Abstract
There is little about the incidence, mortality and risk factors of Esophageal cancer (EC) in the world. Therefore, the purpose of this study was to determine the incidence rate, mortality and EC risk factors in the world. This review study was conducted on published English research by January 2017 with the search in by March 2018 by searching in the databases of PubMed, Scopus, and Science Direct with the keywords "esophagus cancer," "epidemiology," "incidence," "mortality," "risk factor," " world." Based on the findings of this study, the geographical distribution of the EC differs according to subtitles, as AC is more prevalent in developed countries, while SCC is more prevalent in countries in Africa and East Asia. The most important risk factors for EC are the low intake of vegetables and fruits, drinking drinks and hot liquids, reducing the intake of nutritional supplements such as selenium and zinc, smoking, excessive consumption of alcohol, past medical history, obesity and exposure to some environmental factors. According to the findings, it seems that the main cause of EC- is an undesirable lifestyle. Therefore, it is possible to improve the lifestyle and inform the community about EC risk factors and healthy lifestyle education.
Abstract
There is little about the incidence, mortality and risk factors of Esophageal cancer (EC) in the world. Therefore, the purpose of this study was to determine the incidence rate, mortality and EC risk factors in the world. This review study was conducted on published English research by January 2017 with the search in by March 2018 by searching in the databases of PubMed, Scopus, and Science Direct with the keywords "esophagus cancer," "epidemiology," "incidence," "mortality," "risk factor," " world." Based on the findings of this study, the geographical distribution of the EC differs according to subtitles, as AC is more prevalent in developed countries, while SCC is more prevalent in countries in Africa and East Asia. The most important risk factors for EC are the low intake of vegetables and fruits, drinking drinks and hot liquids, reducing the intake of nutritional supplements such as selenium and zinc, smoking, excessive consumption of alcohol, past medical history, obesity and exposure to some environmental factors. According to the findings, it seems that the main cause of EC- is an undesirable lifestyle. Therefore, it is possible to improve the lifestyle and inform the community about EC risk factors and healthy lifestyle education.
Introduction
Esophageal cancer (EC) is the eighth most common cancer in the world and the sixth leading cause of death from cancer in the world 1. An increase in the incidence of EC with its recurrence has led to massive pressures on the health care system 2. Unfortunately, EC is often associated with an unfavorable prognosis for survival of 5 years, which varies from 4% to 40% based on the progression of the disease, with a total 5-year overall survival rate of 18% 3. It has a very good prognosis in the case of early detection 4. In the United States, it is estimated that 17,290 EC cases are diagnosed each year, and 15850 deaths due to the cancer are expected 5. In the whole world, in 2012 the rate of new EC was 455800 cases, and the resulting deaths were reported to be 400200 6. The timing of the incidence of the two EC subtypes is different. The incidence of Esophageal adenocarcinoma (AC) in several Western countries is rising dramatically due to increased risk factors such as overweight and obesity 7. On the contrary, the incidence of squamous cell cancer (SCC) in these countries is steadily declining due to reduced tobacco use and alcohol consumption. However, the incidence of SCC in certain Asian countries, such as Taiwan, is likely to increase due to increased tobacco and alcohol consumption 8. Findings from studies have shown that geographical and racial distribution of BC varies from place to place; As in Asian countries is more than American countries 9. Significant changes in the rate of incidence of EC indicates that various factors affect the increase in EC rates. On the other hand, most studies in this field focus more on clinical challenges and therapeutic approaches to EC management 101112. There is little about the incidence, mortality and risk factors of EC in the world. Therefore, the purpose of this study was to determine the incidence rate, mortality and EC risk factors in the world.
Methods
This review study was conducted on studies published in English by March 2018 by searching in the databases of PubMed, Scopus and Science Direct with the keywords "esophagus cancer", "epidemiology", "incidence", "mortality", "risk factors", " world". In addition, the reference lists of relevant articles were manually searched to find any other potentially eligible articles. The search strategy was tailored due to the requirements of each database. An advanced search was also carried out on cancer-related websites to access specific information for each country. We excluded reviews, commentaries, articles from overlapping samples, conference abstracts, and articles printed in languages other than English. In the initial electronic literature search, 1345 articles were obtained from, and 35 articles were obtained using manual search. After removing duplicates using Endnote X7 (n =850), the title and abstract of the remaining 530 articles were reviewed. After this stage, 102 articles were included in the study and 428 of these articles were removed because of scientific reasons and lack of eligibility criteria or unrelated to our aim, in all, 96 full papers were reviewed.
The incidence and mortality of EC in the world
The incidence of EC based on the geographical area is significantly different. In some countries, it is the second most common cancer, and in some others one of the few common cancers 3. More importantly, the geographical distribution of EC differs according to subtypes; AC is more prevalent in developed countries, while SCC is more prevalent in African and East Asian countries 1314. In terms of regional distribution, the highest standardized incidence rate was observed in both sexes in East Asia (11 per 100,000), East Africa (9.7 per 100,000), South Africa (9.7 per 100,000), Asia (7.7 per 100,000) and Sub-Saharan Africa (4. 5 per 100,000), respectively. In the continental terms have the highest incidence of EC was seen in Asia, Europe, Africa, North America, South America, and Oceania, respectively 215. Ten countries with the highest age-standardized incidence rates for EC include Malawi (24.2 per 100,000) Turkmenistan (19.7 per 100,000) Mongolia (17.6 per 100,000) Kenya (17.6 per 100,000), Uganda (17.1 per 100,000), Lesotho (15.1 per 100,000), Tajikistan (14.7 per 100,000), Burundi (12.8 per 100,000), Bangladesh (12.7 per 100,000) and China (12.5 s per 100,000), respectively 16. Racially, the incidence of ACC and SCC is different between African -Americans, and Asians as compared to Caucasians. The incidence of SCC in African American males is 4.8 times higher than in the Caucasians 17. Conversely, the incidence of AC in Caucasian men is 5 times higher than African Americans 2.
Histologically, the two main EC subtypes are AC and SCC. While SCC has been the most common form of EC for a long time and there is a steady increase in the incidence of AC, especially in developed Western countries 4. Regional incidence rates are low in areas such as Asia, Africa, and South America where the incidence of SCC is high. For both types of subtype, EC is higher in males than in females, with an average SCC and AC ratio of 2.5 and 4.4, respectively 18. The highest SCC rates are in the Asian and AC regions of North America 2. In Asia, the incidence of SCC in China is significantly higher than in other countries 19.
In terms of regional distribution, the highest age-standardized mortality rate was observed in both genders in East Asia (9.1 per 100,000), East Africa (9.1 per 100,000), South Africa (9 per 100,000), Asia (6.7 per 100,000) and Sub-Saharan Africa (5.1 per 100,000), respectively 2. In the continental terms have the highest incidence was seen in Asia (6.7), Africa (6.2), South America (3.4), North America (2.9) and Oceania (2.9), respectively 17. Ten countries with the highest age-standardized incidence rates for EC include Malawi (22.9 per 100,000) Turkmenistan (18.5 per 100,000), Kenya (16.5 per 100,000) Mongolia (15.9 per 100,000), Uganda (15.5 per 100,000), Lesotho (13.9 per 100,000), Tajikistan (13.6 per 100,000), Burundi (12.2 per 100,000), Bangladesh (11.7 per 100,000) and China (10.9 s per 100,000), respectively 16.
Risk factors for BC
In developing countries, the incidence of EC is higher, because of low levels of vitamins and minerals resulting from low consumption of vegetables, and fruits 20. The findings of a meta-analysis study on observational studies show that there is a significant relationship between the high intake of fruits and vegetables and the reduction of SCC risk 21. High incidence of EC was found in families living in Northeastern Iran due to very little consumption of fruits and vegetables in comparison with the families living in areas with low incidence of EC 2223.
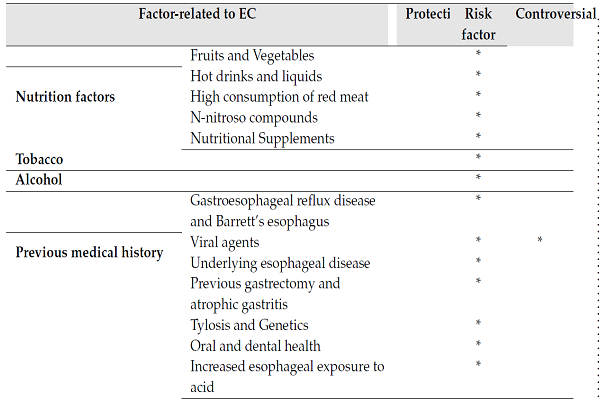
| Factor-related to EC | Protective | Risk factor | Controversial | |
| Nutrition factors | Fruits and Vegetables | * | ||
| Hot drinks and liquids | * | |||
| High consumption of red meat | * | |||
| N-nitroso compounds | * | |||
| Nutritional Supplements | * | |||
| Tobacco | * | |||
| Alcohol | * | |||
| Previous medical history | Gastroesophageal reflux disease and Barrett’s esophagus | * | ||
| Viral agents | * | * | ||
| Underlying esophageal disease | * | |||
| Previous gastrectomy and atrophic gastritis | * | |||
| Tylosis and Genetics | * | |||
| Oral and dental health | * | |||
| Increased esophageal exposure to acid | * | |||
| Drugs | proton pump inhibitors (PPIs) | * | ||
| statins | * | |||
| histamine receptor antagonists (H2RAs) | * | |||
| aspirin and other NSAIDs | * | |||
| bisphosphonate | * | |||
| Obesity | * | |||
| Epidermal growth factor polymorphisms | * | |||
| Polycyclic aromatic hydrocarbons (PAH) | * |
Hot drinks and liquids
One of the main risk factors for EC, especially in South American countries, is tea and coffee consumption. The consumption of hot drinks can also increase the risk of EC due to damage to the esophageal mucosa 2425. Findings from a systematic review of 59 studies showed that more than 50% of studies found that drinking high-temperature fluids is associated with a significant increase in EC risk 24. For tea and coffee, there is little evidence of the relationship between the rate of use (regardless of temperature) and the EC's risk. A few studies have provided separate findings for SCC and AC. The findings of a case-control study in northern Iran indicate that there is a relationship between the use of hot tea (60-64 ° C) or very hot tea (more than 65 ° C) with an increased risk of SCC 23. Findings from a cohort study based on another population in China also showed that a high risk of EC is associated with hot tea 25.
High consumption of red meat
The results of a cohort study on Dutchmen show that high consumption of processed meat is positively correlated with SCC 26. Findings from another prospective study also confirmed this finding 27. Several mechanisms can cause EC and gastric cancer after consuming red meat and processed meat. Heterocyclic amines and polycyclic aromatic hydrocarbons are carcinogenic substances that form during meat cooking at high temperatures 2829. The N-ntitroso compounds and other carcinogenic groups in processed meat contain high levels of nitrite and nitrate compounds 30. The endogenous formation of N-nitroso compounds is influenced by heme in meat, especially red meat 31. The findings of the meta-analysis study in China showed that receiving foods containing nitrogen compounds would increase the incidence of SCC 4.
N-nitroso compounds
Nitrosamines and nitrosamides are both subgroups of N-nitroso compounds that are produced by the reaction of nitrates with amines or amides. N-nitroso compounds increase the incidence of nasal cavity cancers, liver esophagus in many animal models 32. People are exposed to these chemical compounds by diet, tobacco, occupational exposure, or drinking water. Although 45% -75% of cases of exposure to these substances are due to metabolism are developed within the body 33. Directly nitrites are found in sodium nitrite, a preservative in various prepared foods and the metabolites of nitrites digested. Vegetables are the main source of environmental nitrites. However, high levels of nitrite may also be found in water 3435. The conversion of nitrate to nitrite by oral bacteria is a major contributor to the formation of N-nitroso compounds, and the answer may well be why inadequate oral health is associated with a high risk of esophageal and gastric cancers 3236. Contact with high levels of N-nitroso compounds was more common in diets in areas with high probability compared to areas with a lower probability in China for EC 37. A study in high-risk areas in northeastern Iran has shown that the amount of nitrosamine in the saliva of Gonbad's population is four times than the German population 22.
Nutritional Supplements
Findings Several studies have shown that there is a relationship between selenium supplementation and EC risk reduction 383940. Zinc deficiency increases the risk of EC due to increased carcinogens of nitrosamines and overexpression of cyclooxygenase-2 (COX) 41424344. Lack of nutritional intakes also increases the risk of EC 4546.
Tobacco
One of the main risk factors for SCC is smoking. A risk of EC in smokers is 5 times more likely than non-smokers 6. Several studies have found that TS is one of the main risk factors for EC 474849. Several studies have found that the risk of developing ESCC in smokers is 3 to 7 times higher than non-smokers 5051. In 2012, a meta-analysis study found that about 20-30% of esophageal cancer patients were addicted to cigarette smoking 52. Over the course of decades, numerous studies have shown that the incidence of AC in the United States and Great Britain in smokers has increased at a constant rate compared to non-smokers, while this trend is relatively constant for the ESCC and even a decreasing trend 5354. Taking into account the prevalence of smoking in Asia, smoking is also one of the main risk factors for EC in Asia55.
Alcohol
Alcohol is one of the main risk factors for ESCC. The relative risk (RR) of EC increases with increasing alcohol intake ranges from 1.8 to 7.8 based on the volume of alcohol received per week 6. The metabolism of alcohol is regulated by specific enzymes, which their activity and expression are affected by genetic polymorphism 19. Several ecological and retrospective studies in Europe have found that alcohol use has a positive and independent relationship with the increase in the incidence and mortality of adenocarcinoma of the esophagus (EAC). The findings of several prospective studies and meta-analysis revealed a significant and positive correlation between alcohol intake and ESCC risk.
Previous medical history
Gastroesophageal reflux disease and Barrett’s esophagus
The prevalence of gastroesophageal reflux disease in the western population is about 10% and in the United States about 60-30 million people. GERD can cause coughing, violent voice and a bitter taste in the mouth. Also, gastric acid can damage the esophagus and make it difficult to swallow. In more severe cases, GERD causes abnormal cells that can lead to unwanted EC cells 56. In more severe cases, GERD causes abnormal cells that can lead to unwanted EC cells 56. GERD symptoms are often minor and overlooked, but chronic inflammation in the esophagus can lead to irreparable complications. One of the most serious complications is Barrett's esophagus (BE) 57. In BE, the typical cover of the esophagus changes to the membrane, which is similar to the intestinal cover. An increase in the incidence of BE over the past 30 years has been associated with an increase in AC incidence over the same period 58. BE is a pre-cancerous lesion that develops in 6-14% of patients with GERD, and about 0.5-1.5% of these will develop AV 6. The study findings in Spain showed that the incidence of AC in BE patients during follow-up was 48% for each year (95% CI: 0.006% -2.62%) 59.
Viral agents
The only virus known to cause esophageal cancer is the human papillomavirus (HPV). Various types of HPV, especially species 16 and 18, are known to be major risk factors for cervical cancer, as well as in vulval, anal, penicillin and oropharyngeal cancers 60. Over the past 20 years, several studies have been conducted using various methods including isolating HPV DNA in tumor tissue and serologic methods for testing the relationship between HPV exposure and the risk of SCC 61. However, HPV DNA research results are not compatible. Methods in which PCR was used showed evidence of HPV in tumor tissue from 0% to 67% 6263. Epidemiological studies have also shown similar results with serological type-restricted studies 64. While some serological studies have found a positive relationship between SCC and HPV 16 6065, no cases have been found in other studies.
Underlying esophageal disease
The history of the previous disease in the esophagus, such as achalasia and caustic strictures, increases the risk of SCC 6667. In this regard, the findings of a population-based study of 1062 patients with achalasia showed that the risk of SCC increased more than 16-fold in the first 1 to 24-year period after diagnosis. On average, cancer has been detected in these patients 14 years after the diagnosis of achalasia 68. Other study findings of 2414 patients with SCC showed 63 cases had a history of caustic esophageal injury due to the ingestion of lye soap during childhood. The mean time for SCC diagnosis was 41 years after soap ingestion (between 13-71 years) 69.
Previous gastrectomy and atrophic gastritis
The risk of SCC in patients who have previously undergone partial gastrectomy is more than other people. In this regard, findings from a study showed that of 115 patients with relative gastrectomy, 12 cases (10%) were affected by SCC 70. However, other study findings indicated, the risk of SCC and AC was not affected by previous stomach surgery 71. The risk of SCC in patients with atrophic gastritis and other conditions resulting in gastric atrophy is twice that of others 72.
Tylosis and Genetics
Tylosis is a rare disease associated with palm and heel hyperkeratosis, which increases the risk of SCC 73. The hereditary type of Tylosis (Howell-Evans syndrome) is strongly associated with SCC 74. The disease is the most dominant autosomal inheritance; a gene locus is mapped onto the chromosome 17Q25.1, which probably contains a tumor suppressor gene 75. Study findings in China and Japan showed that genetic predisposition to SCC, especially in people who consume a lot of alcohol and cigarettes, is high 4. Also, an increase in areas with a high probability of esophageal squamous cell carcinoma from China to Central Asia to Russia and northeastern Iran and the high incidence of high esophageal cancers among people with Mongolian phenotypes may suggest a genetic involvement in this disease 76. Studies in China and Iran have shown that the likelihood of esophageal SCC carcinoma in first-degree relatives with esophageal cancer is more than twice as likely to have no history of esophageal cancer in the family 227778.
Oral and dental health
Several studies have reported the association between poor oral health and SCC infection, especially in some areas (China and Kashmar, Iran) that tobacco use and high alcohol consumption are not a major risk factor 417980. A few studies do not support this relationship 8182. There is a clear relationship between tooth loss and dysplasia of the esophagus, which is the precursor of SCC in esophagus 80. Oral health may prevent dental caries, oral and gum disease, and tooth loss. It is related with a lower probability to SCC. There are several mechanisms that may increase the risk of oral squamous cell carcinoma by their poor oral health 81. Scratch and physical damage to the epithelium of the esophagus associated with ingestion of unpeeled foods, altered food patterns due to unhealthy teeth, changes in oral flora by increasing carcinogenic microorganisms, oral mucosal infection with oral microorganisms, and genetic factors affecting the mouth. All of them are effective in squamous cell carcinoma 83. In China 80, America 84 and Japan 48, inadequate oral and dental health has been studied as a precursor to the SCC dysplasia.
Increased esophageal exposure to acid
Findings from a study have shown that patients such as the Zollinger-Ellison syndrome, or conditions associated with gastroesophageal reflux such as surgical myotomy or sciatica scleroderma, which are prone to acid secretion, are susceptible to AC 858687.
Also, in patients with BE due to gastroesophageal reflux, the use of proton pump inhibitors will not reduce EC risk 88.
Drugs
Findings from observational studies have shown that the use of proton pump inhibitors (PPIs) and statins in patients with BE may reduce the progression of adenocarcinoma 6. The findings of a meta-analysis and the systematic study showed that there is a significant relationship between the use of PPIs and histamine receptor antagonists (H2RAs) and the reduction of the risk of esophageal adenocarcinoma 89. Regular use of aspirin and other NSAIDs has chemopreventive effects on multiple cancers 9091. The findings of a meta-analysis study showed that regular use of aspirin and NSAID is a protective factor in esophageal cancer with odds ratios of 0.5 (95% CI: 0.38-0.66) and 0.75 (0.1-54), respectively 92. Aspirin and NSAIDs are prostaglandin-endoperoxide synthase 1 and 2; the enzymes involved in the production of prostaglandin. However, the precise biological mechanisms involved in the antinociceptive effects of aspirin are still unknown 93. The findings of a preliminary experimental study that examined the beneficial effect of postoperative use of aspirin on the survival rate of patients with esophageal cancer showed the 5-year survival rate of aspirin users was 51.2%, in the placebo group 41%, and in patients who did not use the pill, 42.3% (p = 0.04 or p = 0.029 when both groups were combined) 94. The use of bisphosphonates is related to AC and SCC 95. A cohort study in United Kingdom on 41,826 people treated with bisphosphonate in the years 1996 to 2006 and individually matched with a control group based on gender, age and general function regardless of the use of bisphosphate showed that after a 4-year follow-up, there was no difference in the risk of EC or stomach cancer combined with EC alone in these two groups (adjusted risk ratio [HR] 1.07, 95 % CI 0.77-1.49) 96.
Obesity
The first reports about the possible relationship between obesity and the EAC were published in the 1990s 9798. This finding was confirmed in studies of large populations, case studies in the United States, Europe, and Australia, and indicate a strong correlation between the increase in BMI and the risk of EA 99100. Findings from epidemiological studies indicate that obesity is one of the main causes of AC 101102. One of the main risk factors for ACE is gastro-esophageal reflux disease (GER and obese people experience GER symptoms repeatedly 101. Two main mechanisms for the development of AC in obese patients have been proposed. First, the physical mechanism involves an increase in the incidence of GERD, and the second is the mechanism of hormone-dependent, which is mainly mediated by inflammatory markers secreted by adipocytes 6.
Other factors
Gender and race
Histologically, SCC is common in blacks and white women, and AC is common in white men 4. The incidence of Esophageal squamous-cell carcinoma (ESCC) is usually higher in males, especially in black males 6.
Epidermal growth factor polymorphisms
Specific polymorphisms of the epidermal growth factor gene are associated with higher levels of serum epidermal growth factor and increased risk of AC, especially in patients with BE 103104.
Polycyclic aromatic hydrocarbons (PAH)
These hydrocarbons are known carcinogenic materials that are created during the incomplete burning of wood and its derivatives such as coal, coal, tobacco, and so on. Several studies have shown that PAH is an etiologic agent associated with gastrointestinal cancers, including SCC 105106. In a study conducted in Lingyan, evidence of tissue pathology consistent with PAH exposure in SCC cases 105, the presence of high levels of PAH in raw and cooked foods 28 and high concentrations of hydroxypyrone glucuronide (OHPG-1), a PAH metabolite in the sample Urinary tract have been reported107. In studies in northeastern Iran, evidence of the potential role of PAH has been found to be an important factor in the creation of SCC 108.
Summary
This study evaluates the incidence, mortality and risk factors of EC based on a review of studies conducted in the world. Based on the findings of this study, the incidence of EC based on the geographical area is significantly different. More importantly, the geographical distribution of the EC differs according to subtitles, as AC is more prevalent in developed countries, while SCC is more prevalent in countries in Africa and East Asia. In terms of regional distribution, the highest standardized rate of incidence and mortality was found in both sexes in East Asia, Africa, South Africa, Asia, and Sub-Saharan Africa. The most important risk factors for EC are the low intake of vegetables and fruits, drinking drinks and hot liquids, reducing the intake of nutritional supplements such as selenium and zinc, smoking, excessive consumption of alcohol, past medical history, obesity and exposure to some environmental factors. According to the findings, it seems that the main cause of EC- is an undesirable lifestyle. Therefore, it is possible to improve the lifestyle and inform the community about EC risk factors and healthy lifestyle education.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CCBY4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
AC: Esophageal adenocarcinoma; EAC: adenocarcinoma of the esophagus; EC: Esophageal cancer; ESCC: Esophageal squamous-cell carcinoma; GERD: Gastroesophageal reflux disease; H2Ras: Histamine receptor antagonists; HPV: Human papillomavirus; PAH: Polycyclic aromatic hydrocarbons; PPIs: proton pump inhibitors; RR: Relative risk; SCC: Squamous cell cancer
Ethics approval and consent to participate
Not to be applied
Competing interests
The authors declare that they have no conflicts of interest.
Authors' contributions
All authors contributed to the design of the research, M.SY, MSE, NPA, MA, OO, extracted the data and summarized it. All authors drafted the first version. HSG, and HS edited the first draft. All authors reviewed, commented and approved the final version.
References
-
Rafiemanesh
H.,
Mehtarpour
M.,
Khani
F.,
Hesami
S. M.,
Shamlou
R.,
Towhidi
F..
Epidemiology, incidence and mortality of lung cancer and their relationship with the development index in the world. Journal of Thoracic Disease.
2016;
8
:
1094-102
.
-
Pakzad
R.,
Mohammadian-Hafshejani
A.,
Khosravi
B.,
Soltani
S.,
Pakzad
I.,
Mohammadian
M..
The incidence and mortality of esophageal cancer and their relationship to development in Asia. Annals of Translational Medicine.
2016;
4
:
29
.
-
Fitzmaurice
C.,
Dicker
D.,
Pain
A.,
Hamavid
H.,
Moradi-Lakeh
M.,
MacIntyre
M. F.,
Global Burden of Disease Cancer
Collaboration.
The global burden of cancer 2013. JAMA Oncology.
2015;
1
:
505-27
.
-
Rafiemanesh
H.,
Maleki
F.,
Mohammadian-Hafshejani
A.,
Salemi
M.,
Salehiniya
H..
The trend in histological changes and the incidence of esophagus cancer in Iran (2003–2008). International Journal of Preventive Medicine.
2016;
7
:
31
.
-
Siegel
R. L.,
Miller
K. D.,
Jemal
A..
Cancer statistics, 2018. CA: a Cancer Journal for Clinicians.
2018;
68
:
7-30
.
-
Wheeler
J. B.,
Reed
C. E..
Epidemiology of esophageal cancer. The Surgical Clinics of North America.
2012;
92
:
1077-87
.
-
Pohl
H.,
Sirovich
B.,
Welch
H. G..
Esophageal adenocarcinoma incidence: are we reaching the peak?. Cancer Epidemiology, Biomarkers & Prevention.
2010;
19
:
1468-70
.
-
Lu
C. L.,
Lang
H. C.,
Luo
J. C.,
Liu
C. C.,
Lin
H. C.,
Chang
F. Y..
Increasing trend of the incidence of esophageal squamous cell carcinoma, but not adenocarcinoma, in Taiwan. Cancer Causes & Control.
2010;
21
:
269-74
.
-
Jemal
A.,
Center
M. M.,
DeSantis
C.,
Ward
E. M..
Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiology, Biomarkers & Prevention.
2010;
19
:
1893-907
.
-
Sihvo
E,
Anttonen
A,
Huuhtanen
R.
Treatment of esophageal cancer. Duodecim; laaketieteellinen aikakauskirja.
2014;
130
:
565-572
.
-
Wouters
M. W.,
Gooiker
G. A.,
van Sandick
J. W.,
Tollenaar
R. A..
The volume-outcome relation in the surgical treatment of esophageal cancer: a systematic review and meta-analysis. Cancer.
2012;
118
:
1754-63
.
-
Tepper
J.,
Krasna
M. J.,
Niedzwiecki
D.,
Hollis
D.,
Reed
C. E.,
Goldberg
R..
Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. Journal of Clinical Oncology.
2008;
26
:
1086-92
.
-
Rustgi
A. K.,
El-Serag
H. B..
Esophageal carcinoma. The New England Journal of Medicine.
2014;
371
:
2499-509
.
-
Arnold
M.,
Soerjomataram
I.,
Ferlay
J.,
Forman
D..
Global incidence of oesophageal cancer by histological subtype in 2012. Gut.
2015;
64
:
381-7
.
-
Torre
L. A.,
Bray
F.,
Siegel
R. L.,
Ferlay
J.,
Lortet-Tieulent
J.,
Jemal
A..
Global cancer statistics, 2012. CA: a Cancer Journal for Clinicians.
2015;
65
:
87-108
.
-
Jemal
A.,
Bray
F.,
Center
M. M.,
Ferlay
J.,
Ward
E.,
Forman
D..
Global cancer statistics. CA: a Cancer Journal for Clinicians.
2011;
61
:
69-90
.
-
Napier
K. J.,
Scheerer
M.,
Misra
S..
Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World Journal of Gastrointestinal Oncology.
2014;
6
:
112-20
.
-
Rice
TW,
Rusch
VW,
Ishwaran
H,
Blackstone
EH.
Worldwide Esophageal Cancer C. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer.
2010;
116
:
3763-73
.
-
Zhang
H. Z.,
Jin
G. F.,
Shen
H. B..
Epidemiologic differences in esophageal cancer between Asian and Western populations. Chinese Journal of Cancer.
2012;
31
:
281-6
.
-
Freedman
N. D.,
Park
Y.,
Subar
A. F.,
Hollenbeck
A. R.,
Leitzmann
M. F.,
Schatzkin
A..
Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. International Journal of Cancer.
2007;
121
:
2753-60
.
-
Liu
J.,
Wang
J.,
Leng
Y.,
Lv
C..
Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. International Journal of Cancer.
2013;
133
:
473-85
.
-
Kamangar
Farin,
Malekzadeh
Reza,
Dawsey
Sanford M,
SAEIDI
F.
Esophageal cancer in Northeastern Iran: a review. 2007
.
-
Islami
F.,
Pourshams
A.,
Nasrollahzadeh
D.,
Kamangar
F.,
Fahimi
S.,
Shakeri
R..
Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ (Clinical Research Ed.).
2009;
338
:
b929
.
-
Islami
F.,
Boffetta
P.,
Ren
J. S.,
Pedoeim
L.,
Khatib
D.,
Kamangar
F..
High-temperature beverages and foods and esophageal cancer risk—a systematic review. International Journal of Cancer.
2009;
125
:
491-524
.
-
Wu
M.,
Liu
A. M.,
Kampman
E.,
Zhang
Z. F.,
Van’t Veer
P.,
Wu
D. L..
Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case-control study. International Journal of Cancer.
2009;
124
:
1907-13
.
-
Keszei
A. P.,
Schouten
L. J.,
Goldbohm
R. A.,
van den Brandt
P. A..
Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2012;
23
:
2319-26
.
-
Cross
A. J.,
Freedman
N. D.,
Ren
J.,
Ward
M. H.,
Hollenbeck
A. R.,
Schatzkin
A..
Meat consumption and risk of esophageal and gastric cancer in a large prospective study. The American Journal of Gastroenterology.
2011;
106
:
432-42
.
-
Phillips
D. H..
Polycyclic aromatic hydrocarbons in the diet. Mutation Research.
1999;
443
:
139-47
.
-
Skog
K. I.,
Johansson
M. A.,
Jägerstad
M. I..
Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food and Chemical Toxicology.
1998;
36
:
879-96
.
-
Tricker
AR.
N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP).
1997;
6
:
226-268
.
-
Cross
A. J.,
Pollock
J. R.,
Bingham
S. A..
Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Research.
2003;
63
:
2358-60
.
-
Mirvish
S. S..
Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Letters.
1995;
93
:
17-48
.
-
Bartsch
H.,
Ohshima
H.,
Pignatelli
B.,
Calmels
S..
Human exposure to endogenous N-nitroso compounds: quantitative estimates in subjects at high risk for cancer of the oral cavity, oesophagus, stomach and urinary bladder. Cancer Surveys.
1989;
8
:
335-62
.
-
Eichholzer
M.,
Gutzwiller
F..
Dietary nitrates, nitrites, and N-nitroso compounds and cancer risk: a review of the epidemiologic evidence. Nutrition Reviews.
1998;
56
:
95-105
.
-
Lijinsky
W..
N-Nitroso compounds in the diet. Mutation Research.
1999;
443
:
129-38
.
-
Loh
Y. H.,
Jakszyn
P.,
Luben
R. N.,
Mulligan
A. A.,
Mitrou
P. N.,
Khaw
K. T..
N-nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk Study–. The American Journal of Clinical Nutrition.
2011;
93
:
1053-61
.
-
Wu
Y.,
Chen
J.,
Ohshima
H.,
Pignatelli
B.,
Boreham
J.,
Li
J..
Geographic association between urinary excretion of N-nitroso compounds and oesophageal cancer mortality in China. International Journal of Cancer.
1993;
54
:
713-9
.
-
Li
B.,
Taylor
P. R.,
Li
J. Y.,
Dawsey
S. M.,
Wang
W.,
Tangrea
J. A..
Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Annals of Epidemiology.
1993;
3
:
577-85
.
-
Blot
W. J.,
Li
J. Y.,
Taylor
P. R.,
Guo
W.,
Dawsey
S.,
Wang
G. Q..
Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. Journal of the National Cancer Institute.
1993;
85
:
1483-92
.
-
Mark
S. D.,
Liu
S. F.,
Li
J. Y.,
Gail
M. H.,
Shen
Q.,
Dawsey
S. M..
The effect of vitamin and mineral supplementation on esophageal cytology: results from the Linxian Dysplasia Trial. International Journal of Cancer.
1994;
57
:
162-6
.
-
Abnet
C. C.,
Lai
B.,
Qiao
Y. L.,
Vogt
S.,
Luo
X. M.,
Taylor
P. R..
Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. Journal of the National Cancer Institute.
2005;
97
:
301-6
.
-
Fong
L. Y.,
Sivak
A.,
Newberne
P. M..
Zinc deficiency and methylbenzylnitrosamine-induced esophageal cancer in rats. Journal of the National Cancer Institute.
1978;
61
:
145-50
.
-
Fong
L. Y.,
Magee
P. N..
Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Letters.
1999;
143
:
63-9
.
-
Fong
L. Y.,
Zhang
L.,
Jiang
Y.,
Farber
J. L..
Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. Journal of the National Cancer Institute.
2005;
97
:
40-50
.
-
Larsson
S. C.,
Giovannucci
E.,
Wolk
A..
Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology.
2006;
131
:
1271-83
.
-
Xiao
Q.,
Freedman
N. D.,
Ren
J.,
Hollenbeck
A. R.,
Abnet
C. C.,
Park
Y..
Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. British Journal of Cancer.
2014;
110
:
1328-33
.
-
Tramacere
I.,
La Vecchia
C.,
Negri
E..
Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology (Cambridge, Mass.).
2011;
22
:
344-9
.
-
Lin
Y.,
Totsuka
Y.,
He
Y.,
Kikuchi
S.,
Qiao
Y.,
Ueda
J..
Epidemiology of esophageal cancer in Japan and China. Journal of Epidemiology.
2013;
23
:
233-42
.
-
Steevens
J.,
Schouten
L. J.,
Goldbohm
R. A.,
van den Brandt
P. A..
Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut.
2010;
59
:
39-48
.
-
Morita
M.,
Kumashiro
R.,
Kubo
N.,
Nakashima
Y.,
Yoshida
R.,
Yoshinaga
K..
Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. International Journal of Clinical Oncology.
2010;
15
:
126-34
.
-
Islami
F.,
Fedirko
V.,
Tramacere
I.,
Bagnardi
V.,
Jenab
M.,
Scotti
L..
Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. International Journal of Cancer.
2011;
129
:
2473-84
.
-
Ferronha
I.,
Bastos
A.,
Lunet
N..
Prediagnosis lifestyle exposures and survival of patients with gastric cancer: systematic review and meta-analysis. European Journal of Cancer Prevention.
2012;
21
:
449-52
.
-
Cook
M. B.,
Kamangar
F.,
Whiteman
D. C.,
Freedman
N. D.,
Gammon
M. D.,
Bernstein
L..
Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. Journal of the National Cancer Institute.
2010;
102
:
1344-53
.
-
Zhang
Z. F.,
Kurtz
R. C.,
Marshall
J. R..
1997.
Google Scholar -
Chen
Z. M.,
Xu
Z.,
Collins
R.,
Li
W. X.,
Peto
R..
Early health effects of the emerging tobacco epidemic in China. A 16-year prospective study. Journal of the American Medical Association.
1997;
278
:
1500-4
.
-
Bergman
J. J..
Gastroesophageal reflux disease and Barrett’s esophagus. Endoscopy.
2005;
37
:
8-18
.
-
Katzka
D. A.,
Rustgi
A. K..
Gastroesophageal reflux disease and Barrett’s esophagus. The Medical Clinics of North America.
2000;
84
:
1137-61
.
-
Shirvani
V. N.,
Ouatu-Lascar
R.,
Kaur
B. S.,
Omary
M. B.,
Triadafilopoulos
G..
Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology.
2000;
118
:
487-96
.
-
Alcedo
J.,
Ferrández
A.,
Arenas
J.,
Sopeña
F.,
Ortego
J.,
Sainz
R..
Trends in Barrett’s esophagus diagnosis in Southern Europe: implications for surveillance. Diseases of the Esophagus.
2009;
22
:
239-48
.
-
Kawaguchi
H.,
Ohno
S.,
Araki
K.,
Miyazaki
M.,
Saeki
H.,
Watanabe
M..
p53 polymorphism in human papillomavirus-associated esophageal cancer. Cancer Research.
2000;
60
:
2753-5
.
-
Bjørge
T.,
Hakulinen
T.,
Engeland
A.,
Jellum
E.,
Koskela
P.,
Lehtinen
M..
A prospective, seroepidemiological study of the role of human papillomavirus in esophageal cancer in Norway. Cancer Research.
1997;
57
:
3989-92
.
-
Li
T.,
Lu
Z. M.,
Chen
K. N.,
Guo
M.,
Xing
H. P.,
Mei
Q..
Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis.
2001;
22
:
929-34
.
-
Suzuk
L.,
Noffsinger
A. E.,
Hui
Y. Z.,
Fenoglio-Preiser
C. M..
Detection of human papillomavirus in esophageal squamous cell carcinoma. Cancer.
1996;
78
:
704-10
.
-
Han
C.,
Qiao
G.,
Hubbert
N. L.,
Li
L.,
Sun
C.,
Wang
Y..
Serologic association between human papillomavirus type 16 infection and esophageal cancer in Shaanxi Province, China. Journal of the National Cancer Institute.
1996;
88
:
1467-71
.
-
Furihata
M.,
Ohtsuki
Y.,
Ogoshi
S.,
Takahashi
A.,
Tamiya
T.,
Ogata
T..
Prognostic significance of human papillomavirus genomes (type-16, -18) and aberrant expression of p53 protein in human esophageal cancer. International Journal of Cancer.
1993;
54
:
226-30
.
-
Knezević
J. D.,
Radovanović
N. S.,
Simić
A. P.,
Kotarac
M. M.,
Skrobić
O. M.,
Konstantinović
V. D..
Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Diseases of the Esophagus.
2007;
20
:
530-4
.
-
Brücher
B. L.,
Stein
H. J.,
Bartels
H.,
Feussner
H.,
Siewert
J. R..
Achalasia and esophageal cancer: incidence, prevalence, and prognosis. World Journal of Surgery.
2001;
25
:
745-9
.
-
Sandler
R. S.,
Nyrén
O.,
Ekbom
A.,
Eisen
G. M.,
Yuen
J.,
Josefsson
S..
The risk of esophageal cancer in patients with achalasia. A population-based study. Journal of the American Medical Association.
1995;
274
:
1359-62
.
-
Appelqvist
P.,
Salmo
M..
Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer.
1980;
45
:
2655-8
.
-
Tachibana
M.,
Abe
S.,
Yoshimura
H.,
Suzuki
K.,
Matsuura
H.,
Nagasue
N..
Squamous cell carcinoma of the esophagus after partial gastrectomy. Dysphagia.
1995;
10
:
49-52
.
-
Birgisson
S.,
Rice
T. W.,
Easley
K. A.,
Richter
J. E..
The lack of association between adenocarcinoma of the esophagus and gastric surgery: a retrospective study. The American Journal of Gastroenterology.
1997;
92
:
216-21
.
-
Islami
F.,
Sheikhattari
P.,
Ren
J. S.,
Kamangar
F..
Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma—a systematic review and meta-analysis. Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2011;
22
:
754-60
.
-
Stevens
H. P.,
Kelsell
D. P.,
Bryant
S. P.,
Bishop
D. T.,
Spurr
N. K.,
Weissenbach
J..
Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. Literature survey and proposed updated classification of the keratodermas. Archives of Dermatology.
1996;
132
:
640-51
.
-
Saarinen
S.,
Vahteristo
P.,
Lehtonen
R.,
Aittomäki
K.,
Launonen
V.,
Kiviluoto
T..
Analysis of a Finnish family confirms RHBDF2 mutations as the underlying factor in tylosis with esophageal cancer. Familial Cancer.
2012;
11
:
525-8
.
-
Iwaya
T.,
Maesawa
C.,
Ogasawara
S.,
Tamura
G..
Tylosis esophageal cancer locus on chromosome 17q25.1 is commonly deleted in sporadic human esophageal cancer. Gastroenterology.
1998;
114
:
1206-10
.
-
Zhang
X.,
Miao
X.,
Tan
W.,
Ning
B.,
Liu
Z.,
Hong
Y..
Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology.
2005;
129
:
565-76
.
-
Ye
Y.,
Wang
K. K.,
Gu
J.,
Yang
H.,
Lin
J.,
Ajani
J. A..
Genetic variations in microRNA-related genes are novel susceptibility loci for esophageal cancer risk. Cancer Prevention Research (Philadelphia, Pa.).
2008;
1
:
460-9
.
-
Tran
G. D.,
Sun
X. D.,
Abnet
C. C.,
Fan
J. H.,
Dawsey
S. M.,
Dong
Z. W..
Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. International Journal of Cancer.
2005;
113
:
456-63
.
-
Dar
N. A.,
Islami
F.,
Bhat
G. A.,
Shah
I. A.,
Makhdoomi
M. A.,
Iqbal
B..
Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. British Journal of Cancer.
2013;
109
:
1367-72
.
-
Abnet
C. C.,
Kamangar
F.,
Islami
F.,
Nasrollahzadeh
D.,
Brennan
P.,
Aghcheli
K..
Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiology, Biomarkers & Prevention.
2008;
17
:
3062-8
.
-
Michaud
D. S.,
Liu
Y.,
Meyer
M.,
Giovannucci
E.,
Joshipura
K..
Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. The Lancet. Oncology.
2008;
9
:
550-8
.
-
Abnet
C. C.,
Kamangar
F.,
Dawsey
S. M.,
Stolzenberg-Solomon
R. Z.,
Albanes
D.,
Pietinen
P..
Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scandinavian Journal of Gastroenterology.
2005;
40
:
681-7
.
-
Guha
N.,
Boffetta
P.,
Wünsch Filho
V.,
Eluf Neto
J.,
Shangina
O.,
Zaridze
D..
Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. American Journal of Epidemiology.
2007;
166
:
1159-73
.
-
Brown
L. M.,
Hoover
R.,
Silverman
D.,
Baris
D.,
Hayes
R.,
Swanson
G. M..
Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. American Journal of Epidemiology.
2001;
153
:
114-22
.
-
Roy
P. K.,
Venzon
D. J.,
Shojamanesh
H.,
Abou-Saif
A.,
Peghini
P.,
Doppman
J. L..
Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine.
2000;
79
:
379-411
.
-
Miller
L. S.,
Vinayek
R.,
Frucht
H.,
Gardner
J. D.,
Jensen
R. T.,
Maton
P. N..
Reflux esophagitis in patients with Zollinger-Ellison syndrome. Gastroenterology.
1990;
98
:
341-6
.
-
Gibril
F.,
Jensen
R. T..
Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Current Gastroenterology Reports.
2004;
6
:
454-63
.
-
Hu
Q.,
Sun
T. T.,
Hong
J.,
Fang
J. Y.,
Xiong
H.,
Meltzer
S. J..
Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One.
2017;
12
:
e0169691
.
-
Singh
Siddharth,
Garg
Sushil Kumar,
Singh
Preet Paul,
Iyer
Prasad G,
El-Serag
Hashem B.
Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett\'s oesophagus: a systematic review and meta-analysis. Gut.
2013;
:
gutjnl-2013-305997
.
-
Din
F. V.,
Theodoratou
E.,
Farrington
S. M.,
Tenesa
A.,
Barnetson
R. A.,
Cetnarskyj
R..
Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut.
2010;
59
:
1670-9
.
-
Huang
T. B.,
Yan
Y.,
Guo
Z. F.,
Zhang
X. L.,
Liu
H.,
Geng
J..
Aspirin use and the risk of prostate cancer: a meta-analysis of 24 epidemiologic studies. International Urology and Nephrology.
2014;
46
:
1715-28
.
-
Corley
D. A.,
Kerlikowske
K.,
Verma
R.,
Buffler
P..
Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology.
2003;
124
:
47-56
.
-
Bruno
A.,
Dovizio
M.,
Tacconelli
S.,
Patrignani
P..
Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract. Best Practice & Research. Clinical Gastroenterology.
2012;
26
:
e1-13
.
-
Liu
J. F.,
Jamieson
G. G.,
Wu
T. C.,
Zhu
G. J.,
Drew
P. A..
A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Annals of Surgical Oncology.
2009;
16
:
1397-402
.
-
Wysowski
D. K..
Reports of esophageal cancer with oral bisphosphonate use. The New England Journal of Medicine.
2009;
360
:
89-90
.
-
Cardwell
C. R.,
Abnet
C. C.,
Cantwell
M. M.,
Murray
L. J..
Exposure to oral bisphosphonates and risk of esophageal cancer. Journal of the American Medical Association.
2010;
304
:
657-63
.
-
Brown
L. M.,
Swanson
C. A.,
Gridley
G.,
Swanson
G. M.,
Schoenberg
J. B.,
Greenberg
R. S..
Adenocarcinoma of the esophagus: role of obesity and diet. Journal of the National Cancer Institute.
1995;
87
:
104-9
.
-
Vaughan
T. L.,
Davis
S.,
Kristal
A.,
Thomas
D. B..
Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. 1995;
4
:
85-92
.
-
Chow
W. H.,
Blot
W. J.,
Vaughan
T. L.,
Risch
H. A.,
Gammon
M. D.,
Stanford
J. L..
Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. Journal of the National Cancer Institute.
1998;
90
:
150-5
.
-
Whiteman
D. C.,
Sadeghi
S.,
Pandeya
N.,
Smithers
B. M.,
Gotley
D. C.,
Bain
C. J..
Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut.
2007
.
-
Thrift
Aaron P,
Shaheen
Nicholas J,
Gammon
Marilie D,
Bernstein
Leslie,
Reid
Brian J,
Onstad
Lynn,
Risch
Harvey A,
Liu
Geoffrey,
Bird
Nigel C,
Wu
Anna H.
Obesity and risk of esophageal adenocarcinoma and Barrett’s esophagus: a Mendelian randomization study. JNCI: Journal of the National Cancer Institute.
2014;
106
.
-
Hoyo
Cathrine,
Cook
Michael B,
Kamangar
Farin,
Freedman
Neal D,
Whiteman
David C,
Bernstein
Leslie,
Brown
Linda M,
Risch
Harvey A,
Ye
Weimin,
Sharp
Linda.
Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. International journal of epidemiology.
2012;
41
:
1706-1718
.
-
Lanuti
Michael,
Liu
Geoffrey,
Goodwin
Jonathan M,
Zhai
Rihong,
Fuchs
Bryan C,
Asomaning
Kofi,
Su
Li,
Nishioka
Norman S,
Tanabe
Kenneth K,
Christiani
David C.
A functional epidermal growth factor (EGF) polymorphism, EGF serum levels, and esophageal adenocarcinoma risk and outcome. Clinical Cancer Research.
2008;
14
:
3216-3222
.
-
Zhu
Jian,
Meng
Xiaoxin,
Yan
Fu,
Qin
Chao,
Wang
Meilin,
Ding
Qi,
Li
Pu,
Yang
Jian,
Ju
Xiaobing,
Zhang
Zhengdong.
A functional epidermal growth factor (EGF) polymorphism, EGF serum levels and renal cell carcinoma risk in a Chinese population. Journal of human genetics.
2010;
55
:
236
.
-
Kamangar
Farin,
Strickland
Paul T,
Pourshams
Akram,
Malekzadeh
Reza,
Boffetta
Paolo,
Roth
Mark J,
Abnet
Christian C,
Saadatian-Elahi
Mitra,
Rakhshani
Nasser,
Brennan
Paul.
High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer research.
2005;
25
:
425-428
.
-
Kamangar
Farin,
Schantz
Michele M,
Abnet
Christian C,
Fagundes
Renato B,
Dawsey
Sanford M.
High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiology and Prevention Biomarkers.
2008;
17
:
1262-1268
.
-
Ramesh
Aramandla,
Walker
Stormy A,
Hood
Darryl B,
Guillén
Maria D,
Schneider
Klaus,
Weyand
Eric H.
Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. International journal of toxicology.
2004;
23
:
301-333
.
-
Abedi-Ardekani
Behnoush,
Kamangar
Farin,
Hewitt
Stephen M,
Hainaut
Pierre,
Sotoudeh
Masoud,
Abnet
Christian C,
Taylor
Philip R,
Boffetta
Paolo,
Malekzadeh
Reza,
Dawsey
Sanford M.
Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut.
2010;
59
:
1178-1183
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 7 (2018)
Page No.: 2504-2517
Published on: 2018-07-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 19329 times
- Download PDF downloaded - 3436 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress

