Abstract
Background: Cyclosporine (CYC) is an immunosuppressant drug used widely in kidney transplant patient. The major side effect of CYC is nephrotoxicity. In this study, three different doses of CYC alone or accompanied with zinc (Zn) supplement were administrated in male and female rats to determine the kidney tissue damages and functions.
Methods: Male and female rats were treated with 10, 50 or 100 mg/kg/day of CYC alone or accompanied with 10 mg /kg/day of Zn sulfate for 10 days. The parameters related to renal function were determined and the kidney tissues were subjected to histological evaluation.
Results: All male and female animals were treated with high dose CYC (100 mg/kg/day) alone or accompanied with Zn supplement during the experiment. The data obtained for the serum levels of creatinine (Cr) and blood urea nitrogen/Cr ratio, clearance of Cr, kidney weight (KW), sodium (Na) filtration rate, Na excretion rate and Na excretion fraction (%) in surviving animals suggest a role of gender in the variation of these factors. The kidney tissue damage score (KTDS) was increased as the dosage of CYC was elevated, and the Zn supplement attenuated the KTDS in animals treated with low dose CYC (10 mg/kg/day).
Conclusion: The CYC-induced nephrotoxicity may be gender-related, and the 10 mg/kg dose of Zn sulphate as a supplement may possibly prevent the induced nephrotoxicity in males due to its antioxidant effects.
Introduction
Calcineurin inhibitors (CNIs) include cyclosporine (CYC), tacrolimus, voclosporin and pimecrolimus. Patients with organ transplant usually receive CYC and tacrolimus as immunosuppressant agents to avoid organ transplant rejection, but these drugs can cause acute and chronic nephrotoxicity 1. Among them, the major adverse effects of CYC are nephrotoxicity, hepatoxicity, hypertension and risk of malignancy, thereby limiting its clinical use. The optimal use dosage of CYC for patient monitoring is one of the major difficulties in the clinic, thus dose adjustment strategy has been suggested for CYC therapy 2. In addition, many research studies have been conducted to suggest supplemental agents to confer additional protection for the kidney against CYC-induced toxicity. These supplemental agents may include herbal drugs 345, endothelin-1 receptor antagonists 6, antihypertensive drugs or β-blockers 789, NADPH oxidase activity inhibitor 10, antioxidants 11, omega-3 fatty acids 12, apelin peptide 13, and/or renin inhibitor 14.
Gender and sex hormones are also important factors that influence the effects of CYC 1516171819. CYC-mediated side effects may be gender-related in kidney 20 and heart 21. El-Bassossy and Eid reported that 21 days of CYC treatment in rats led to sex-related nephrotoxicity due to different responses to inflammatory factors 20. Until now, the role of gender or sex hormones in kidney toxicity induced by other drugs, such as cisplatin and gentamicin, have been documented 222324252627282930313233, although their mechanisms have not been completely understood.
In addition, the antioxidant supplements, such as zinc (Zn), have been widely used in laboratory research to protect the kidney against injury 343536373839404142. It seems that the administration of this trace element Zn could be considered as a safe protective agent for transplant patients. Therefore, in the current study, we evaluated three different doses of CYC, alone or accompanied with Zn supplement; moreover, kidney tissue damages and functions were investigated.
Methods
Animals
This research was conducted on 110 male (n=55, 268±4 g) and female (n=55, 209±2 g) Wistar rats split among 14 experimental groups. The protocol for our study was approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1397.2.053).
Experimental group design
Group 1 (n=8; 252±11g): male rats treated with vehicle (sesame oil) as a solvent for CYC.
Groups 2, 3, and 4 (n=8 per group; 280±11g, 256±15g, and 282±7g, respectively): male rats treated with 10, 50 and 100 mg/kg/day of CYC dissolved in sesame oil, respectively.
Groups 5, 6, and 7 (n=8 per group except group 5 (n=7); 266±8g, 272±11g, and 266±13g, respectively): male rats co-treated with 10, 50 and 100 mg/kg/day of CYC dissolved in sesame oil, respectively, plus 10 mg/kg/day of Zn sulfate as a supplement.
Groups 8-14 (n=8 per group except group 11 (n=7); 205±5g, 204±6g, 211±6g, 217±10g, 211±4g, 205±6g, and 210±7g, respectively): female rats were given the same regimen as male rats in Groups 1-7. The treatment duration for each group was 10 days.
Drugs
CYC was purchased from Zahravi Pharm Co. (Tabriz, Iran). Each capsule of CYC contains 100 mg of CYC. To prepare the desired concentrations, the drug was dissolved in sesame oil (Barij Esans Co, Isfahan, Iran). The Zn sulfate used in this study was from BDH Co. (London, England) with 99% purity.
Treatments
Based on the design of the experimental groups, CYC was administrated daily by subcutaneous injection and for 10 consecutive days. The Zn sulfate was also given daily by intra-peritoneal injection (i.p.) for 10 consecutive days based on body weight.
Measurements
Mortality rate for each group was recorded daily, and the remaining surviving animals (until the 11th day) were subjected to placement in metabolic cages for 3 hour urine collection. The volume of urine was determined by scaled micro tube. Finally, the blood samples were obtained and the animals were sacrificed humanely. Then, kidney tissue was fixed in 10% formalin to perform histological evaluation using H&E staining. The tissue damage in the stained tissue (as kidney tissues damage score; KTDS) was scored by a pathologist who was blinded to the study protocol. The score was assigned from 1 to 4 based on intensity of tissue damage while zero was considered as normal. The intensity of tissue damage was considered based on criteria of vacuolization, dilatation, hyaline cast, debris or degeneration.
The levels of blood urea nitrogen (BUN) and creatinine (Cr) were determined using quantitative diagnostic kits (Pars Azmoon, Iran) by automatic analyzer RA-1000 (Technicon, Ireland). The levels of sodium (Na) in serum and urine were determined by flame photometer assay using Flam Fp20 Model (Seac Co, Italy). The Cr clearance (ClCr) was calculated by the clearance formula: ClCr= urine flow (UF)* urine Cr level/ serum Cr level.
Statistical Analysis
Data were reported as mean±SEM. The one way ANOVA and Student’s t-test for quantitative data were used to compare the measured parameters between the groups. The Kruskal Wallis H and Mann-Whitney tests were applied to compare the histology findings between the groups. Indeed, p-value <0.05 was considered statistically significant.
Results
Animal survival and weight change
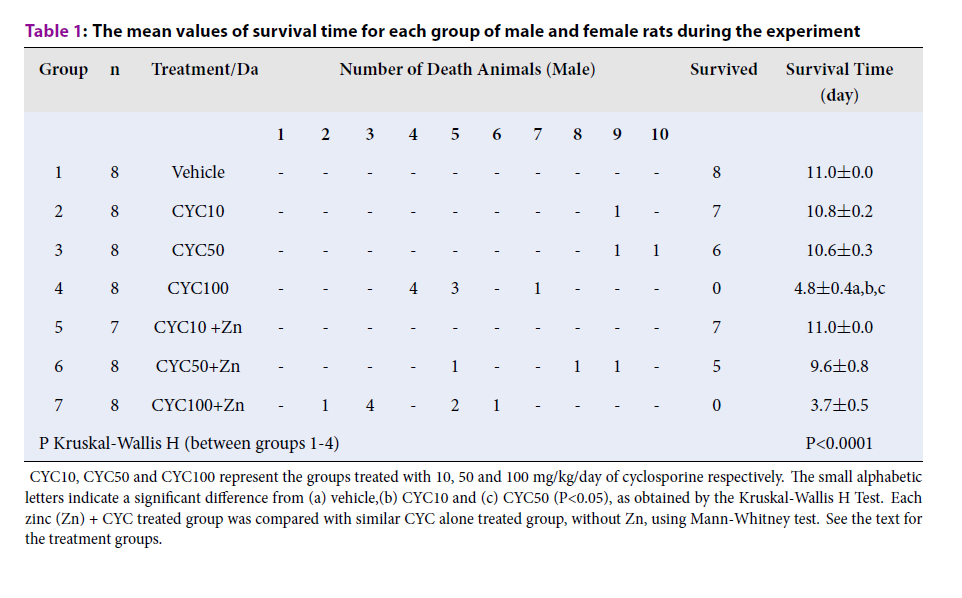
The data for animal survival time were tabulated in Table 1Table 2. The entire male and female animals treated with high dose CYC (100 mg/kg/day) expired during the experiment and no animals survived on the last day of experiment (11th day- sacrifice day). The survival time of male rats in groups 1-7 were 11.0±0.0, 10.8±0.2, 10.6±0.3, 4.8±0.4, 11.0±0.0, 9.6±0.8 and 3.7±0.5 days (P<0.0001), while the survival time of female rats in groups 8-14 were 11.0±0.0, 11.0±0.0, 10.5±0.3, 7.0±0.7, 11.0±0.0, 9.7±0.8 and 5.1±0.4 days (P<0.0001), respectively (Table 1Table 2).
| Group | n | Treatment/Day | Number of Death Animals (Male) | Survived | Survival Time (day) | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
| 1 | 8 | Vehicle | - | - | - | - | - | - | - | - | - | - | 8 | 11.0±0.0 |
| 2 | 8 | CYC10 | - | - | - | - | - | - | - | - | 1 | - | 7 | 10.8±0.2 |
| 3 | 8 | CYC50 | - | - | - | - | - | - | - | - | 1 | 1 | 6 | 10.6±0.3 |
| 4 | 8 | CYC100 | - | - | - | 4 | 3 | - | 1 | - | - | - | 0 | 4.8±0.4 a,b,c |
| 5 | 7 | CYC10 +Zn | - | - | - | - | - | - | - | - | - | - | 7 | 11.0±0.0 |
| 6 | 8 | CYC50+Zn | - | - | - | - | 1 | - | - | 1 | 1 | - | 5 | 9.6±0.8 |
| 7 | 8 | CYC100+Zn | - | 1 | 4 | - | 2 | 1 | - | - | - | - | 0 | 3.7±0.5 |
| P Kruskal-Wallis H (between groups 1-4) | P<0.0001 | |||||||||||||
| Group | n | Treatment/Day | Number of Death Animals (Female) | Survived | Survival Time (day) | P-value (between genders) Mann-Whitney Test | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||
| 8 | 8 | Vehicle | - | - | - | - | - | - | - | - | - | - | 8 | 11.0±0.0 | 1.0 |
| 9 | 8 | CYC10 | - | - | - | - | - | - | - | - | - | - | 8 | 11.0±0.0 | 0.32 |
| 10 | 8 | CYC50 | - | - | - | - | - | - | - | - | 1 | 2 | 5 | 10.5±0.3 a,b | 0.65 |
| 11 | 8 | CYC100 | - | - | - | - | 3 | 1 | - | 2 | 1 | 1 | 0 | 7.0±0.7 a,b,c | 0.06 |
| 12 | 8 | CYC10 +Zn | - | - | - | - | - | - | - | - | - | - | 8 | 11.0±0.0 | 1.0 |
| 13 | 8 | CYC50+Zn | - | - | - | - | - | 2 | - | - | - | 1 | 5 | 9.7±0.8 | 0.70 |
| 14 | 8 | CYC100+Zn | - | - | 1 | 1 | 2 | 4 | - | - | - | - | 0 | 5.1±0.4 | 0.05 |
| P Kruskal-Wallis H (between groups 8-11) | P<0.0001 | ||||||||||||||
In male rats, the percentage change of weight of animals in the CYC alone treatment groups were significantly less than vehicle-treated group (vehicle: 11.52±2.3%, CYC 10: 1± 4.3%, CYC 50: -1±4.5%, P<0.05). In addition, the percentage change of weight loss in male rats co-treated with CYC (50 mg/kg/day) and Zn (-16.15±1.9 %) was significantly greater than CYC (50 mg/kg) alone treated group (-1±4.50 %) (P<0.05). However, in female rats a greater percentage of weight loss was observed in CYC (50 mg/kg/day), with or without Zn, treatment groups (-14.39±1.89 and -15.50±2.69 %, P<0.001) (Figure 1).
CYC alone administration increased the kidney weight (KW) normalized to body weight (BW) in male rats significantly when compared with vehicle group (vehicle; 0.67±0.02, CYC 10: 0.72±0.01 g/ 100 g BW, CYC 50: 0.76±0.02 g/ 100 g BW, P<0.05), while addition of Zn supplement did not alter KW towards normal in CYC 10 (0.70±0.01g/ 100 g BW) or CYC 50 (0.80±0.05 g/
100 g BW) groups. In female rats, the normalized KW in vehicle, CYC10, CYC50, CYC10+Zn, and CYC 50+Zn groups were 0.66±0.01, 0.69±0.01, 0.84±0.04, 0.66±0.01 and 0.81±0.03g/ 100 g BW, respectively. These findings indicated that the high dose of CYC (50 mg/kg/day) alone increased the normalized KW significantly (P<0.05), and that Zn supplement did not alter it.
The serum levels of blood urea nitrogen (BUN) and Creatinine (Cr)
In male rats, the levels of BUN in vehicle, CYC10, CYC50, CYC10+Zn and CYC50+Zn groups were 18.69±1.42, 30.14±7.62, 27.95±2.02, 20.82±3.43 and 39.81± 11.31 mg/dL, respectively, while the serum levels of Cr in these groups were 0.76±0.06, 0.81±0.03, 0.82±0.11, 0.58±0.06 and 0.69±0.08 mg/dl. The serum levels of BUN/Cr ratio were also evaluated in male rats groups of vehicle; CYC10, CYC50, CYC10+Zn and CYC50+Zn were 25.88± 3.04, 37.94± 9.84, 37.92± 6.79, 36.01± 4.07 and 55.83± 13.07, respectively. The result indicated that the serum level of BUN, Cr and BUN/Cr ratio increased insignificantly in male rats treated with CYC, and that Zn supplement decreased the serum levels of BUN insignificantly and Cr significantly (P<0.05) in low dose of CYC-treated rats (Figure 2, left panel).
In female rats, the levels of BUN in vehicle, CYC10 and CYC 50, respectively, were 17.84±1.18, 17.96±1.16 and 35.37±9.83 mg/dL. However, the Zn supplement did not attenuate these markers toward normal levels. The serum level of BUN in CYC 10+Zn and CYC50+Zn groups were 18.86±1.78 and 53.73±10.11mg/dL, respectively (Figure 2, right panel). These results indicated that the CYC (50 mg/kg/day) increased the serum levels of BUN and BUN/Cr ratio significantly (P<0.05) in females.
Renal function parameters
The findings related to renal function parameters were normalized to KW. The normalized UF in male rats for vehicle, CYC10, CYC50, CYC10+Zn and CYC50+Zn groups were 6.91±1.81, 2.84±1.35, 3.42±1.21, 2.87±0.48 and 4.74±0.62 µL/min/g tissue, while the normalized ClCr in these groups were 465.90±120.29, 249.19±54.09, 305.27±90.80, 460.82±107.20 and 417.58±51.54 µL/min/g tissue, respectively. The results revealed that CYC decreased UF and ClCr in male rats insignificantly.
The normalized UF in female rats for vehicle, CYC10, CYC50, CYC10+Zn and CYC50+Zn groups were 3.05±0.60, 3.83±1.04, 2.67±1.26, 6.43±2.36 and 3.89±1.78 µL/min/g tissue, and the normalized ClCr in the mentioned groups were 201.01±33.10, 366.03±50.18, 138.63±74.82, 435.75±100.60 and 300.53±186.55 µL/min/g tissue, respectively. These findings showed that CYC (at dose of 10 mg/kg/day) alone increased ClCr significantly in female rats when compared with vehicle group (P<0.05) (Figure 3). However, Zn supplement did not provide the protective effect in either males or females.
In male rats, the normalized Na filtration rate in the vehicle, CYC10, CYC50, CYC10+Zn, and CYC50+Zn groups were 75.52±18.98, 42.69±9.38, 51.39±15.71, 80.66±20.69, 93.73±9.12 µmoL/min/g tissue, respectively, while the normalized Na excretion rate in these groups were 1.19±0.18, 0.38±0.16, 0.36±0.11, 0.60±0.11, 0.39±0.16 µmole/min/g tissue and the percentage of Na excretion fraction were 1.96±0.29, 0.72±0.19, 0.72±0.16, 0.88±0.21 and 0.40±0.15%, respectively. The results analyses indicated that the CYC alone decreased Na filtration rate (insignificantly), Na excretion rate, and percentage of Na excretion fraction (significantly, P<0.05) in male rats (Figure 4, left panel).
Similar observation for the normalized Na excretion rate and percentage of Na excretion fraction were also observed in female rats (Figure 4, right panel). The normalized Na filtration rate for the vehicle, CYC10, CYC50, CYC10+Zn, CYC50+Zn female groups were 34.19±5.27, 67.31±9.82, 24.72±13.44, 77.43±18.55, 51.20±31.60 µmoL/min/g tissue, while the Na excretion rate in these groups were 0.59±0.09, 0.30±0.05, 0.06±0.05, 0.59±0.13, 0.47±0.23 µmoL/min/g tissue, respectively. In the similar female rat groups, the percentage of Na excretion fractions were 1.81±0.28, 0.46±0.07, 0.25±0.08, 0.85±0.11, and 1.05±0.19%, respectively. As the data show, the Zn supplement increased the Na excretion rate and the percentage of Na excretion fraction, only in female rats.
The kidney histology findings
In the male rats, the mean value of KTDS for vehicle, CYC10, CYC50, CYC100, CYC10+Zn and CYC50+Zn, CYC100+Zn groups were 0.5±0.18, 1.37±0.18, 1.75±0.16, 2.42±0.20, 0.66±0.21, 2± 0.18, 2.7±0.18, while in the female gender of above groups these findings were 0.5±0.18, 1.62±0.26, 2±0.20, 2.71±0.18, 1±0.18, 2.25±0.26, 2.83±0.16, respectively. The histology data indicated that KTDS was increased by CYC dose dependently of both male and female rats (Figure 5). Hence, Zn supplement decreased the KTDS in male and female rats treated with CYC (10 mg/kg/day) significantly (P<0.05). The samples of images from the animals in each group of experiment are shown in Figure 6.
Discussion
The major findings of this study indicated that CYC-induced nephrotoxicity was dose-related in both male and female rats. In addition, Zn supplementation accompanied with low dose of CYC (10 mg/kg/day) attenuated CYC and induced tissue damage. The protective role of Zn in male rats treated with low dose of CYC (10 mg/kg/day) also was observed by attenuation of serum level of Cr. In addition, when UF, ClCr, Na filtration rate, Na excretion rate and Na excretion fraction are considered, the data indicated that low dose of CYC accompanied with Zn increased all the mentioned markers insignificantly. On the contrary, when KW and body weight change were targeted, Zn supplementation with low dose of CYC did not alter weight loss and KW. Finally, if pathological findings are looked as ultimate findings, the interpretation of the data will be easier, and we can assumed that Zn could be a protectant agent against CYC (10 mg/kg/day) induced nephrotoxicity. CYC reduced the body weight percentage change and increased the normalized KW. The weight gain by CYC was reported before 43. Under conditions of induced nephrotoxicity, both body weight loss and KW gain occurred 252627. Together with BUN, Cr and BUN/Cr ratio data, it seems that weight loss and KW gain are related to CYC induced nephrotoxicity, which are confirmed by pathology findings. The weight loss and KW gain by CYC (50 mg/kg) was different in female rats; this difference may be related to body water content. Male and female rats have different distribution of body fluid compartment, and this fact may affect the alteration of markers, such as body weight loss, KW gain and the serum level of BUN. In addition, Zn supplementation decreases the serum levels of BUN and Cr in male rats treated with low dose of CYC (10 mg/kg/day) when compared with CYC alone treated rats (Figure 2). Such observation was not detected in female. The protective effect of Zn in kidney injury may be dose and gender related 3439, and actually the involved mechanisms is not well clear. Some of the renal function markers also were different between genders. Collectively, this difference possibly is related to sex hormones. CYC affects sex hormones 4445, and in-vitro study demonstrated that the therapeutic dose of CYC may alter ovarian function 45. The Zn also may protect the kidney gender dependently 39. Therefore, the different responses from the measured markers to CYC and Zn in male and female rats may be expected, but the exact mechanisms need to be defined. There is one limitation in our study related to dose of Zn. In the current study, we only used 10 mg/kg/day of Zn sulphate, and possibly this dose of Zn supplement may be appropriate for a lower dose of CYC. A higher dose of CYC (50 mg/kg/day) needs higher doses of Zn sulphate.
Conclusions
The high dose of CYC (100 mg/kg) demonstrated the highly toxic effect. No animals survived on the last day of experiment, and the other dose of CYC induced nephrotoxicity gender dependently. However, the 10 mg/kg of Zn sulphate as a supplement may prevent induced nephrotoxicity in males, possibly due to its antioxidant effects.
Competing Interests
The authors have declared that no conflict of interest exists
Authors' Contributions
SC, SK and AT conducted the experimental procedures and data analysis, MM, YG, and MM conducted study design, and finalized the article. MN conducted study design, experimental procedures, data analysis and finalized the article.
References
-
Sharma
A.,
Jain
S.,
Gupta
R.,
Guleria
S.,
Agarwal
S.,
Dinda
A..
Calcineurin inhibitor toxicity in renal allografts: morphologic clues from protocol biopsies. Indian Journal of Pathology & Microbiology.
2010;
53
:
651-7
.
View Article Google Scholar -
Kokuhu
T.,
Fukushima
K.,
Ushigome
H.,
Yoshimura
N.,
Sugioka
N..
Dose adjustment strategy of cyclosporine A in renal transplant patients: evaluation of anthropometric parameters for dose adjustment and C0 vs. C2 monitoring in Japan, 2001-2010. International Journal of Medical Sciences.
2013;
10
:
1665-73
.
View Article Google Scholar -
Aziz
M. M.,
Eid
N. I.,
Nada
A. S.,
Amin
N. E.,
Ain-Shoka
A. A..
Possible protective effect of the algae spirulina against nephrotoxicity induced by cyclosporine A and/or gamma radiation in rats. Environmental Science and Pollution Research International.
2018;
25
:
9060-70
.
View Article Google Scholar -
Mostafa-Hedeab
G.,
Sati
L. M.,
Elnaggar
H. M.,
Elgatlawey
Z. O.,
Eltwab
A. A.,
Elsaghayer
W. A..
Ameliorating effect of olive leaf extract on cyclosporine-induced nephrotoxicity in rats. Iranian Journal of Kidney Diseases.
2015;
9
:
361-8
.
-
Shin
B. C.,
Kwon
Y. E.,
Chung
J. H.,
Kim
H. L..
The antiproteinuric effects of green tea extract on acute cyclosporine-induced nephrotoxicity in rats. Transplant Proc.
2012;
44
:
1080-2
.
-
Caires
A.,
Fernandes
G. S.,
Leme
A. M.,
Castino
B.,
Pessoa
E. A.,
Fernandes
S. M..
Endothelin-1 receptor antagonists protect the kidney against the nephrotoxicity induced by cyclosporine-A in normotensive and hypertensive rats. Brazilian Journal of Medical and Biological Research.
2017;
51
:
e6373
.
View Article Google Scholar -
Raeisi
S.,
Ghorbanihaghjo
A.,
Argani
H.,
Dastmalchi
S.,
Ghasemi
B.,
Ghazizadeh
T..
The effects of valsartan on renal glutathione peroxidase expression in alleviation of cyclosporine nephrotoxicity in rats. BioImpacts.
2016;
6
:
119-24
.
View Article Google Scholar -
Hewedy
W. A.,
Mostafa
D. K..
Nebivolol suppresses asymmetric dimethylarginine and attenuates cyclosporine-induced nephrotoxicity and endothelial dysfunction in rats. Pharmacological Reports.
2016;
68
:
1319-25
.
View Article Google Scholar -
El-Gowilly
S. M..
Metoprolol ameliorates cyclosporine a-induced hypertension and nephrotoxicity in rats. Journal of Cardiovascular Pharmacology.
2011;
58
:
639-46
.
View Article Google Scholar -
Ciarcia
R.,
Damiano
S.,
Florio
A.,
Spagnuolo
M.,
Zacchia
E.,
Squillacioti
C..
The Protective Effect of Apocynin on Cyclosporine A-Induced Hypertension and Nephrotoxicity in Rats. Journal of Cellular Biochemistry.
2015;
116
:
1848-56
.
View Article Google Scholar -
Al-Malki
A. L.,
Moselhy
S. S..
The protective effect of epicatchin against oxidative stress and nephrotoxicity in rats induced by cyclosporine. Human and Experimental Toxicology.
2011;
30
:
145-51
.
View Article Google Scholar -
Mariee
A. D.,
Abd-Ellah
M. F..
Protective effect of docosahexaenoic acid against cyclosporine A-induced nephrotoxicity in rats: a possible mechanism of action. Renal Failure.
2011;
33
:
66-71
.
View Article Google Scholar -
Kim
J. S.,
Yang
J. W.,
Han
B. G.,
Kwon
H. J.,
Kim
J. H.,
Choi
S. O..
Protective Role of Apelin Against Cyclosporine-Induced Renal Tubular Injury in Rats. Transplant Proc.
2017;
49
:
1499-1509
.
-
Saraswat
M. S.,
Addepalli
V.,
Jain
M.,
Pawar
V. D.,
Patel
R. B..
Renoprotective activity of aliskiren, a renin inhibitor in cyclosporine A induced hypertensive nephropathy in dTG mice. Pharmacological Reports.
2014;
66
:
62-7
.
View Article Google Scholar -
Tornatore
K. M.,
Brazeau
D.,
Dole
K.,
Danison
R.,
Wilding
G.,
Leca
N..
Sex differences in cyclosporine pharmacokinetics and ABCB1 gene expression in mononuclear blood cells in African American and Caucasian renal transplant recipients. Journal of Clinical Pharmacology.
2013;
53
:
1039-47
.
View Article Google Scholar -
Gottschalk
S.,
Cummins
C. L.,
Leibfritz
D.,
Christians
U.,
Benet
L. Z.,
Serkova
N. J..
Age and sex differences in the effects of the immunosuppressants cyclosporine, sirolimus and everolimus on rat brain metabolism. Neurotoxicology.
2011;
32
:
50-7
.
View Article Google Scholar -
Muller
V.,
Szabo
A. J.,
Erdely
A.,
Tain
Y. L.,
Baylis
C..
Sex differences in response to cyclosporine immunosuppression in experimental kidney transplantation. Clinical and Experimental Pharmacology & Physiology.
2008;
35
:
574-9
.
View Article Google Scholar -
Hirasawa
K.,
Kamada
N..
Female sex hormone, estradiol, antagonizes the immunosuppressive activity of cyclosporine in rat organ transplantation. Transplant Proc.
1992;
24
:
408-9
.
-
Robson
S. C.,
Neuberger
J. M.,
Williams
R..
The influence of cyclosporine A therapy on sex hormone levels in pre- and post-menopausal women with primary biliary cirrhosis. Journal of Hepatology.
1994;
21
:
412-6
.
View Article Google Scholar -
Kramer
B. K.,
Neumayer
H. H.,
Stahl
R.,
Pietrzyk
M.,
Kruger
B.,
Pfalzer
B.,
Bourbigot
B.,
Campbell
S.,
Whelchel
J.,
Eris
J.,
Vitko
S.,
Budde
K..
Graft function, cardiovascular risk factors, and sex hormones in renal transplant recipients on an immunosuppressive regimen of everolimus, reduced dose of cyclosporine, and basiliximab. Transplant Proc.
2005;
37
:
1601-4
.
-
Dh
V. T..
R S, JS G, A F. Hepatic regeneration. Digestive Diseases and Sciences.
1991;
36
:
1309-12
.
-
El-Bassossy
H. M.,
Eid
B. G..
Cyclosporine A exhibits gender-specific nephrotoxicity in rats: effect on renal tissue inflammation. Biochemical and Biophysical Research Communications.
2018;
495
:
468-72
.
View Article Google Scholar -
El-Bassossy
H. M.,
Awan
Z.,
El-Mas
M. M..
Perinatal ciclosporin A exposure elicits sex-related cardiac dysfunction and inflammation in the rat progeny. Toxicology Letters.
2017;
281
:
35-43
.
View Article Google Scholar -
Nematbakhsh
Mehdi,
Pezeshki
Zahra,
Jazi
Fatemeh Eshraghi,
Mazaheri
Bahar,
Moeini
Maryam,
Safari
Tahereh,
Azarkish
Fariba,
Moslemi
Fatemeh,
Maleki
Maryam,
Rezaei
Alireza.
Cisplatin-induced nephrotoxicity; protective supplements and gender differences. Asian Pacific journal of cancer prevention: APJCP.
2017;
18
:
295
.
-
Nematbakhsh
M.,
Ebrahimian
S.,
Tooyserkani
M.,
Eshraghi-Jazi
F.,
Talebi
A.,
Ashrafi
F..
Gender difference in Cisplatin-induced nephrotoxicity in a rat model: greater intensity of damage in male than female. Nephro-Urology Monthly.
2013;
5
:
818-21
.
View Article Google Scholar -
Eshraghi-Jazi
F.,
Nematbakhsh
M.,
Nasri
H.,
Talebi
A.,
Haghighi
M.,
Pezeshki
Z.,
Safari
T.,
Ashrafi
F..
The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci.
2011;
16
:
1389-96
.
-
Haghighi
M.,
Nematbakhsh
M.,
Talebi
A.,
Nasri
H.,
Ashrafi
F.,
Roshanaei
K..
The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Renal Failure.
2012;
34
:
1046-51
.
View Article Google Scholar -
Eshraghi-Jazi
F.,
Talebi
A.,
Mirsaeedi
F. S.,
Ahmadian
S.,
Moslemi
F.,
Nematbakhsh
M..
Gentamicin Induced Nephrotoxicity: The Role of Sex Hormones in Gonadectomized Male and Female Rats. Scientifica.
2016;
5025097
:
26
.
-
Chen
W. Y.,
Hsiao
C. H.,
Chen
Y. C.,
Ho
C. H.,
Wang
J. J.,
Hsing
C. H..
Cisplatin Nephrotoxicity Might Have a Sex Difference. An analysis Based on Women\'s Sex Hormone Changes. Journal of Cancer.
2017;
8
:
3939-44
.
View Article Google Scholar -
Eshraghi-Jazi
F.,
Nematbakhsh
M.,
Pezeshki
Z.,
Nasri
H.,
Talebi
A.,
Safari
T..
Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iranian Journal of Kidney Diseases.
2013;
7
:
383-9
.
-
Pezeshki
Z.,
Nematbakhsh
M.,
Nasri
H.,
Talebi
A.,
Pilehvarian
A. A.,
Safari
T..
Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicology International.
2013;
20
:
43-7
.
View Article Google Scholar -
Nematbakhsh
M.,
Nasri
H..
Cisplatin nephrotoxicity may be sex related. Kidney International.
2013;
83
:
1201
.
View Article Google Scholar -
Wongtawatchai
T.,
Agthong
S.,
Kaewsema
A.,
Chentanez
V..
Sex-related differences in cisplatin-induced neuropathy in rats. Journal of the Medical Association of Thailand.
2009;
92
:
1485-91
.
-
Rao
K.,
Sethi
K.,
Ischia
J.,
Gibson
L.,
Galea
L.,
Xiao
L..
Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One.
2017;
12
:
e0180028
.
View Article Google Scholar -
Xu
Z.,
Zhou
J..
Zinc and myocardial ischemia/reperfusion injury. Biometals.
2013;
26
:
863-78
.
View Article Google Scholar -
Shuttleworth
C. W.,
Weiss
J. H..
Zinc: new clues to diverse roles in brain ischemia. Trends in Pharmacological Sciences.
2011;
32
:
480-6
.
View Article Google Scholar -
Ogawa
T.,
Mimura
Y..
Antioxidant effect of zinc on acute renal failure induced by ischemia-reperfusion injury in rats. American Journal of Nephrology.
1999;
19
:
609-14
.
View Article Google Scholar -
Guo
L.,
Li
P.,
Meng
C.,
Lu
R.,
Yang
Y.,
Zhou
Y..
Protective effect of zinc on mouse renal ischemia-reperfusion injury by anti-apoptosis and antioxidation. Current Pharmaceutical Biotechnology.
2014;
15
:
577-82
.
View Article Google Scholar -
Moslemi
F.,
Talebi
A. M..
The protective effect of zinc supplementation on renal ischemia/reperfusion injury in rat: gender-related difference. International Journal of Preventive Medicine.
2018
.
-
Yilmaz
M.,
Mogulkoc
R.,
Baltaci
A. K..
Effect of Three-Week Zinc and Melatonin Supplementation on the Oxidant-Antioxidant System in Experimental Renal Ischemia-Reperfusion in Rats. Acta Clinica Croatica.
2015;
54
:
395-401
.
-
Guo
L.,
Li
P.,
Meng
C.,
Lu
R.,
Yang
Y.,
Zhou
Y..
Protective effect of zinc on mouse renal ischemia-reperfusion injury by anti-apoptosis and antioxidation. Current Pharmaceutical Biotechnology.
2014;
15
:
577-82
.
View Article Google Scholar -
Barekat
F.,
Talebi
A. M..
The protective roles of zinc and estradiol in renal ischemia/ reperfusion injury in ovariectomized rats. Journal of Nephropathology.
2018;
7
:
5
.
-
Ersoy
A.,
Baran
B.,
Ersoy
C.,
Kahvecioglu
S.,
Akdag
I..
Calcineurin inhibitors and post-transplant weight gain. Nephrology (Carlton, Vic.).
2008;
13
:
433-9
.
View Article Google Scholar -
Robson
S. C.,
Neuberger
J. M.,
Williams
R..
The influence of cyclosporine A therapy on sex hormone levels in pre- and post-menopausal women with primary biliary cirrhosis. Journal of Hepatology.
1994;
21
:
412-6
.
View Article Google Scholar -
Gore-Langton
R. E..
Cyclosporine differentially affects estrogen and progestin synthesis by rat granulosa cells in vitro. Molecular and Cellular Endocrinology.
1988;
57
:
187-98
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 12 (2018)
Page No.: 2888-2897
Published on: 2018-12-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6344 times
- Download PDF downloaded - 1985 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress







