Abstract
The objective: to investigate the relationship between levels of growth differentiation factor-15 (GDF-15) and circulating number of endothelial progenitor cells (EPCs) with angiopoietin phenotypes: CD34+CD14+CD309+, and CD34+CD14+CD309+Tie2+ in patients with type 2 DM.
Methods: The study was retrospectively involved 76 patients with type 2 DM aged 38 to 55 years and 30 healthy volunteers. Data collection included demographic and anthropometric information, hemodynamic performances and biomarkers of the diseases. Flow cytometry was used to determine EPCs' populations.
Results: The levels of GDF-15 in peripheral blood of diabetics associated with age (r = 0.31, P = 0.044), high-sensitive C-reactive protein [hs-CRP] (r = 0.40, P = 0.001), smoking (r = 0.38, P = 0.001), body mass index [BMI] (r = 0.34, P = 0.001), LDL cholesterol (r = 0.28, P = 0.001), glycated hemoglobin [HbA1c] (r = -0.28, P = 0.001), number of CV risk factors (r = 0.26, P = 0.001). In univariate logistic regression analysis we found that level of GDF-15 ≥ 618 pg/mL, hs-CRP ≥7.12 mg/L, HbA1c ≥6.4%, fasting glucose ≥6.7 mmol/L, and BMI ≥27.3 kg/m2 predicted deficiency of both angiopoetic phenotypes of EPCs. In multivariate logistic regression model GDF-15 ≥618 pg/mL demonstrated the best odds ratio values for declining of EPCs in diabetics in comparison with other predictors including BMI, HbA1c and hs-CRP.
Conclusion: GDF-15 was remarkably evaluated in type 2 DM population to healthy volunteers, and it was an independent factor that contributes to mobilization and probably proliferation of endothelial precursors with high angiopoetic activity.
Introduction
Prevalence of diabetes mellitus (DM) increases rapidly worldwide reaching pandemic proportion in developing and developed countries 123. The impact of DM on the public health strong associated with vascular complications, which predominantly determine morbidity and mortality from the disease 4. Indeed, DM regardless of phenotypes exhibit close link with atherosclerosis, stable ischemic heart disease, myocardial infarction / acute coronary syndrome, peripheral artery disease (PAD), retinopathy, stroke, heart failure chronic kidney disease and occur death prematurely due to cardiovascular (CV) causes 56. Despite being well-designed programs for lifestyle modification, glycemic, lipids and blood pressure control patients with type 2 DM (T2DM) have demonstrated 2-8-fold higher CV mortality rate to those who did not have T2DM 78. In this context, early stratification of people with diabetes at high risk of vascular complication based on biomarker assay was recognized as promising 9. Because developing of T2DM associated with glycaemia, insulin resistance, overweight / abdominal obesity, adipocytokine dysfunction, and other co-existing abnormalities (mitochondrial energy deficiency, hypercoagulation, oxidative stress, protein glycation, immune responses, inflammatory activity, some hormonal changes and metabolic memory phenomenon) 1011, there needs a biological marker reflecting several faces of pathophysiological process and corresponding to metabolic syndrome and T2DM.
Growth differentiation factor-15 (GDF-15) belongs to the transforming growth factor-beta/bone morphogenetic protein superfamily that involves in the pathogenesis of several diseases (CV disease, infective and inflammatory bowel diseases, metabolic diseases, rheumatic and autoimmune diseases) and it was recently found an independent predictor of all-cause and CV mortality in general population and T2DM patients 121314. The levels of circulating GDF-15 were positively related to traditional CV risk factors (age, smoking, hypertension, DM, body mass index), non-traditional CV risk factors (uric acid, pro-inflammatory cytokines, oxidative stress biomarkers, telomere length) 15. GDF-15 releases from broad spectrum of the cells (cardiomyocytes, endothelial cells, adipocytes, macrophages / mononuclear, vascular smooth muscle cells, astrocytes) due to direct pro-inflammatory cytokine stimulation and after tissue injury and hypoxia 16. Most of the investigators reported that GDF-15 plays a pivotal role in the protection of tissue from different injuries, although the innate molecular mechanisms of the effect remain unclear. The hypothesis of the study based on the assumption that GDF-15 could regulate vascular function and restores endothelial integrity through mobilization of endothelial progenitor cells (EPCs) with angiopoietin phenotypes and thereby mediate tissue repair activity.
According to molecular characteristics, EPCs may express appropriate surface antigens, such as CD31, CD 144, CD309 (vascular endothelial growth factor receptor-2), and CD133, but in the absence of CD45. The CD45(-) cells, which are detected and isolated based on single or combined expression of CD34, CD133 and CD309, referred to as EPCs 17, while after differentiation EPCs lose CD133 antigen and begin to be positively on CD31, vascular endothelial cadherin, endothelial NO synthase (eNOS) and von Willebrand factor 18. Depending on ability to appear in the fibronectin-coated dish all EPCs were divided into early outgrowth or late outgrowth endothelial cells. Interestingly, the late outgrowth precursors originated from peripheral blood, and ex vivo demonstrated appropriate immune phenotype CD31+CD146+CD105+CD309+ and Tei2 and functional properties suitable mature endothelial cells. There are two distinct populations of late outgrowth progenitor cells based on differential expression of the cell surface marker CD34. The population of EPCs with co-expression of CD34 antigen additionally to CD31(+), CD146(+), CD105(+), and CD309(+) exhibited higher proliferative capability and angiopoietin activity to CD34(-) EPCs 19. There is evidence regarding that the absence of CD34(+) EPC in the colony led to cultures collapsed within one or two passages that confirm an idea of strong hierarchy in self-renewal EPCs may be an essential functional feature of precursors. Finally, late outgrowth precursors may differentiate into functionally mature endothelial cells and progenitor-like angiogenesis-promoting cells (CD34+ EPCs).
Numerous clinical studies have shown the lowered number and weak function of CD34+ EPCs in patients with pre-diabetes and T2DM 202122. Moreover, vascular complications of DM including retinopathy and atherosclerosis, muscular related to declined circulating the number of CD34+ EPCs with high proliferative capacity and ability to restore vascular integrity and function 23. Thus, GDF-15 and CD34+EPCs could coordinate vascular repair and restore endothelial function in diabetics. This study aimed to investigate the relationship between levels of GDF-15 and circulating number of EPCs with proliferative and angiopoietin phenotypes: CD34+CD14+CD309+, and CD34+CD14+CD309+Tie2+ in patients with T2DM without known CV diseases.
Methods
The study cohort consisted of 76 patients with type 2 DM (41 males) aged 38 to 55 years who were retrospectively involved between March 2014 and July 2017. All DM patients included in the study have no known CV diseases including angina pectoris, previous myocardial infarction/stroke, heart failure, and asymptomatic atherosclerosis (defined by the negative result of the contrast-enhanced multiple spiral tomography angiography). Apart from established CV disease, the criteria of non-inclusion were acute infections; active inflammation; pulmonary edema; tachyarrhythmia; valvular heart disease; thyrotoxicosis; ischemic stroke; intracranial hemorrhage; surgery; trauma, autoimmune disease, malignancy, before the study entry. As a control cohort, we enrolled 30 healthy volunteers matched for age and sex with type 2 DM patients.
Ethical declaration
All the patients have given their written informed consent for participation in the study. The study was approved by the Local Ethical Committee. The investigators followed strictly all the requirements to clinical trials in conformity with the World Medical Association Declaration of Helsinki, 1964, good clinical practice provided by International Conference on Harmonization, Council of Europe Convention for the Protection of Human Rights and Dignity of the Human Being in view of using achievements in biology and medicine, convention on Human Rights and Biomedicine, including Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research, and legislation of Ukraine.
Diagnosis of T2DM was checked and confirmed according to the current recommendation of ADA (2018) 24. Patients with type 2 DM were treated according to current clinical guideline 6. All DM patients were treated with metformin in individually adjusted daily doses under continuous control of fasting glycemia, the daily profile of glucose concentration and glycated hemoglobin level (HbAc1). No insulin given patients with T2DM were selected in the study. Twenty-five T2DM individuals with mild-to-moderate arterial hypertension were treated with angiotensin-II receptor antagonist valsartan in daily doses 80 mg to 160 mg depending on office systolic and diastolic BP values. Dyslipidemic T2DM patients have treated with statins predominantly atorvastatin in averaged doses 40-80 mg/daily.
Demographic data, smoking status, and anthropometric measurements
Demographic factors such as age, gender, height, weight, body mass, body mass index, waist circumference, and waist-to-hip ratio past medical and medication history were collected at baseline. Current smoking was defined as consumption of one cigarette daily for three months 25.
Anthropometric data were measured by professional health attendants with the participants standing without shoes and heavy outer garments with a wall-mounted stadiometer (OMRON, Japan). Body mass index (BMI) was calculated by the staff person as weight (kg) divided by height squared (m2). Waist and hip circumference were measured in a standing position per protocol 2627.
Cardiac ultrasound and Doppler procedures
Transthoracic echocardiography was performed on ACUSON ultrasound system (Siemens, Germany) in В-mode regimen and Tissue Doppler Imaging (TDI) regimen from parasternal, subcostal, and apical positions over the short and long axis using 5 МHz phased transducers. Left ventricular ejection fraction (LVEF) was measured by the modified Simpson's method 28. Left ventricular (LV) mass was estimated using the formula recommended by American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group 28. LV hypertrophy (LVH) was defined as an LV mass/body surface area (BSA) ≥ 96 g/m2, for women, and ≥ 116 g/m2, for men 29. To measure peak systolic (Sm), early diastolic (Em), and late diastolic (Аm) myocardial velocities TDI was carried out according to the American Society of Echocardiography 29.
Calculation of glomerular filtration rate
CKD-EPI formula was used to calculate Glomerular Filtration Rate (GFR) 30.
Blood sampling
Blood samples were drawn in the morning following overnight fasting (at 7-8 a.m.) into barcoded silicone test tubes (Thermo Fisher Scientific, Waltham, MA, USA) wherein two mL of 5% Trilon B solution were added. Then samples were centrifuged upon permanent cooling at 6,000 rpm for 3 minutes, and then plasma was collected to be immediately refrigerated. Each aliquot was stored at a temperature -70оС.
The GDF-15 level was measured by ELISA assay using commercial kit manufacturing by LifeSpan BioSciences (USA) on Elecsys 1010 analyzer (Roche, Mannheim, Germany). Detection range was 31.2 to 2,000 pg/mL.
High-sensitivity C-reactive protein (C-RP) levels were measured by the nephelometric technique with the commercial kit (Eagle Biosciences, Nashua, NH, USA) and obtained with “AU640 Analyzer” (Olympus Diagnostic Systems Group, Japan).
High-performance liquid chromatography method was performed to determine hemoglobin A1c (HbA1c) in 5% Trilon B anticoagulated blood samples.
Concentrations of total cholesterol (TC), cholesterol of high-density lipoproteins (HDL-C), low-density lipoproteins (LDL-C) and triglycerides (TG) were measured by the direct enzymatic method with commercial kits (DIALAB, Neudorf, Austria) using automatic analyzer Roche P800 (F. Hoffmann-La Roche AG, Basel, Switzerland).
Sample preparation for isolating peripheral blood mononuclear cells
We used a standard method for isolating of peripheral blood mononuclear cells from collected blood samples by means density gradient centrifugation using “Lympholyte” solution (Cedarlane Laboratories, Burlington, ON, Canada). Each prepared sample contained 5 mL of peripheral blood were previously exposed to 1:2 dilution with Phosphate-Buffered Saline (PBS, Gibco™ PBS buffers, Thermo Fisher Scientific, Waltham, MA, USA) and stratified onto 5 mL of “Lympholyte” solution. All received samples were centrifuged 30 min at room temperature at 3,000 rpm without a brake. After separation, white blood cells were recollected, diluted with PBS+0.02% Tween and centrifuged at 1,200 rpm for 5 min at room temperature. For erythrocytes removal pellet was treated with RBC lysis buffer and washed 6X with 300µL in PBS before analysis.
Determining endothelial progenitor cells
Cell populations were phenotyped by multicolor flow cytometry in a single-tube panel consisting of CD45, CD34, CD14, СD309(VEGFR2) and Tie-2 antigens as per HD-FACS (High-Definition Fluorescence Activated Cell Sorter) methodology according to gating strategy of International Society of Hematotherapy and Graft Engineering sequential (ISHAGE protocol of gating strategy) 31. To determine appropriate antigens on the surfaces of EPCs we used FITC-CD34 (BD Biosciences, Franklin Lakes, NJ, USA), FITC-CD14 (BD Biosciences, Franklin Lakes, NJ, USA),APC-CDTie2 (Miltenyi Biotec, Bergisch Gladbach, Germany), PE-CD45 (R&D Systems, Minneapolis, MN, USA), and PE-CD309 (R&D Systems, Minneapolis, MN, USA). Angiopoietin immune phenotypes of EPC have been identified as CD45- CD34+CD14+CD309+ and CD45-CD34+CD14+CD309+Tie2+. For each sample, 500 thousand events have been analyzed. As evident from Figure, representative dot-plots reported coherent EPC phenotyping.
Statistical Analysis
Statistical analysis of the results obtained was carried out in SPSS system for Windows, Version 20 (SPSS Inc, Chicago, IL, USA). The data were expressed as mean (М) and error of the mean (±m) or a 95% confidence interval (CI); the median (Ме) and the interquartile range (IQR). Categorical variables were reported as numerous (n) and percentages (%). Shapiro–Wilk test and Kolmogorov-Smirnov test were used to assay the normality of continuous variables. To compare the main parameters of patients’ groups (subject to the type of distribution of the parameters analyzed), one-tailed Student t-test or Mann-Whitney U-test were used. The two-tailed version of the Wilcoxon test was used for paired comparison of parameter values inside the group. Categorical variables between groups were compared with Chi2 test (χ2) and Fisher F exact test. The factors, which could be associated potentially with the number of circulating EPCs, were determined by means of univariate analysis of variance (ANOVA); and then, the identified factors with Р< 0.1 also were studied by means of multivariate analysis of variance (MANOVA). The odds ratio (OR), Wald x2 and 95% CI were calculated for all the independent predictors of declining of the circulating number of EPCs with angiogenic phenotypes. The calculated difference of P<0.05 was considered statistically significant.
Results
Table 1 shows a general characteristic of the individuals included in the study. Patients with type 2 DM and healthy volunteers were matched for age, sex, smoking status, heart rate, left ventricular ejection fraction (LVEF) and frequency of left ventricular hypertrophy. However, at least 32% of all patients with established type 2 DM had hypertension, 36.8% of individuals had dyslipidemia, and 31.5% / 27.6% demonstrated overweight / obesity. Expectedly, body mass index and weight to hip ratio were significantly lower in healthy volunteers when compared with people with diabetes. Systolic and diastolic blood pressure levels in people with diabetes were slightly higher for healthy individuals.
| Parameters | Healthy volunteers (n=30) | Entire group of T2DM patients (n=76) | P value |
| Age, years | 45.30±5.30 | 47.90±5.10 | 0.66 |
| male, n (%) | 16 (53.3%) | 41 (53.9%) | 0.82 |
| Adherence to smoking, n (%) | 6 (20.0%) | 18 (23.7%) | 0.16 |
| Hypertension, n (%) | - | 25 (32.9%) | 0.001 |
| Dyslipidemia, n (%) | - | 28 (36.8%) | 0.001 |
| Overweight, n (%) | - | 24 (31.5%) | 0.001 |
| Obesity, n (%) | - | 21 (27.6%) | 0.001 |
| BMI, kg/m2 | 23.2 (21.7–25.2) | 27.3 (24.3–29.5) | 0.04 |
| WHR, units | 0.85 (0.82 – 0.87) | 1.02 (0.96 – 1.10) | 0.001 |
| Systolic BP, mm Hg | 121±4 | 132±7 | 0.044 |
| Diastolic BP, mm Hg | 68±4 | 78±5 | 0.042 |
| Heart rate, beat per min. | 65.25±4.18 | 70.15±5.20 | 0.12 |
| LVEF, % | 67.2 (61.9 – 72.8) | 60.3 (53.1 – 67.2) | 0.22 |
| Е/Аm, U | 8.6±0.54 | 11.1±1.61 | 0.12 |
| Е/Em, U | 7.6±0.70 | 11.0±1.68 | 0.12 |
| LVH, % | - | 31 (40.8%) | 0.001 |
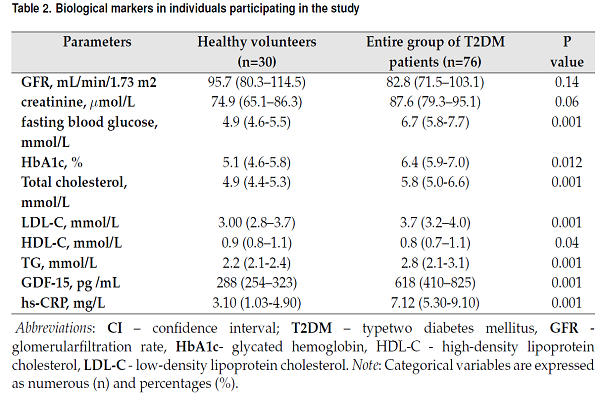
Biological markers in individuals participating in the study are reported in Table 2. There were no significant differences between both cohorts in estimated GFR as well as in levels of creatinine. Fasting glucose, HbA1c, hs-CRP, and GDF-15 in blood were sufficiently higher in people with diabetes to healthy volunteers. Additionally, lipid profile abnormality was found in people with diabetes rather than in healthy volunteers. Total cholesterol (TC), cholesterol of low-density lipoproteins (LDL) and triglycerides (TG) were higher, but cholesterol of high-density lipoproteins (HDL) was lower in people with diabetes in comparison with healthy individuals.
| Parameters | Healthy volunteers (n=30) | Entire group of T2DM patients (n=76) | P value |
| GFR, mL/min/1.73 m2 | 95.7 (80.3–114.5) | 82.8 (71.5–103.1) | 0.14 |
| creatinine, μmol/L | 74.9 (65.1–86.3) | 87.6 (79.3–95.1) | 0.06 |
| fasting blood glucose, mmol/L | 4.9 (4.6-5.5) | 6.7 (5.8-7.7) | 0.001 |
| HbA1c, % | 5.1 (4.6-5.8) | 6.4 (5.9-7.0) | 0.012 |
| Total cholesterol, mmol/L | 4.9 (4.4-5.3) | 5.8 (5.0-6.6) | 0.001 |
| LDL-C, mmol/L | 3.00 (2.8–3.7) | 3.7 (3.2–4.0) | 0.001 |
| HDL-C, mmol/L | 0.9 (0.8–1.1) | 0.8 (0.7–1.1) | 0.04 |
| TG, mmol/L | 2.2 (2.1-2.4) | 2.8 (2.1-3.1) | 0.001 |
| GDF-15, pg /mL | 288 (254–323) | 618 (410–825) | 0.001 |
| hs-CRP, mg/L | 3.10 (1.03-4.90) | 7.12 (5.30-9.10) | 0.001 |
The number of EPCs with angiopoietin phenotypes in individuals participating in the study presented in Table 3. There was significant difference (P=0.001) between cohorts in the number of circulating EPCs labeled CD45-CD34+CD14+CD309+ and CD45-CD34+CD14+CD309+Tie2+. The deficiency of these EPCs was found in people with diabetes in comparison with healthy volunteers.
| Cell phenotypes | Healthy volunteers (n=30) | Entire group of T2DM patients (n=76) | P value |
| CD45- CD34+CD14+CD309+, cells/µL | 10.54 (6.33-18.12) | 6.20 (4.30-9.15) | 0.001 |
| CD45- CD34+CD14+CD309+Tie2+, cells/µL | 7.23 (5.10-10.20) | 1.85 (1.01-2.95) | 0.001 |
The univariate linear regression analysis has shown an association between number of EPCs with immune phenotypes determined CD45-CD34+CD14+CD309+ and CD45-CD34+CD14+CD309+Tie2+ and CV risk factors, hemodynamic performances, and various biomarkers. In diabetics the number of CD45-CD34+CD14+CD309+ cells in peripheral blood inversely related to GDF-15 (r = -0.42, P = 0.001), BMI (r = -0.40, P = 0.001), hs-CRP (r = -0.38, P = 0.001), LV hypertrophy(r = -0.36, P = 0.012), fasting glucose (r = -0.36, P = 0.001), number of CV risk factors (r = -0.38, P = 0.001), LDL cholesterol (r = -0.30, P = 0.002), TG (r = -0.30, P = 0.001), age (r = -0.24, P = 0.014). Number of EPCs with immune phenotype CD45-CD34+CD14+CD309+Tie2+ related inversely to GDF-15 (r = -0.44, P = 0.001), BMI (r = -0.44, P = 0.001), hs-CRP (r = -0.42, P = 0.003), LV hypertrophy(r = -0.36, P = 0.012), fasting glucose (r = -0.38, P = 0.001), HbA1c (r = -0.34, P = 0.001), TG (r = -0.32, P = 0.001), number of CV risk factors (r = -0.36, P = 0.002), smoking (r = -0.32, P = 0.003), LDL cholesterol (r = -0.26, P = 0.002) and age (r = -0.24, P = 0.014).
Therefore, levels of GDF-15 in peripheral blood of diabetics associated with age (r = 0.31, P = 0.044), hs-CRP (r = 0.40, P = 0.001), smoking (r = 0.38, P = 0.001), BMI (r = 0.34, P = 0.001), LDL cholesterol (r = 0.28, P = 0.001), HbA1c (r = -0.28, P = 0.001), number of CV risk factors (r = 0.26, P = 0.001), whereas in healthy individuals concentrations of GDF-15 were not associated with age, but it related to adherence to smoke (r = 0.32, P = 0.001).
Multivariate linear regression analysis has shown that the number of CD45-CD34+CD14+CD309+ and CD45-CD34+CD14+CD309+Tie2+ EPCs in peripheral blood related to BMI (r = -0.42, P = 0.001 and r = -0.44, P = 0.002 respectively), concentration of GDF-15 (r = -0.40, P = 0.001 and r = -0.42, P = 0.001 respectively), hs-CRP (r = -0.34, P = 0.001 and r = -0.36, P = 0.001 respectively), fasting glucose (r = -0.32, P = 0.002 and r = -0.34, P = 0.002 respectively), HbA1c (r = -0.24, P = 0.001 and r = -0.30, P = 0.001 respectively), LDL cholesterol (r = -0.26, P = 0.002 and r = -0.30, P = 0.001 respectively). After BMI and LDL cholesterol adjusting multivariate linear regression analysis has demonstrated that the number of CD45-CD34+CD14+CD309+ and CD45-CD34+CD14+CD309+Tie2+ EPCs significantly related to concentration of GDF-15 (r = -0.38, P = 0.001 and r = -0.40, P = 0.001 respectively), hs-CRP (r = -0.32, P = 0.001 and r = -0.36, P = 0.001 respectively), HbA1c (r = -0.28, P = 0.001 and r = -0.30, P = 0.001 respectively) and fasting glucose (r = -0.32, P = 0.001 and r = -0.33, P = 0.003 respectively).
In univariate logistic regression analysis we found that level of GDF-15 above the median of plasma concentration (≥ 618 pg/mL), hs-CRP ≥7.12 mg/L, HbA1c ≥6.4%, fasting glucose ≥6.7 mmol/L, and BMI ≥27.3 kg/m2 predicted deficiency of both angiopoietin phenotypes of circulating EPCs. In multivariate logistic regression model GDF-15 ≥ 618 pg/mL demonstrated the best odds ratio (OR) values for declining of EPCs in people with diabetes in comparison with other predictors including BMI, HbA1c, and hs-CRP Table 4. Thus, our hypothesis regarding that level of GDF-15 could relate to the circulating number of EPCs with angiopoietin phenotypes was found confirmation in the results of the study.
| Variables | Univariate OR (95% CI) | Wald x2 | P-value | Multivariate OR (95% CI) | Wald x2 | P-value |
| Dependent variable: number of CD45- CD34+CD14+CD309+ EPCs | ||||||
| GDF-15 (≥ 618 pg / mL versus < 618 pg / mL) | 1.12 (1.02-1.28) | 18.2 | 0.001 | 1.10 (1.04-1.19) | 12.3 | 0.001 |
| hs-CRP (≥ 7.12 mg / L versus < 7.12 mg / L) | 1.04 (1.02-1.07) | 9.3 | 0.048 | 1.02 (1.00-1.04) | 8.3 | 0.06 |
| Fasting glucose ≥ 6.7 mmol/L versus <6.7 mmol/L | 1.03 (1.00-1.10) | 4.4 | 0.062 | - | - | - |
| HbA1c ≥ 6.4% versus <6.4% | 1.08 (1.02-1.16) | 8.5 | 0.001 | 1.03 (1.01-1.07) | 7.9 | 0.05 |
| BMI ≥ 27.3 kg/m2 versus <27.3 kg/m2 | 1.14 (1.05-1.23) | 19.1 | 0.003 | 1.08 (1.02-1.17) | 9.4 | 0.014 |
| Dependent variable: CD45- CD34+CD14+CD309+Tie2+ EPCs | ||||||
| GDF-15 (≥ 618 pg / mL versus < 618 pg / mL) | 1.16 (1.06-1.31) | 19.5 | 0.001 | 1.12 (1.03-1.26) | 15.1 | 0.001 |
| hs-CRP (≥ 7.12 mg / L versus < 7.12 mg / L) | 1.08 (1.04-1.16) | 11.6 | 0.001 | 1.04 (1.00-1.10) | 10.4 | 0.052 |
| Fasting glucose ≥ 6.7 mmol/L versus <6.7 mmol/L | 1.04 (1.02-1.09) | 4.1 | 0.012 | 1.03 (1.00-1.06) | 3.8 | 0.058 |
| HbA1c ≥ 6.4% versus <6.4% | 1.09 (1.04-1.18) | 9.4 | 0.001 | 1.05 (1.02-1.09) | 6.6 | 0.048 |
| BMI ≥ 27.3 kg/m2 versus <27.3 kg/m2 | 1.12 (1.01-1.22) | 16.4 | 0.001 | 1.10 (1.02-1.23) | 14.4 | 0.001 |
Discussion
This is the first study that determines the inverse association between levels of GDF-15 in peripheral blood and circulating number of EPCs with angiopoietin capacity labeled CD45-CD34+CD14+CD309+ and CD45-CD34+CD14+CD309+Tie2+ in type 2 DM patients without established CV diseases. We confirmed that elevated levels of GDF-15 in diabetic population positively related to age, BMI, smoking, and hs-CRP and that circulating number of EPCs in people with diabetes was significantly lower to healthy volunteers.
In this study, we have been hypothesized that deficiency of EPCs with high proliferative and angiopoietin activity in patients with type 2 DM associating with altered vascular repair could be compensated with the protective capability of GDF-15. Indeed, previous studies have revealed that elevated GDF-15 level could be a protector from injury of numerous tissues, such as heart, adipose tissue, and endothelium, by inhibiting c-Jun N-terminal kinase, Bcl-2-associated death promoter, and epidermal growth factor receptor and activating Smad, eNOS, PI3K, and AKT signaling pathways 323334. However, there are severe controversies in this issue, because few reports in opposite revealed that lowered GDF-15 levels are beneficial against endothelial injury and microvascular inflammation 3536. In contrast, there is evidence that successful glycemic control and treatment of dyslipidemia with statins in moderate-to-high doses did not cause changes in plasma levels of GDF-15 3738. Although traditionally achieving of full control for hyperglycemia and hyperlipidemia is considered a predictor of better clinical outcomes in people with diabetes, lack of GDF-15 dynamic requires to be clarified. We suggest that GDF-15 as stress-induced cytokine interplays PI3K / AKT signaling in EPCs supporting their proliferative capacity. On the other hand, inhibiting c-Jun N-terminal kinase and Bcl-2-associated death promoter with GDF-15 could protect EPCs epigenetically modified by LDL cholesterol, TG, pro-inflammatory cytokines, phospholipases, reactive oxygen species and improve survival of proangiogenic EPCs. Therefore, activating Smad / eNOS in early growth EPCs may accompany by eliminating apoptotic EPCs from circulation that prevent vascular injury 39. Nevertheless, stimulating of Tie2/PI3K/Akt/eNOS signaling in late growth EPCs supports their re-endothelialization capacity and survival 40. The final effect of GDF-15 on EPCs is high likely positive and associate with vascular reparation and protection of resident cells from “metabolic memory” phenomenon 41.
Additionally, there is a large body of evidence regarding that other CV risk factors corresponding to DM development and progression could directly influence on vascular wall and endothelium. Indeed, hypertension, dyslipidemia, hyperglycemia, and inflammation are established risk factors for multiple focus atherosclerosis and vascular calcification 6. Whether GDF-15 could be involved as the protector from atherosclerotic injury with similar molecular mechanisms as mentioned above is not sufficiently clear. Although some scientists reported that levels of GDF-15 in people with diabetes demonstrated the close positive association with traditional CV risk factors, such as LDL cholesterol, BMI, age, inflammatory activity, but not with TG 1514. The results of our study well correspond with these findings, and we yielded that GDF-15 could be a component of the endogenous vascular repair system that maintains vascular integrity and function. In fact, the role of GDF-15 in DM is not entirely understood and requires to be investigated in the large clinical trials focusing on protective mechanisms of GDF-15 on EPCs and other components of vascular repair system.
Study limitations
There were several limitations of the study predominantly relating to a small number of patients and no randomized design. Although it was a small sample size in this study, statistical power was adequate. To note, the duration of T2DM for each patient included in the study was not known and hence. It can be significant because there was a strong negative correlation between the number of circulating angiogenic EPCs and severity of the disease, as well as fluctuation of fasting glucose and the level of HbAc1. Therefore, in the study, we included T2DM patients without established CV disease including asymptomatic atherosclerosis. Probably, an association between GDF-15 and circulating number of angiogenic EPCs requires to be compared in people with diabetes with known atherosclerosis and/or CV disease. Additionally, our findings regarding protective role of GDF-15 remains to be confirmed via validation in external cohorts, particularly in large clinical trials.
Conclusions
The results of the study clarified that GDF-15 was an independent factor influenced on number of endothelial precursors with high angiopoetic activity in T2DM patients. These findings could take into consideration in risk stratification and individualized care of the T2DM patients.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CCBY4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
Аm: late diastolic myocardial velocityl; BMI: body mass index; BP: blood pressure; CI: confidence interval; CV: cardiovascular; DM: diabetes mellitus; E: peak velocity of early diastolic left ventricular filling; Em: early diastolic myocardial velocity; EPCs: endothelial progenitor cells; GDF-15: growth / differential factor-15; GFR: glomerular filtration rate; HD-FACS: High-Definition Fluorescence Activated Cell Sorter; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high sensitive C-reactive protein; LDL-C: low-density lipoprotein cholesterol; LV: left ventricular; LVEF: left ventricular ejection fraction; LVH: LV hypertrophy; OR: odds ratio; SSC: side scatter characteristic; WHR: weight to hip ratio
Ethics approval and consent to participate
All the patients have given their written informed consent for participation in the study. The study was approved by the Local Ethical Committee. The investigators followed strictly all the requirements to clinical trials in conformity with the World Medical Association Declaration of Helsinki, 1964, good clinical practice provided by International Conference on Harmonization, Council of Europe Convention for the Protection of Human Rights and Dignity of the Human Being in view of using achievements in biology and medicine, convention on Human Rights and Biomedicine, including Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research, and legislation of Ukraine.
Competing interests
The authors declare that they have no conflicts of interest.
Authors' contributions
The authors have contributed equally to this paper.
Acknowledgments
We thank all patients for their participation in the investigation, staff of the Regional Zaporozhye Hospital (Ukraine) and the doctors, nurses, and administrative staff in City hospital #6 (Zaporozhye, Ukraine), City hospital #10 (Zaporozhye, Ukraine), Private Clinic “Vita Center” (Zaporozhye, Ukraine), Regional Center of Cardiovascular Diseases (Zaporozhye, Ukraine), general practices, and site-managed organizations that assisted with the study.
References
-
Naseribafrouei
A.,
Eliassen
B. M.,
Melhus
M.,
Svartberg
J.,
Broderstad
A. R..
Prevalence of pre-diabetes and type 2 diabetes mellitus among Sami and non-Sami men and women in Northern Norway - The SAMINOR 2 Clinical Survey. International Journal of Circumpolar Health.
2018;
77
:
1463786
.
-
Barreto
M.,
Kislaya
I.,
Gaio
V.,
Rodrigues
A. P.,
Santos
A. J.,
Namorado
S.,
Prevalence, awareness, treatment and control of diabetes in Portugal: Results from the first National Health examination Survey (INSEF 2015). Diabetes Research and Clinical Practice.
2018;
140
:
271-8
.
-
Bullard
K. M.,
Cowie
C. C.,
Lessem
S. E.,
Saydah
S. H.,
Menke
A.,
Geiss
L. S..
Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR. Morbidity and Mortality Weekly Report.
2018;
67
:
359-61
.
-
López-Leal
J.,
Cueto-Manzano
A. M.,
Martínez-Torres
J.,
de la O-Peña
D.,
Téllez-Agraz
E. U.,
Cortés-Sanabria
L..
Prevalence and risk factors of chronic kidney disease in the comprehensive care program DiabetIMSS. Revista Medica del Instituto Mexicano del Seguro Social.
2017;
55
:
S210-8
.
-
Sarwar
N.,
Gao
P.,
Seshasai
S. R.,
Gobin
R.,
Kaptoge
S.,
Di Angelantonio
E.,
Emerging Risk Factors
Collaboration.
Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet.
2010;
375
:
2215-22
.
-
Piepoli
M. F.,
Hoes
A. W.,
Agewall
S.,
Albus
C.,
Brotons
C.,
Catapano
A. L.,
2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal.
2016;
37
:
2315-81
.
-
Chellapan
D. K.,
Sheng Yap
W.,
Bt Ahmad Suhaimi
N. A.,
Gupta
G.,
Dua
K..
Current therapies and targets for type 2 diabetes mellitus: a review. Panminerva Medica.
2018;
•••
.
-
Burggraaf
B.,
Castro Cabezas
M..
Interventions in type 2 diabetes mellitus and cardiovascular mortality-An overview of clinical trials. European Journal of Internal Medicine.
2017;
42
:
1-15
.
-
Berezin
A. E..
Cardiac biomarkers in diabetes mellitus: new dawn for risk stratification?. Diabetes & Metabolic Syndrome.
2017;
11
:
S201-8
.
-
Morrow
R. M.,
Picard
M.,
Derbeneva
O.,
Leipzig
J.,
McManus
M. J.,
Gouspillou
G..
Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America.
2017;
114
:
2705-10
.
-
Lin
J. F.,
Wu
S.,
Hsu
S. Y.,
Yeh
K. H.,
Chou
H. H.,
Cheng
S. T..
Growth-differentiation factor-15 and major cardiac events. The American Journal of the Medical Sciences.
2014;
347
:
305-11
.
-
Hsu
L. A.,
Wu
S.,
Juang
J. J.,
Chiang
F. T.,
Teng
M. S.,
Lin
J. F..
Growth Differentiation Factor 15 May Predict Mortality of Peripheral and Coronary Artery Diseases and Correlate with Their Risk Factors. Mediators of Inflammation.
2017;
2017
:
9398401
.
-
Wang
X.,
Zhu
L.,
Wu
Y.,
Sun
K.,
Su
M.,
Yu
L..
Plasma growth differentiation factor 15 predicts first-ever stroke in hypertensive patients. Medicine.
2016;
95
:
e4342
.
-
Resl
M.,
Clodi
M.,
Vila
G.,
Luger
A.,
Neuhold
S.,
Wurm
R..
Targeted multiple biomarker approach in predicting cardiovascular events in patients with diabetes. Heart (British Cardiac Society).
2016;
102
:
1963-8
.
-
Shin
M. Y.,
Kim
J. M.,
Kang
Y. E.,
Kim
M. K.,
Joung
K. H.,
Lee
J. H..
Association between Growth Differentiation Factor 15 (GDF15) and Cardiovascular Risk in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Journal of Korean Medical Science.
2016;
31
:
1413-8
.
-
Berezin
A. E..
Biomarkers for cardiovascular risk in patients with diabetes. Heart (British Cardiac Society).
2016;
102
:
1939-41
.
-
Haberzettl
P.,
Conklin
D. J.,
O’Toole
T. E..
Comprehensive Toxicology (Third Edition) 2018.
Google Scholar -
Ferreras
C.,
Cole
C. L.,
Urban
K.,
Jayson
G. C.,
Avizienyte
E..
Segregation of late outgrowth endothelial cells into functional endothelial CD34- and progenitor-like CD34+ cell populations. Angiogenesis.
2015;
18
:
47-68
.
-
Patel
J.,
Donovan
P.,
Khosrotehrani
K..
Concise Review: Functional Definition of Endothelial Progenitor Cells: A Molecular Perspective. Stem Cells Translational Medicine.
2016;
5
:
1302-6
.
-
Berezin
A. E.,
Kremzer
A. A.,
Berezina
T. A.,
Martovitskaya
Y. V.,
Gronenko
E. A..
Data regarding association between serum osteoprotegerin level, numerous of circulating endothelial-derived and mononuclear-derived progenitor cells in patients with metabolic syndrome. Data in Brief.
2016;
8
:
717-22
.
-
Falay
M.,
Aktas
S..
Endothelial Progenitor Cells (EPC) Count by Multicolor Flow Cytometry in Healthy Individuals and Diabetes Mellitus (DM) Patients. Clinical Laboratory.
2016;
62
:
2161-6
.
-
Berezin
A. E.,
Samura
T. A.,
Kremzer
A. A.,
Berezina
T. A.,
Martovitskaya
Y. V.,
Gromenko
E. A..
An association of serum vistafin level and number of circulating endothelial progenitor cells in type 2 diabetes mellitus patients. Diabetes & Metabolic Syndrome.
2016;
10
:
205-12
.
-
Bakogiannis
C.,
Tousoulis
D.,
Androulakis
E.,
Briasoulis
A.,
Papageorgiou
N.,
Vogiatzi
G..
Circulating endothelial progenitor cells as biomarkers for prediction of cardiovascular outcomes. Current Medicinal Chemistry.
2012;
19
:
2597-604
.
-
American Diabetes
Association.
Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clinical Diabetes.
2018;
36
:
14-37
.
-
Lindson-Hawley
N.,
Begh
R.,
McDermott
M. S.,
McEwen
A.,
Lycett
D..
The importance of practitioner smoking status: a survey of NHS Stop Smoking Service practitioners. Patient Education and Counseling.
2013;
93
:
139-45
.
-
World Health
Organization.
2008.
Google Scholar -
Ashwell
M.,
Gunn
P.,
Gibson
S..
Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity Reviews.
2012;
13
:
275-86
.
-
Quiñones
M. A.,
Douglas
P. S.,
Foster
E.,
Gorcsan
J.,
Lewis
J. F.,
Pearlman
A. S.,
American College of
Cardiology,
American Heart
Association,
American College of
Physicians,
American Society of Internal Medicine Task Force on Clinical
Competence.
American College of Cardiology/American Heart Association clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians—American Society of Internal Medicine Task Force on Clinical Competence. Circulation.
2003;
107
:
1068-89
.
-
Lang
R. M.,
Bierig
M.,
Devereux
R. B.,
Flachskampf
F. A.,
Foster
E.,
Pellikka
P. A.,
Chamber Quantification Writing
Group,
American Society of Echocardiography’s
Guidelines,
Standards
Committee,
European Association of
Echocardiography.
Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography.
2005;
18
:
1440-63
.
-
Levey
A. S.,
Stevens
L. A.,
Schmid
C. H.,
Zhang
Y. L.,
Castro
A. F.,
Feldman
H. I.,
Ckd
E. P. I..
A new equation to estimate glomerular filtration rate. Annals of Internal Medicine.
2009;
150
:
604-12
.
-
Tung
J. W.,
Parks
D. R.,
Moore
W. A.,
Herzenberg
L. A.,
Herzenberg
L. A..
New approaches to fluorescence compensation and visualization of FACS data. Clinical Immunology (Orlando, Fla.).
2004;
110
:
277-83
.
-
Adela
R.,
Banerjee
S. K..
GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. Journal of Diabetes Research.
2015;
2015
:
490842
.
-
Hong
J. H.,
Chung
H. K.,
Park
H. Y.,
Joung
K. H.,
Lee
J. H.,
Jung
J. G..
GDF15 Is a Novel Biomarker for Impaired Fasting Glucose. Diabetes & Metabolism Journal.
2014;
38
:
472-9
.
-
Dominguez-Rodriguez
A.,
Abreu-Gonzalez
P.,
Avanzas
P..
Usefulness of growth differentiation factor-15 levels to predict diabetic cardiomyopathy in asymptomatic patients with type 2 diabetes mellitus. The American Journal of Cardiology.
2014;
114
:
890-4
.
-
Berezin
A. E..
Diabetes mellitus related biomarker: the predictive role of growth-differentiation factor-15. Diabetes & Metabolic Syndrome.
2016;
10
:
S154-7
.
-
Krintus
M.,
Kozinski
M.,
Kubica
J.,
Sypniewska
G..
Critical appraisal of inflammatory markers in cardiovascular risk stratification. Critical Reviews in Clinical Laboratory Sciences.
2014;
51
:
263-79
.
-
Murakami
T.,
Ueba
Y.,
Shinoto
Y.,
Koga
Y.,
Kaneda
D.,
Hatoko
T..
Successful Glycemic Control Decreases the Elevated Serum FGF21 Level without Affecting Normal Serum GDF15 Levels in a Patient with Mitochondrial Diabetes. The Tohoku Journal of Experimental Medicine.
2016;
239
:
89-94
.
-
Kim
J. M.,
Back
M. K.,
Yi
H. S.,
Joung
K. H.,
Kim
H. J.,
Ku
B. J..
Effect of Atorvastatin on Growth Differentiation Factor-15 in Patients with Type 2 Diabetes Mellitus and Dyslipidemia. Diabetes & Metabolism Journal.
2016;
40
:
70-8
.
-
Zeng
H.,
Jiang
Y.,
Tang
H.,
Ren
Z.,
Zeng
G.,
Yang
Z..
Abnormal phosphorylation of Tie2/Akt/eNOS signaling pathway and decreased number or function of circulating endothelial progenitor cells in prehypertensive premenopausal women with diabetes mellitus. BMC Endocrine Disorders.
2016;
16
:
13
.
-
Yang
Z.,
Xia
W. H.,
Zhang
Y. Y.,
Xu
S. Y.,
Liu
X.,
Zhang
X. Y..
Shear stress-induced activation of Tie2-dependent signaling pathway enhances reendothelialization capacity of early endothelial progenitor cells. Journal of Molecular and Cellular Cardiology.
2012;
52
:
1155-63
.
-
Berezin
A. E..
Endothelial progenitor cells dysfunction and impaired tissue reparation: the missed link in diabetes mellitus development. Diabetes & Metabolic Syndrome.
2017;
11
:
215-20
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 7 (2018)
Page No.: 2480-2492
Published on: 2018-07-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4928 times
- Download PDF downloaded - 1989 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress


