Abstract
Background: Chromosome Xq11-12 is the place that the androgen receptor (AR) sequence appears. Herein, the prevalence of this biomarker and its relation with pT stage and tumor grade was reported.
Methods: Four online sites (PubMed, Scopus, Web of Science, and Cochrane Library) have been searched up to Sep 2018 systematically. Meta-Analysis software version 2.0 (CMA 2.0) and STATA 14.0 statistical software were utilized. Publication bias did not exist.
Results: From the initial 1141 articles identified from the systematically searches. At last, nine of them remained for analysis. The meta-analysis included 1447 patients that 345 of them had AR expression. AR expression significantly correlated with low tumor grade and low tumor pT stage.
Conclusion: AR expression was 28.2%, and it had the relationship with tumor low grade and low pT stage. Additional studies required to figure out the role of it on RCC patients.
Introduction
Renal cell carcinoma (RCC) is the foremost different urological malignancy among adults 1. The most prevalent form of malignancy RCC is clear cell RCC (ccRCC) and represents over 90% of malignant kidney tumors 2. The androgen receptor (AR) sequence is found at chromosome Xq11-12 and includes of eight exons 3. Prevalence of the AR is appeared in several parts of the body, principally in sexual parts of men. Also, it was reported in breast, bladder, liver, gastrointestinal 4. High AR expression is potential prognosis factor in bladder cancer 5, and it created longer life in patients with serous carcinoma of the ovary 6, an agent for progressive the squamous cell carcinoma of head and neck 78. We tend to are responsive to only rare studies evaluating AR immunohistochemical staining in RCC 91011. Though the targeted therapy creates an effective route for advanced RCC patients 12, the relationship between AR expression and RCC progression stay uncertain until now 13. This meta-analysis reports the expression of AR and its relation with some pathological factors in RCC.
Methods
Search Strategy
PubMed, Scopus, Web of science, and Cochran library included in this study. Search terms included systematically up to September 2018 were “androgen receptor”, “AR”, “kidney”, “renal cell carcinoma”, “cancer”, “carcinoma”, and “tumor. The studies were searched for the assessment of expression of the AR in RCC patients in English abstract.
Inclusion and Exclusion Criteria
Inclusion criteria:
1) Cohort studies
2) Human studies
Exclusion criteria:
1) Case-control, case report, and review studies; conference paper and letter to editor
2) The study with incomplete data
Data extraction
First author, year of publication, nation, RCC samples, expression, grade, pT stage, number of males, and the mean age were extracted. We could not have analysis on types of survival as a result of the studies didn't have the same data.
Statistical analysis
Comprehensive Meta-Analysis software version 2.0 (CMA 2.0) and STATA 14.0 statistical software (StataCorp LP, College Station, TX, USA) were used for random-effect analysis. P-value (2-sided) <0.05 is significant.
CMA 2.0
The event rate (ER) with 95% confidence interval (95% CI) was calculated for estimation of the expression of AR mutations in RCC patients. Begg’s and Egger’s tests were calculated for bias.
STATA 14.0
P<0.1 is significant for heterogeneity. Heterogeneity is assessed by the Q and I 2 statistics.
Results
After primary searches, 1141 articles identified. Twenty one studies remained after we excluded the non-relevant studies. Then, twelve studies were removed based on reasons (five conference papers, three reviews, two articles without full text, one letter to editor, and one database). At last, nine of them remained for analysis (Figure 1).
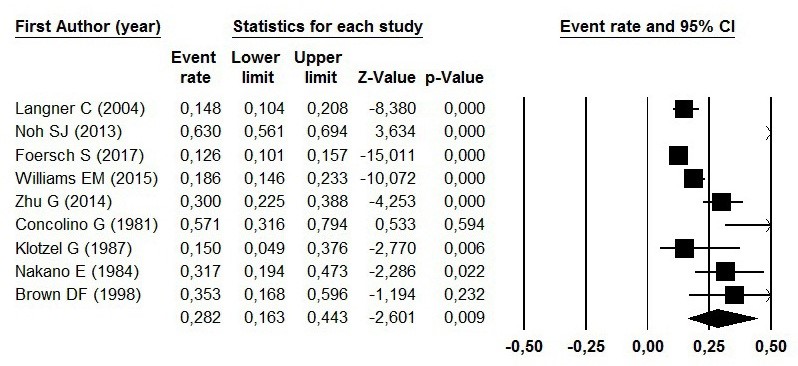
From nine studies 91011141516171819, two studies 1417 were Germany, two studies 1519 were USA and others were from Austria 9, Korea 10, China 11, Italy 16 and Japan 18. From 1447 patients 91011141516171819, 345 AR expression exist. One hundred seventy-one cases in four studies 9101118 of meta-analysis study were male, and 59 cases were female (sum=202). Mean age (min-max) in two studies was 60.06 (29-82). Three studies 9111418 on pT stage in 132 patients with expression AR showed 51 cases pT stage 1, 20 cases pT stage 2, 41 cases pT stage 3. And also, 160 low grade versus 42 high-grade cases exist in four studies 9101118 (Table 1).
| First Author (year) | Nation | Patients | Total androgen expression | Sex | pT Stage | Grade | AgeMean (Min – Max) | |||
| Male | Female | Low | High | Low | High | |||||
| Langner C, 2004 9 | Austria | 182 | 27 | 24 | 3 | 102(24) | 80(3) | 99(21) | 83(6) | - |
| Noh SJ, 2013 10 | Korea | 200 | 126 | 93 | 33 | - | - | 158(101) | 42(25) | 58.13 29–82) |
| Foersch S, 2017 14 | Germany | 546 | 69 | - | - | 348(64) | 198(36) | 463(NA) | 83(NA) | -- |
| Williams EM, 2015 15 | USA | 307 | 57 | - | - | - | - | - | - | -- |
| Zhu G, 2014 11 | China | 120 | 36 | 24 | 12 | 95(34) | 25(2) | 89(32) | 31(4) | 63 (35-82) |
| Concolino G, 1981 16 | Italy | 14 | 8 | - | - | - | - | - | - | -- |
| Klotzl G, 1987 17 | Germany | 20 | 3 | - | - | - | - | - | - | -- |
| Nakano E, 1984 18 | Japon | 41 | 13 | 30 | 11 | - | - | 20(6) | 21(7) | - (46-76) |
| Brown DF, 1998 19 | USA | 17 | 6 | - | - | - | - | - | - | -- |
AR Expression
The prevalence of AR expression in RCC patients has been reported in Figure 2 by the ER. The pooled ER of the studies was 28.2% (95% CI=16.3%-44.3%; P=0.009). The Begg’s and Egger’s tests didn’t show publication bias (P=0.53 and P=0.75, respectively) (Figure 3).
AR expression and tumor grade
Among three studies (502 cases), heterogeneity was existed (I2 = 85.80%, p =0.0) in a fixed‑effects model. Correlation between AR expression and low grade was positive (OR, 1.98; 95% CI, 1.44-2.71; P<0.01) (Figure 4).
AR and pT stage
Among three studies (848 cases), heterogeneity was not existed (I2 = 4.3%, p = 0.353). Correlation between AR expression and low pT-stage was positive (OR, 4.04; 95% CI, (3.10-5.26); P< 0.01) (Figure 5).
Discussion
Radiotherapy, chemotherapy, and immunotherapy used for treat metastatic RCC patients 12. But, a lot of controversial for treatment of this malignancy are existed until now 1317. Moreover, AR expression has been related to chemo-responsiveness 20. Exaggerated AR expression was related to attenuate responsiveness and exaggerated migration of tumor cells 21. The clear cell RCC resists chemotherapy and radiation with a restricted therapeutic period of the anti-angiogenesis targeted therapy (6–15 months) 22. Clinical researchers have disclosed that some patients reply to endocrine therapy objectively or subjectively 2324. AR can help to the pathobiology of breast malignancy 25, and maybe inhibition of androgen sign had a therapeutic role in it 26. Firstly, AR was observed in cancerous renal tissues and then appears in other urinary organs like prostate and then breast cancer 27. Nakano et al., who noted that patients with RCC showing immunoreactivity for one or a lot of hormone receptors (ER, PR and/or AR) had a considerably higher survival rate 18. A cohort found that AR expression in clear cell RCC was prognostic which high expression 28. Cut off for express AR reported from 12.9% to 100% 293031. AR expression found in non-invasive urothelial malignancies as compared to invasive urothelial malignance 3233.
Consequently, AR immunoreactivity was related to renowned favorable prognostic factors, like tiny tumor size, low pT stage (more than 30% in pT1a tumors) in addition as low histological grade 34. The AR-positive rate ranged from 16.3%-44.3% in RCC tissues in this study. Noh et al., 10 and Concolino et al., 16 showed the level of AR-positive is higher than our meta-analysis. In other hands Langne et al., 9 and Klotzl et al., 17 demonstrated that the AR-positive is lower than in this study among RCC patients. Nine studies (1,447 cases) surveyed for AR expression in RCC patients in the meta-analysis and also expressed relationship tumor grades and pT stages with it. Zhu et al., 11 incontestable that AR expression rate was negatively accompanied pT stage and Fuhrman’s grade in RCC patients. Also, this relationship was indicated by Foersch et al., 14 and Langner et al. 9, Zhu et al., 11 reported prevalence of AR had a negative relationship with pT stage and grade in RCC patients. Foersch et al., 14 and Langner et al., 9 reported the results opposite of him. Noh et al., 10 said AR expression related with the histological nuclear grade (p<0.027) and TNM stage (p<0.002). The meta-analysis showed AR expression correlated with low grade and pT stage positively.
C onclusion
As our knowledge, this study is first meta-analysis to research the impact of AR expression on RCC patients. The current study showed the level of expression of AR and its relative with tumor grade and pT stage in RCC patients. Additional studies are needed to the role of AR expression in these patients.
Competing Interests
The author(s) declared no conflicts of interest.
Authors' Contributions
Conceptualization: ES MP
Formal analysis: ES
Methodology: ES HA
Project administration: ES MP HA HM
Software: ES
Validation: HM HA
Writing — original draft: ES
Writing — review & editing: ES MP HA HM
Abbreviations
AR: Androgen Receptor
ccRCC: Clear Cell Renal Cell Carcinoma
CI: Confidence Interval
ER: Estrogen Receptor pT Stage: Pathologic Tumor Stage PR: Progesterone Receptor RCC: Renal Cell Carcinoma
References
-
Foersch
S.,
Schindeldecker
M.,
Keith
M.,
Tagscherer
K. E.,
Fernandez
A.,
Stenzel
P. J..
Prognostic relevance of androgen receptor expression in renal cell carcinomas. Oncotarget.
2017;
8
:
78545-55
.
View Article PubMed Google Scholar -
Langner
C.,
Ratschek
M.,
Rehak
P.,
Schips
L.,
Zigeuner
R..
Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. The Journal of Urology.
2004;
171
:
611-4
.
View Article PubMed Google Scholar -
Noh
S. J.,
Kang
M. J.,
Kim
K. M.,
Bae
J. S.,
Park
H. S.,
Moon
W. S..
Acetylation status of P53 and the expression of DBC1, SIRT1, and androgen receptor are associated with survival in clear cell renal cell carcinoma patients. Pathology.
2013;
45
:
574-80
.
View Article PubMed Google Scholar -
Williams
E. M.,
Higgins
J. P.,
Sangoi
A. R.,
McKenney
J. K.,
Troxell
M. L..
Androgen receptor immunohistochemistry in genitourinary neoplasms. International Urology and Nephrology.
2015;
47
:
81-5
.
View Article PubMed Google Scholar -
Concolino
G.,
Marocchi
A.,
Toscano
V.,
Silverio
F. Di.
Nuclear androgen receptor as marker of responsiveness to medroxyprogesterone acetate in human renal cell carcinoma. Journal of Steroid Biochemistry.
1981;
15
:
397-402
.
View Article PubMed Google Scholar -
Klotzl
G.,
Otto
U.,
Becker
H.,
Klosterhalfen
H..
Determination of androgen, progestin and estrogen receptors with two different assays in renal cell carcinoma. Urologia Internationalis.
1987;
42
:
100-4
.
View Article PubMed Google Scholar -
Nakano
E.,
Tada
Y.,
Fujioka
H.,
Matsuda
M.,
Osafune
M.,
Kotake
T..
Hormone receptor in renal cell carcinoma and correlation with clinical response to endocrine therapy. The Journal of Urology.
1984;
132
:
240-5
.
View Article PubMed Google Scholar -
Brown
D. F.,
Dababo
M. A.,
Hladik
C. L.,
Eagan
K. P.,
White
C. L.,
Rushing
E. J..
Hormone receptor immunoreactivity in hemangioblastomas and clear cell renal cell carcinomas. Modern Pathology.
1998;
11
:
55-9
.
PubMed Google Scholar -
Zhu
G.,
Liang
L.,
Li
L.,
Dang
Q.,
Song
W.,
Yeh
S..
The expression and evaluation of androgen receptor in human renal cell carcinoma. Urology.
2014;
83
:
510.e19-24
.
View Article PubMed Google Scholar -
Payandeh
M.,
Sadeghi
E.,
Sadeghi
M..
Two Side Effects of Sutent Therapy in Renal Cell Carcinoma: A Case Report. 2017;
10
:
e4040
.
View Article Google Scholar -
Eble
J. L.,
Sauter
G.,
Epstein
J.,
Sesterhenn
I..
2004.
Google Scholar -
Brinkmann
A. O.,
Faber
P. W.,
van Rooij
H. C.,
Kuiper
G. G.,
Ris
C.,
Klaassen
P..
The human androgen receptor: domain structure, genomic organization and regulation of expression. Journal of Steroid Biochemistry.
1989;
34
:
307-10
.
View Article Google Scholar -
Kimura
N.,
Mizokami
A.,
Oonuma
T.,
Sasano
H.,
Nagura
H..
Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. The Journal of Histochemistry and Cytochemistry.
1993;
41
:
671-8
.
View Article PubMed Google Scholar -
Nam
J. K.,
Park
S. W.,
Lee
S. D.,
Chung
M. K..
Prognostic value of sex-hormone receptor expression in non-muscle-invasive bladder cancer. Yonsei Medical Journal.
2014;
55
:
1214-21
.
View Article PubMed Google Scholar -
Nodin
B.,
Zendehrokh
N.,
Brandstedt
J.,
Nilsson
E.,
Manjer
J.,
Brennan
D. J..
Increased androgen receptor expression in serous carcinoma of the ovary is associated with an improved survival. Journal of Ovarian Research.
2010;
3
:
14
.
View Article PubMed Google Scholar -
Rades
D.,
Seibold
N. D.,
Schild
S. E.,
Bruchhage
K. L.,
Gebhard
M. P.,
Noack
F..
Androgen receptor expression: prognostic value in locally advanced squamous cell carcinoma of the head and neck. Strahlentherapie und Onkologie.
2013;
189
:
849-55
.
View Article PubMed Google Scholar -
Vera-Badillo
F. E.,
Templeton
A. J.,
de Gouveia
P.,
Diaz-Padilla
I.,
Bedard
P. L.,
Al-Mubarak
M..
Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. Journal of the National Cancer Institute.
2014;
106
:
djt319
.
View Article Google Scholar -
W.M.
Murphy,
J.B.
Beckwith,
J.B.
Beckwith.
Atlas of Tumor PathologyArmed Forces Institute of Pathology: Washington, D. C.; 1994.
Google Scholar -
Nolan
L. P.,
Heatley
M. K..
The value of immunocytochemistry in distinguishing between clear cell carcinoma of the kidney and ovary. International Journal of Gynecological Pathology.
2001;
20
:
155-9
.
View Article PubMed Google Scholar -
Elattar
A.,
Warburton
K. G.,
Mukhopadhyay
A.,
Freer
R. M.,
Shaheen
F.,
Cross
P..
Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecologic Oncology.
2012;
124
:
142-7
.
View Article PubMed Google Scholar -
Shyr
C. R.,
Chen
C. C.,
Hsieh
T. F.,
Chang
C. H.,
Ma
W. L.,
Yeh
S..
The expression and actions of androgen receptor in upper urinary tract urothelial carcinoma (UUTUC) tissues and the primary cultured cells. Endocrine.
2013;
43
:
191-9
.
View Article PubMed Google Scholar -
Rini
B. I.,
Atkins
M. B..
Resistance to targeted therapy in renal-cell carcinoma. The Lancet. Oncology.
2009;
10
:
992-1000
.
View Article Google Scholar -
Paine
C. H.,
Wright
F. W.,
Ellis
F..
The use of progestogen in the treatment of metastatic carcinoma of the kidney and uterine body. British Journal of Cancer.
1970;
24
:
277-82
.
View Article Google Scholar -
Wagle
D. G.,
Murphy
G. P..
Hormonal therapy in advanced renal cell carcinoma. Cancer.
1971;
28
:
318-21
.
View Article Google Scholar -
Niemeier
L. A.,
Dabbs
D. J.,
Beriwal
S.,
Striebel
J. M.,
Bhargava
R..
Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Modern Pathology.
2010;
23
:
205-12
.
View Article Google Scholar -
Barton
V. N.,
D\'Amato
N. C.,
Gordon
M. A.,
Lind
H. T.,
Spoelstra
N. S.,
Babbs
B. L..
Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Molecular Cancer Therapeutics.
2015;
14
:
769-78
.
View Article Google Scholar -
Sasaki
M.,
Nomoto
M.,
Yonezawa
S.,
Nakagawa
M.,
Sakuragi
N.,
Fujimoto
S..
Distribution of a single nucleotide polymorphism on codon 211 of the androgen receptor gene and its correlation with human renal cell cancer in Japanese patients. Biochemical and Biophysical Research Communications.
2004;
321
:
468-71
.
View Article PubMed Google Scholar -
Zhao
H.,
Leppert
J. T.,
Peehl
D. M..
A Protective Role for Androgen Receptor in Clear Cell Renal Cell Carcinoma Based on Mining TCGA Data. PLoS One.
2016;
11
:
e0146505
.
View Article Google Scholar -
Kominea
A.,
Konstantinopoulos
P. A.,
Kapranos
N.,
Vandoros
G.,
Gkermpesi
M.,
Andricopoulos
P..
Androgen receptor (AR) expression is an independent unfavorable prognostic factor in gastric cancer. Journal of Cancer Research and Clinical Oncology.
2004;
130
:
253-8
.
View Article Google Scholar -
Segawa
N.,
Mori
I.,
Utsunomiya
H.,
Nakamura
M.,
Nakamura
Y.,
Shan
L..
Prognostic significance of neuroendocrine differentiation, proliferation activity and androgen receptor expression in prostate cancer. Pathology International.
2001;
51
:
452-9
.
View Article Google Scholar -
Mir
C.,
Shariat
S. F.,
Kwast
T. H. van der,
Ashfaq
R.,
Lotan
Y.,
Evans
A..
Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU International.
2011;
108
:
24-30
.
View Article PubMed Google Scholar -
Boorjian
S.,
Ugras
S.,
Mongan
N. P.,
Gudas
L. J.,
You
X.,
Tickoo
S. K..
Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology.
2004;
64
:
383-8
.
View Article Google Scholar -
Tuygun
C.,
Kankaya
D.,
Imamoglu
A.,
Sertcelik
A.,
Zengin
K.,
Oktay
M..
Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: a comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urologic Oncology.
2011;
29
:
43-51
.
View Article PubMed Google Scholar -
Mejean
A.,
Oudard
S.,
Thiounn
N..
Prognostic factors of renal cell carcinoma. The Journal of Urology.
2003;
169
:
821-7
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 11 (2018)
Page No.: 2820-2826
Published on: 2018-11-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5881 times
- Download PDF downloaded - 2005 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress







