotic lesion in the bleomycin-treated mice which were not present in the control or the mice treated with MSC. Cell infiltration was observed in all the treatment groups.
Discussion
The beneficial effect of MSC administration in pulmonary fibrosis has been demonstrated in multiple studies [3]. MSCs secrete various factors such as hepatocyte growth factor, epithelial growth factor and fibroblast growth factor and tumor growth factor-β to improve cell proliferation. They modulate the immune response by secreting an array of cytokines including interleukins, tumor necrosis factor-a and interferon-g [17]. Further MSCs are also known to have
anti-apoptotic properties which facilitate the survival of damaged cells [18][19]. Additionally, they also regulate T-cell activity [20][21]. Along with MSC administration, the extracellular vesicles or microvesicles secreted by the cells and conditioned medium may also be used for disease treatment [22][23]. In the present study, we examined the effect of administration of pre-conditioned MSC in a mouse model of idiopathic pulmonary fibrosis.
We observed increased cell viability in the lung upon MSC administration which again demonstrates the anti-apoptotic and regenerative ability of MSC. Perhaps these effects may be first seen at the site of injury, and therefore a similar effect could not be observed in peripheral blood and bone marrow. The response to tissue injury results in inflammatory processes followed by fibrosis [24]. MSC transplantation led to a reduction in inflammation in all the tissues. We found that similar to untreated MSC, pre-conditioned MSC reduced nitric oxide levels and increased cell proliferation as compared to the diseased tissues. Cells release nitric oxide as a mechanism to combat oxidative stress. NO levels in the cells increase in conditions associated with inflammation [25]. Lee et al. reported a similar time-dependent reduction in nitric oxide metabolites upon administration of MSC in a model of lung fibrosis [6]. The similar reduction in inflammation was observed in NBT assay.
Upon further analysis using flow cytometry, we found a significant increase in granulocytes and macrophage population in peripheral blood in the bleomycin-treated mice which reduced upon administration of MSC. Granulocytes, specifically neutrophil, and macrophages are known to be increased in an inflammatory condition which leads to the generation of swelling, redness, pain, and heat [26]. These results are consistent with previous reports which found a reduction in neutrophil and macrophage infiltration upon MSC administration [6].
MSC have been reported to reduce fibrosis in the kidney [27]. Other studies have observed improvement in lung histology upon MSC administration. Lee and colleagues found that intravenously administered MSCs decreased bleomycin-induced lung edema, neutrophil infiltration, collagen deposition, and overall mortality [6]. Similarly, Ortiz and colleagues reported that after bleomycin exposure in mice, MSCs home to sites of lung injury, lead to less inflammation, decrease fibrosis and extracellular matrix collagen deposition, and contribute to tissue repair [28]. We observed reduction in collagen upon MSC administration as seen in the hydroxyproline assay. We did not observe significant structural changes in MSC transplantation. This may be due to differences in time of administration of MSC.
Previously, hypoxic pre-conditioning using a hypoxia chamber was shown to attenuate lung disease in mice [13]. Earlier studies have demonstrated the potential of hypoxic pre-conditioning in improving cell survival, proliferation, homing and differentiation [29]. Hypoxic pre-conditioning of umbilical cord-derived MSC has also been shown to increase cell differentiation [30]. There haven’t been many studies demonstrating the use of oxidative stress pre-conditioning in pulmonary fibrosis. However, we did not observe any significant change upon administration of MSC and pre-conditioned MSC. Perhaps alteration of the dose of CoCl2 and H2O2 used to induce hypoxia and oxidative stress respectively may demonstrate improvement in lung histology. Increasing the duration of pre-conditioning to 48 or 72 hours may alter the efficacy of cells in ameliorating fibrosis. Further studies to examine the expression of cytokines and other growth factors will assist in understanding the mechanism of action of MSC and pre-conditioning of these cells.
Conclusion
Our results show that transplantation of MSCs reduced the inflammation and increased cell viability in the lung. The pre-conditioning of MSC with CoCl2 or H2O2 for 24 hours did not significantly improve the beneficial effect of MSC administration. Moreover, our results again demonstrate that MSC transplantation results in paracrine effects.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
BALF: Broncheoalveolar lavage fluid, BM-MSC: Bone marrow derived mesenchymal stem cells, MSC: Mesenchymal stem cells, MTT: (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium bromide, NBT: Nitroblue tetrazolium, NO: Nitric oxide
Ethics approval and consent to participate
Not be applied
Competing interests
The authors have no conflict of interest.
Funding
Not be applied
Authors’ contributions
AD performed the literature searches, experiments, data analysis and drafted the manuscript. SK performed the literature searches and experiments. ERB conceptualized the study and edited/reviewed the manuscript.
References
-
S
Geiger,
D
Hirsch,
FG
Hermann.
Cell therapy for lung disease. Eur Respir Rev.
2017;
26
.
-
L
Hagmeyer,
M
Treml,
C
Priegnitz,
WJ
Randerath.
Successful Concomitant Therapy with Pirfenidone and Nintedanib in Idiopathic Pulmonary Fibrosis: A Case Report. Respiration.
2016;
91
:
327-32
.
View Article Google Scholar -
X
Li,
S
Yue,
Z
Luo.
Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget.
2017;
8
:
102600-102616
.
View Article Google Scholar -
F
Zhao,
YF
Zhang,
YG
Liu,
JJ
Zhou,
ZK
Li,
CG
Wu,
HW
Qi.
Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc.
2008;
40
:
1700-5
.
View Article PubMed Google Scholar -
M
Choi,
T
Ban,
T
Rhim.
Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Mol Cells.
2014;
37
:
133-9
.
View Article PubMed PMC Google Scholar -
SH
Lee,
AS
Jang,
YE
Kim,
JY
Cha,
TH
Kim,
S
Jung,
SK
Park,
YK
Lee,
JH
Won,
YH
Kim,
CS
Park.
Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res.
2010;
11
:
16
.
View Article PubMed PMC Google Scholar -
LA
Ortiz,
M
Dutreil,
C
Fattman,
AC
Pandey,
G
Torres,
K
Go,
DG
Phinney.
Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A.
2007;
104
:
11002-7
.
View Article PubMed PMC Google Scholar -
Y
Moodley,
V
Vaghjiani,
J
Chan,
S
Baltic,
M
Ryan,
J
Tchongue,
CS
Samuel,
P
Murthi,
O
Parolini,
U
Manuelpillai.
Anti-inflammatory effects of adult stem cells in sustained lung injury: a comparative study. PLoS One.
2013;
8
:
e69299
.
View Article Google Scholar -
JM
Hare,
JH
Traverse,
TD
Henry,
N
Dib,
RK
Strumpf,
SP
Schulman,
G
Gerstenblith,
AN
DeMaria,
AE
Denktas,
RS
Gammon,
JH
J. B.,
MA
Reisman,
GL
Schaer,
W
Sherman.
A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol.
2009;
54
:
2277-86
.
View Article Google Scholar -
JH
Park,
DY
Kim,
IY
Sung,
GH
Choi,
MH
Jeon,
KK
Kim,
SR
Jeon.
Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery.
2012;
70
:
1238-47; discussion 1247
.
View Article PubMed Google Scholar -
VK
Prasad,
KG
Lucas,
GI
Kleiner,
JA
Talano,
D
Jacobsohn,
G
Broadwater,
R
Monroy,
J
Kurtzberg.
Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant.
2011;
17
:
534-41
.
View Article Google Scholar -
SP
Yu,
Z
Wei,
L
Wei.
Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res.
2013;
4
:
76-88
.
View Article PubMed PMC Google Scholar -
YW
Lan,
KB
Choo,
CM
Chen,
TH
Hung,
YB
Chen,
CH
Hsieh,
HP
Kuo,
KY
Chong.
Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther.
2015;
6
:
97
.
View Article PubMed PMC Google Scholar -
AV
Boopathy,
KD
Pendergrass,
PL
Che,
YS
Yoon,
ME
Davis.
Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res Ther.
2013;
4
:
43
.
View Article PubMed PMC Google Scholar -
S
Kar,
S
Biswas,
ER
Banerjee.
Evaluating the ameliorative potential of plant flavonoids and their nanocomposites in bleomycin induced idiopathic pulmonary fibrosis. Biomedical Research and Therapy.
2016;
3
:
707-722
.
View Article Google Scholar -
ER
Banerjee,
MA
Laflamme,
T
Papayannopoulou,
M
Kahn,
CE
Murry,
JH
W. R..
Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One.
2012;
7
:
e33165
.
View Article PubMed PMC Google Scholar -
A
Uccelli,
L
Moretta,
V
Pistoia.
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
2008;
8
:
726-36
.
View Article PubMed Google Scholar -
GJ
Block,
S
Ohkouchi,
F
Fung,
J
Frenkel,
C
Gregory,
R
Pochampally,
G
DiMattia,
DE
Sullivan,
DJ
Prockop.
Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells.
2009;
27
:
670-681
.
View Article PubMed PMC Google Scholar -
Z
Zhang,
A
Deb,
Z
Zhang,
A
Pachori,
W
He,
J
Guo,
R
Pratt,
VJ
Dzau.
Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol.
2009;
46
:
370-7
.
View Article PubMed PMC Google Scholar -
G
Ren,
L
Zhang,
X
Zhao,
G
Xu,
Y
Zhang,
AI
Roberts,
RC
Zhao,
Y
Shi.
Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell.
2008;
2
:
141-50
.
View Article PubMed Google Scholar -
Z
Selmani,
A
Naji,
I
Zidi,
B
Favier,
E
Gaiffe,
L
Obert,
C
Borg,
P
Saas,
P
Tiberghien,
N
Rouas- Freiss,
ED
Carosella,
F
Deschaseaux.
Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells.
2008;
26
:
212-22
.
View Article Google Scholar -
SR
Baglio,
DM
Pegtel,
N
Baldini.
Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol.
2012;
3
:
359
.
View Article PubMed PMC Google Scholar -
Q
Shen,
B
Chen,
Z
Xiao,
L
Zhao,
X
Xu,
X
Wan,
M
Jin,
J
Dai,
H
Dai.
Paracrine factors from mesenchymal stem cells attenuate epithelial injury and lung fibrosis. Mol Med Rep.
2015;
11
:
2831-7
.
View Article PubMed Google Scholar -
DJ
Prockop.
Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol.
2016;
51
:
7-13
.
View Article PubMed PMC Google Scholar -
JN
Sharma,
A
Al-Omran,
SS
Parvathy.
Role of nitric oxide in inflammatory diseases. Inflammopharmacology.
2007;
15
:
252-9
.
View Article PubMed Google Scholar -
R
Medzhitov.
Inflammation 2010: new adventures of an old flame. Cell.
2010;
140
:
771-6
.
View Article PubMed Google Scholar -
P
Semedo,
M
Correa-Costa,
MA
Cenedeze,
DMAC
Malheiros,
MA
dos Reis,
MH
Shimizu,
AC
Seguro,
A
Pacheco-Silva,
NOS
Camara.
Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells.
2009;
27
:
3063-73
.
View Article Google Scholar -
LA
Ortiz,
F
Gambelli,
C
McBride,
D
Gaupp,
M
Baddoo,
N
Kaminski,
DG
Phinney.
Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A.
2003;
100
:
8407-11
.
View Article PubMed PMC Google Scholar -
R
Das,
H
Jahr,
GJ
van Osch,
E
Farrell.
The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev.
2010;
16
:
159-68
.
View Article PubMed Google Scholar -
DJ
Griffon,
J
Cho,
JR
Wagner,
C
Charavaryamath,
J
Wei,
AW
Johnson.
Effects of Hypoxia and Chitosan on Equine Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int.
2016;
2016
:
2987140
.
View Article PubMed PMC Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 4 (2018)
Page No.: 2208-2222
Published on: 2018-04-26
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8148 times
- Download PDF downloaded - 1666 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress