Ex-vivo cytotoxic, antibacterial and DPPH free radical scavenging assay with ethanolic leaf extract of Glycosmis pentaphylla to justify its traditional use
Abstract
Aim: Glycosmis pentaphylla belongs to the family Rutaceae. It is a shrub and locally common in the treatment of hepatic impairment. We have designed this study to provide a scientific basis with the traditional use of leaf of G. pentaphylla in the treatment of hepatitis.
Methods: The well-established DPPH free radical scavenging activity was tested for antioxidant property evaluation. On the other hand, disk diffusion and brine shrimp method was respectively used to determine antibacterial and cytotoxic activity.
Results & Discussion: In the evaluation of antioxidant property IC50 found 204.91 ± 2.223 µg/ml, in cytotoxicity testing, it is found that the plant part shows 30.49 ± 1.976 µg/ml of LC50. The ethanolic extract of G. pentaphylla leaves also have efficiency in bacterial growth inhibition; this extract is effective against for both gram, negative and positive. The zone of inhibition at 500 µg/ml dose in E. coli and C. albican culture was 18 mm and 15 mm, respectively. In thin layer chromatography analysis, we found presence of couple of non-polar and polar component, presence of three non-chromatophoric component are also evident.
Conclusion: Appropriate isolation and identification of mechanism is suggested in further study.
Introduction
From the ancient era, it is human’s nature to find cure in the herb source. This practice is still popular among people on all continents, and most of them have their own enriched prehistory. There is evidence that plants are still widely used in ethnomedicine around the world. There is around 250,000 to 500,000 species of plants on Earth Borris, 1996. Only a small fraction of them most likely 1-10% of them are used as food by both humans and other animals. Therefore, there is huge possibility to use plants in medical practice and remedy purposes Moerman, 1996.
An antibacterial agent that either kills microorganism or suppresses its growth is often termed as antibiotic. The term antibiotic covers a broad range of agents like antimicrobials, including antifungal and other compounds Dorland, 2010. Waksmanin first used antibiotic in 1942; he use dit to describe any substance that intersect the replicationor kills microorganisms Waksman, 1947. With the application of modern science, most of today’s antibioticsare either structural modification or use of optical isomerism of the 1st generation antibiotics that used to be natural compounds, for example, Penicillin, Cephalosporin, Sulfonamide, Quinolone, and so forth von Nussbaum et al., 2006. Plant chemicals that are supposed to be responsiblefor antibacterial effects, likely to have phenolic ring, alkaloid, tannins. For example, common herbs thyme and tarragon possess effective antibacterial, antifungal, and antiviral activities, containing caffeicacid in phytochemical list Brantner et al., 1996Duke, 1985Mason and Bruce P, 1987Thomson, 1978. The mechanisms areyet not clear but might be due to phenolictoxicity to microorganisms that inhibit enzymesby the oxidation, possibly through reaction with sulfhydryl groups or through other nonspecific interaction with the proteins Ya C., 1988.
The liver is a highly sensitive organ, which plays a major role in maintenance and performance of the homeostasis in our body. It is the major organ where processes like metabolism and detoxification takes place. Therefore, there is a chance of injury because of chronic exposure to drugs, environmental toxicants and other xenobiotics Amacher, 2002. Liver disorders are one of the serious health issue, at present time. Ethanol is a lipid-soluble non-electrolyte and is readily absorbed from the skin and gastrointestinaltract. It quickly diffuses to the circulatory system, dispersed evenly through out the body McDonough, 2003. Ethanolis metabolized in the liver and person who consume regularly and get addicted to alcohol (drinks 4 to 5 per day) are at risk of chronic liver diseases Zakhari and Li, 2007. Moreover, both acute and chronicin take of ethanol produces cytokines in large amounts, particularly TNF-α by hepatic κ-cells, which plays a major role in causing liver injury Thurman, 1998Tsukamoto et al., 2001Zhou et al., 2003. These things results into accumulation of hepatic lipids also the lipid peroxides and lead to auto-oxidation of hepatic cells either by acting asa pro-oxidant or by decreasing the antioxidant levels, thereby resulting in a remarkable hepatotoxicity. Lipid peroxidation by ethanol induces hepatic oxidative stress, which identified as a reason to play a pathogenic role in Alcoholic Liver Disease (ALD) Bunout, 1999. There is evidence that almost 5% of oxygen, from total oxygen consumed, converts into oxygen derived free radicles Halliwell, 1988Yu, 1994. Meanwhile, those free radicals are known as reactive oxygen species or ROS (e.g., O2-, H2O2, OH-), that are formed in body as a byproduct of different metabolism process and from exogenous sources. ROS molecules produces a stressed condition in human body that causes each cell to face about 10000 hits per second Lata, 2003. If the generation of ROS exceeds the antioxidative defense of body, cells become saturated. Then the free radicals targets macromolecule (like lipid, protein, carbohydrate) of human body and different disease condition appears Byung et al., 1992Campbell and Abdulla, 1995Cotran, 1999. Free radicals are responsible for pathogenic condition of degenerative disease like Alzheimer’s, they also involved in consequence of diabetes, cardiovascular disease, nephrotoxicity, neurotoxicity and so far Marx, 1987. Many plants contains molecules like vitamin C and E, flavonoids, carotenoids, phenolic content etc. that have ability to prevent oxidation and remove excess free radicals from body Pratt, 1992.
Glycosmis pentaphylla is an evergreen shrub or small tree that reaches up to 5 m. The branches are hairless, unarmed, young parts, finely rusty and puberulent. Leaves are alternate, pinnate with an unpaired terminal leaflet. The plant is locally known as Motali. The whole plant has medicinal value and used locally as an anti-pyretic and anti-diarrheal agent. Particularly, its leaves extract are important in the treatment and recovery from Hepatitis. This folkloric use of this plant makes us interested to carry out the present evaluation with this plant.
Material and Methods
Collection and identification of plant
The plant part was collected from Madhupur of Tangail forest region of Bangladesh, in between April-May 2013. Taxonomist of National Herbarium Bangladesh, Dhaka, identified the plant and an accession number was submitted (35483).
Extraction
Extract was prepared from leaf part of the collected plant by usingorganic solvent Ghani, 2005. The fresh leaves of Glycosmis pentaphylla were pieced; washed and air-dried at room temperature (24 ± 2°C) for about 10 days. Dried leaves was milled into coarsepowder. Coarse powder, weighing about 200 grams, takenin a bottle and dissolved in ethanol. Then the mixture kept for 2 days with uninterrupted shaking. The extract was collected using Buckner funnel, where theethanolic mix of the powder was poured under vacuumsuction. The filtrate contained the crude drug extract ofethanol. The ethanol was evaporated and a concentrated crude drug extract of Glycosmis pentaphylla leaves was obtained, which wasweighed to be 29 grams and was preserved into alpine tubefor further use at 4°C. The percent yield was 14.5%.
Antioxidant assay
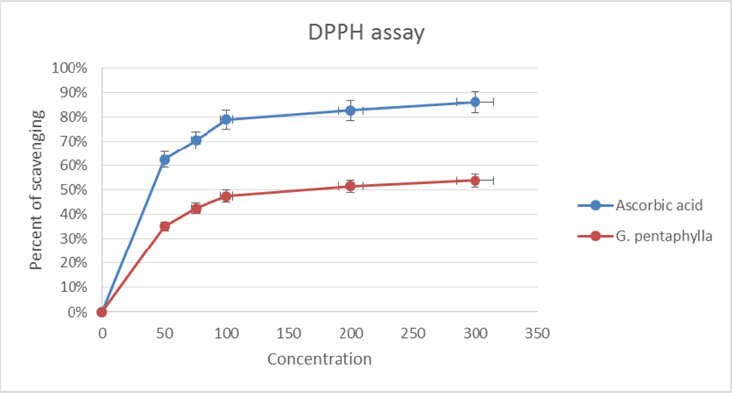
DPPH scavenging assay: The DPPH scavenging activity of G. pentaphylla was measured according to the method of Liu and Zhao (2006) Liu and Zhao, 2006. The reaction mixture contained 2 ml of 95% ethanol, 0.1 M DPPH and 2 ml of the ethanolic leaf extract of G. pentaphylla (50, 75, 100, 200, 300 μg/ml). The solution was incubated at 25°C for 15 min, and the absorbance of G. pentaphylla was determined at 517 nm. The antioxidant activity of G. pentaphylla extract was evaluated according to the following formula:
Scavenging rate (%) = [1-A]/ A0 X 100
Where A is absorbance of G. pentaphylla extract and A0 is the absorbance of negative control (DPPH solution). Ascorbic acid used in this method as positive control, to compare the effectiveness.
Cytotoxic assay
In vitro Brine shrimp lethality bioassay Rahman and Rashid, 2008 technique applied, using nauplii of Artemia salina, for the determination of general toxic property of G. pentaphylla. In this method Vincristinsulphate was used as a positive control, for the comparison. Eggs kept in a small tank containing 3.8% NaCl solution for hatching, a light source was attached to that tank, we hatched eggs for 2 days and then it is ready for experiment. Four milligrams of the extract was dissolved in DMSO to geta concentration of varying concentrations 100, 50, 25, 12.50 and 6.25 μg/ml. 10 brine shrimp nauplii were then placed in each vial and allowed to stand for 24 hour. The vials were observed using a magnifying glass and the number of survivors in each vial were counted and noted. From these data, the percentage of mortality of the nauplii was calculated for each concentration and the 50% lethal concentration (LC50) values were determined.
Antimicrobial property investigation
Antimicrobial Activity: Stock solution was prepared by dissolving 10 mg of the ethanolic crude drug extract in ethanol. The disk for drug dissolving was prepared using sterilized filter paper. Papers were punched uniformly to exactly 6mm in diameter. Sample solutions of desired concentrations (100, 200, 400 and 500 μg/disk) were applied with the help of the micropipette in an aseptic condition. These disks were left for a few minutes in aseptic condition for complete evaporation of the solvent. In this study, commercially prepared Kanamycin disk, K-30 disks containing 30 μg/disk, was used as a standard for comparison purpose. The in vitro disk diffusion assay (Perez C, et al., 1990), of antibacterial screening was used to determine the susceptibility of the pathogenic microorganisms to the test compound applied.
Preparation of fresh culture of the pathogenic organisms: The nutrient agar medium was prepared and dispersed in a number of test tubes to prepare slants (5 ml in each test tube). This was done to prepare (Axenic) cultures from the supplied cultures (Madigan M and Martinko J, 2005). The test tubes were sterilized at 121°C temperature and a pressure of 15lbs/sq inch for 15 minutes. After sterilization, they were kept in an inclined position, for solidification, and then was incubated at 37.5°C. The test organisms were transferred to the agar slants from the supplied cultures with the help of an inoculating loop in aseptic condition. The culture was kept at 4°C or less for bacterial growth for 12 hours. Then incubated at 37°C for 24 hours to assure the growth of test organisms. These fresh (Axenic) cultures were then used for the sensitivity test.
The test plates were prepared for the disc diffusion test of the test samples. Bacterial suspensions were transferred to the sterile petri dishes in an aseptic area. The petri dishes were rotated several times, first clockwise and then anticlockwise to assure homogenous distribution of the test organisms. The media was poured into petri dishes in such a way, in order to give a uniform depth of approximately 4mm.
Finally, the medium was cooled to room temperature in laminar airflow unit and it was kept in refrigerator at (4°C) and the sample impregnated discs and standard disc were seeded, in the sub-solidified medium. The medium was congealed to room temperature in laminar airflow unit, then refrigerate at (4°C) for 24 hours in order to provide sufficient time to diffuse the antibiotics into the medium. Hence, the zones of inhibition of different samples were compared Brown and Kothari, 1975.
TLC analysis of the fraction
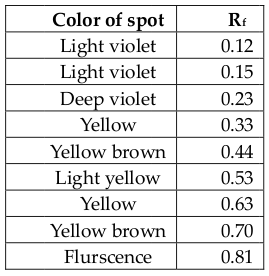
Extracts were checked by thin layer chromatography (TLC) on analytical plates over silical gel. The solvent systems used was H-EA = 2:1 where, H = hexane, EA = ethyl acetate. In this case, the spots were visualized by exposure of the plates to UV lamp. Different bands were observed and corresponding Rf values are determined. Rf value of each spot was calculated, Rf = (Distance traveled by solute/Distance traveled by solvent)
Statistical Analysis
The statistical analysis was performed using Graph-Pad Prism-6 software. Values are represented in tabular sheet as mean ± SD and ANOVA was performed for anti-microbial assay. The significant limit for that particular case was set p<0.05.
Results
Antioxidant assay
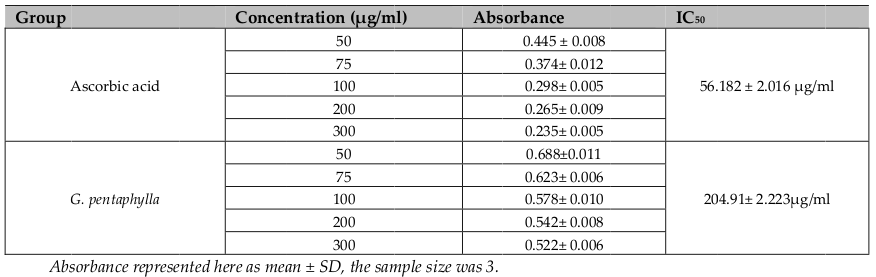
DPPH is a relatively stable free radical and the assay determines the ability of ethanolic extract of G. pentaphyllato reduce DPPH free radicals to the corresponding hydrazine by converting the unpaired electrons to paired ones. Antioxidant can act by converting the unpaired electron to paired one. The dose dependent inhibition of DPPH radicals ( Figure 1 ) indicates that selected extract causes reduction of DPPH radical in a stoichiometric manner Murray, 1999Sanchez-Moreno, 2002Vani et al., 1997; with the inhibitory concentration (IC50) 204.91 ± 2.223 μg/ml; where the comparable standard have 56.182 ± 2.016 μg/ml of IC50value ( Table 1 ). From this point of view, it is clear that the extract have moderate antioxidative capacity, through which it can yet reduce the exacerbation free radicals.
Antimicrobial assay
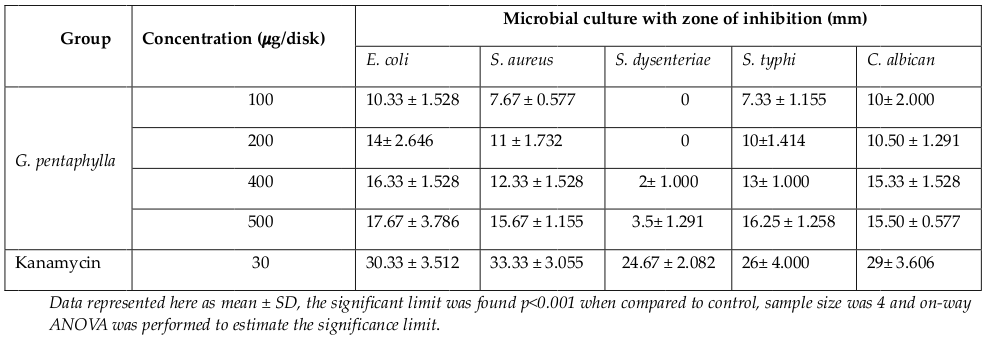
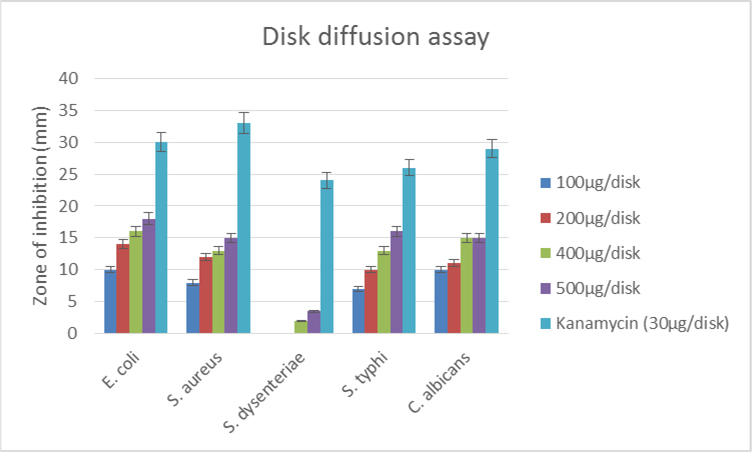
The antimicrobial activity of the ethanolic extract of-leaves of G. pentaphylla was measured by disc diffusion method. Different concentrations of 100 μg/disk, 200 μg/disk, 400 μg/disk, and 500 μg/disk were measured and compared with the zone of inhibitions, which was produced by the standard. The zones of inhibition were seen against selective bacteria at a particular concentration ( Table 2 ). The studied ethanolic extract of leaves of plant G. pentaphylla showed higher activity against E. coli. At higher concentrations of 400 μg/disc and 500 μg/disc, the extract also showed goodinhibitions against other studied microorganism. However, the extract showed negligible or no activity against S. dysenteriae, which is a gram-negative bacteria.
Cytotoxic assay
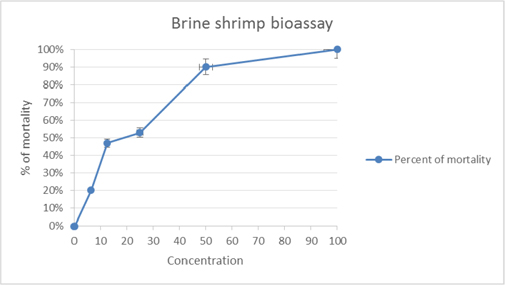
In cytotoxic test activity, percent of mortality increased gradually with the increase in concentration of the test samples. LC50 values obtained from the best-fitline slope ( Figure 3 ) were 30.49 ± 1.976 μg/ml and 24.879 ± 2.413 μg/ml for G. pentaphylla and vincristine sulphate, respectively.
The brine shrimp lethality bioassay is very useful toassess the bioactivity of the plant extracts, which inmost cases correlates reasonably well with cytotoxicand anti-tumor properties McLaughlin et al., 1993. LC50 values of G. pentaphylla revealed its considerable cytotoxic potency. Sufficient amount of phenolics and flavonoidsmay be present and it might be responsible for its promising cytotoxic activity Moreira et al.,2007Okwori, 2007 and the possible mechanism of cytotoxicityagainst brine shrimp nauplii due to poisonous effecton cell mitosis.
TLC assay for compound detection
Observation of the TLC plates under UV lamp results following ( Table 3 ). Four non-polar compounds were present with Rf values of 0.12, 0.15 and 0.23 and 0.33. Two compounds are in between polar and non-polar with Rf value of 0.44 and 0.53. Two polar compounds were present with Rf values of 0.63 and 0.70. A fluorescent compound with an Rf value of 0.81 could also be detected. Three non-chromatophoric compounds with Rf values of 0.04 (nonpolar) and 0.23 (nonpolar) and 0.64 (partially polar or polar). Thus, many compounds were present and isolation of pure compound is necessary.
Discussion
The extractive preliminary phytochemical analysis that was performed earlier, results the presence of alkaloid, flavonoid, steroid, saponin etc. Ansari P, et al., 2015 Flavonoids have the hepatoprotective reputation as anti-oxidant phytoagent (Faure et al., 1990). Tannins Hong et al., 1995 and polyphenols Toda et al., 1991 are also reported as they have significant antioxidant properties. Accordingly, these compounds have shown to have antioxidant activity Dong, 2003Leung, 2000. Total phenolic constitutes are one of the major groups responsible for primary antioxidant or free radical termination, detected in the herbal preparation. Flavonoids are the most widespread group of natural compounds and probably the most important natural phenolics. The medicinal effects of plants are often attributed to the antioxidant activity of phytochemical constituents mainly phenolics, flavonoids and flavonols Miliauskas et al., 2004. It is claimed that the phenolic compounds are powerful chain breaking antioxidants Shahidi et al., 1992. Herbal preparation revealed well effects in DPPH scavenging in this present study.
Figure 2 Figure 2 Schematic presentation of bacterial growth inhibition; crude extract revealed its potent effectiveness at 500 μg/disk concentration; there was two gram-positive and three gram-negative microbes used, studied extract found similar effective for both class of organism.
The crude extracts of plants are pharmacologically potent may bedue to presence of various components in the whole extract, that are claimed to possess antioxidant activity by several investigator Hamburger and Hostettmann, 1991.
In the present study, we evaluated the antibacterial activity of the ethanolic crude extracts of G. pentaphylla. The study of antimicrobial activity was carried out against E. coli, S. aureus, S. dysenteriae, S. typhi and C. albican. The results are showed in Table 2 . In this study, crude extract of G. pentaphylla leaves have more potent antimicrobial activity against gram positive thangram-negative bacteria.The antibacterial activity-demonstrated by ethanolic extract of G. pentaphylla may be due to presence of flavonoids. Many crude extracted from plants by several research groups have a history of use in folk medicine, as antibacterial agent. Most of the time it is reported that the flavonoid rich plant extracts possess better activity. Flavonoid enriched species ofHypericum Dall’Agnol et al., 2003, Capsella and Chromolaena El-Abyad et al., 1989 have been reported to have antibacterial activity. Many other phytochemical preparations with high flavonoid content have also been reported to exhibit antibacterial activity Al-Saleh et al., 1997Aladesanmi et al., 1986Mahmoud et al., 1989Quarenghi, 2000Rauha et al., 2000Singh and Nath, 1999Tarle and Dvorzak, 1990Tereschuk et al., 1997Torrenegra et al., 1989, and so forth. From phytochemical analysis, reported earlier Ansari et al., 2015, it is clear that the antimicrobial activity possessed by our plant extract may be due to presence of flavonoid content.
Based on the present study, the brine shrimp lethality of the crude extract was found to be concentration-dependent. The observed lethality plant extracts against brine shrimp indicates the presence of potent cytotoxic and probably antitumor components of the plant. According to Meyer et al. (1982) Meyer et al., 1982, crude plant extract is toxic if the LC50 value isbe-low 1000 μg/ml, but the plant extract is non-toxic if LC50 is higher than 1000 μg/ml. The LC50 value we obtained from this study was 30.49 ± 1.976 μg/ml, which means it is more potent according to Meyer et al. and probably containing active anti-tumor constituents.
Conclusion
This work has demonstrated that the ethanolic extracts of G. pentaphylla leaves possesses different pharmacological property. This plant extracts contains several active constituents. The antioxidant, cytotoxic and antimicrobial potentiality is the result or evidence of their presence. However, this plant has been used in traditional medicine for many years, our present study reportalso support the traditional use of the plant in infectious and inflammatory disorders. Further study need to be carried onto under stand the exact mechanisms of suchactions and to isolate the active principles responsible for the observed activity.
References
-
G.F.S.
Al-Saleh,
A.Y.
Gamal El-Din,
J.A.
Abbas,
N.A.
Saeed.
Phytochemical and Biological Studies of Medicinal Plants in Bahrain: The Family Chenopodiaceae—Part 2. Pharmaceutical Biology.
1997;
35
:
38-42
.
-
A.
Aladesanmi,
A.
Sofowora,
J.
Leary.
Preliminary biological and phytochemical investigation of two Nigerian medicinal plants. Pharmaceutical Biology.
1986;
24
:
147-153
.
-
D.E.
Amacher.
A toxicologist’s guide to biomarkers of hepatic response. Human & Experimental Toxicology.
2002;
21
:
253-262
.
-
P.
Ansari,
A.
Ulla,
A.R.U.
Islam,
M.
Sultana,
M.N.
Alam,
M.
Mustakim,
M.N.
Uddin.
Ex-vivo cytotoxic, antibacterial and DPPH free radical scavenging assay with ethanolic leaf extract of Glycosmis pentaphylla to justify its traditional use. Biomedical Research and Therapy.
2015;
2
.
-
R.P.
Borris.
Natural products research: perspectives from a major pharmaceutical company. Journal of Ethnopharmacology.
1996;
51
:
29-38
.
-
A.
Brantner,
Ž.
Maleš,
S.
Pepeljnjak,
A.
Antolić.
Antimicrobial activity of Paliurus spina-christi Mill. (Christ’s thorn). Journal of Ethnopharmacology.
1996;
52
:
119-122
.
-
D.F.
Brown,
D.
Kothari.
Comparison of antibiotic discs from different sources. Journal of Clinical Pathology.
1975;
28
:
779-783
.
-
D.
Bunout.
Nutritional and metabolic effects of alcoholism: their relationship with alcoholic liver disease. Nutrition.
1999;
15
:
583-589
.
-
P.Y.
Byung,
E.A.
Suescun,
S.Y.
Yang.
Effect of age-related lipid peroxidation on membrane fluidity and phospholipase A 2: modulation by dietary restriction. Mechanisms of ageing and development.
1992;
65
:
17-33
.
-
I.C.
Campbell,
E.M.
Abdulla.
Strategic Approaches to in Vitro Neurotoxicology. In Neurotoxicology (Elsevier BV).
1995;
:
495-505
.
-
R.S.
Cotran,
V.
Kumar,
T.
Collins.
Robin’s pathological basis of diseases, 6th ed. Noida, India: Thomson press.
1999
.
-
R.
Dall’Agnol,
A.
Ferraz,
A.P.
Bernardi,
D.
Albring,
C.
Nör,
L.
Sarmento,
L.
Lamb,
M.
Hass,
G.
von Poser,
E.E.S.
Schapoval.
Antimicrobial activity of some Hypericum species. Phytomedicine.
2003;
10
:
511-516
.
-
Z.
Dong.
Molecular mechanism of the chemopreventive effect of resveratrol. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis.
2003;
523-524
:
145-150
.
-
N.W.
Dorland.
Dorlands Medical Dictionary: antibacterial. 2010
.
-
J.A.
Duke.
Handbook of Medicinal Herbs. CRC Press.
1985
.
-
M.S.
El-Abyad,
N.M.
Morsi,
D.A.
Zaki,
M.T.
Shaaban.
Preliminary screening of some Egyptian weeds for antimicrobial activity. Microbios.
1989;
62
:
47-57
.
-
M.
Fauré,
E.
Lissi,
R.
Torres,
L.A.
Videla.
Antioxidant activities of lignans and flavonoids. Phytochemistry.
1990;
29
:
3773-3775
.
-
A.
Ghani.
Textbook of Pharmacognosy, 1st ed edn. Dhaka, Bangladesh: Institution of Medical Technology.
2005
.
-
B.
Halliwell,
J.M.C.
Gutteirdge.
Free radicals in biology and medicine, 2nd edn. Oxford: Clarendon Press.
1988
.
-
M.
Hamburger,
K.
Hostettmann.
7. Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry.
1991;
30
:
3864-3874
.
-
C.-Y.
Hong,
C.-P.
Wang,
S.-S.
Huang,
F.-L.
Hsu.
The Inhibitory Effect of Tannins on Lipid Peroxidation of Rat Heart Mitochondria. Journal of Pharmacy and Pharmacology.
1995;
47
:
138-142
.
-
H.
Lata,
G.K.
Ahuja.
Role of free radicals in health and disease. Ind J Phys App Sci.
2003;
125
.
-
I.K.
Leung,
Y.
Su,
R.
Chen,
A.
Zang,
Y.
Huang,
Z.Y.
Chen.
The flavins in black and catechins in green tea are equally effective antioxidants. J Nutr.
2000;
:
2248-2251
.
-
X.
Liu,
M.
Zhao.
Antioxidant activities and functional composition content of selected Phyllanthus emblica fruits juice. Food and Fermentation Industries.
2006;
5
:
151-154
.
-
M.J.
Mahmoud,
A.-L.M.
Jawad,
A.M.
Hussain,
M.
Al-Omari,
A.
Al- Naib.
In vitro Antimicrobial Activity of Salsola rosmarinus and Adiantum capillus-veneris. Pharmaceutical Biology.
1989;
27
:
14-16
.
-
J.
Marx.
Oxygen free radicals linked to many diseases. Science.
1987;
235
:
529-531
.
-
T.L.
Mason,
W.
Bruce P.
Inactivation of red beet β-glucan synthase by native and oxidized phenolic compounds. Phytochemistry.
1987;
26
:
2197-2202
.
-
K.
McDonough.
Antioxidant nutrients and alcohol. Toxicology.
2003;
189
:
89-97
.
-
J.L.
McLaughlin,
C.-j.
Chang,
D.L.
Smith.
Simple Bench- Top Bioassays (Brine Shrimp and Potato Discs) for the Discovery of Plant Antitumor Compounds. In ACS Symposium Series (American Chemical Society (ACS)).
1993;
:
112-137
.
-
B.
Meyer,
N.
Ferrigni,
J.
Putnam,
L.
Jacobsen,
D.
Nichols,
J.
McLaughlin.
Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med.
1982;
45
:
31-34
.
-
G.
Miliauskas,
P.R.
Venskutonis,
T.A.
van Beek.
Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry.
2004;
85
:
231-237
.
-
D.E.
Moerman.
An analysis of the food plants and drug plants of native North America. Journal of Ethnopharmacology.
1996;
52
:
1-22
.
-
M.D.
Moreira,
M.C.
Picanço,
L.C.A.
Barbosa,
R.N.C.
Guedes,
E.C.
Barros,
M.R.
Campos.
Compounds fromAgeratum conyzoides: isolation, structural elucidation and insecticidal activity. Pest Manag Sci.
2007;
63
:
615-621
.
-
P.R.
Murray,
E.J.
Baron,
M.A.
Pfaller,
F.C.
Tenover,
R.H.
Yolke.
Manual of clinical microbiology, 7th ed. edn (Washington: ASM).
1999
.
-
A.E.J.
Okwori,
C.O.
Dina,
S.
Junaid,
I.O.
Okeke,
J.A.
Adetunji,
A.O.
Olabode.
Antibacterial activities of Ageratum conyzoides extracts on selected bacterial pathogens. Int J Micro.
2007;
:
1937-1949
.
-
D.E.
Pratt.
Natural antioxidant from plant materials; Phenolic compounds in food and their effects on health II. In Antioxidants and Cancer prevention (ACS symposium series) M. Hang, Ho, C., and Lee, C., ed. (Washington D. C.: American Chemical Society).
1992
.
-
M.
Quarenghi.
Antimicrobial activity of flowers from Anthemis cotula. Fitoterapia.
2000;
71
:
710-712
.
-
M.S.
Rahman,
M.A.
Rashid.
. Oriental Pharmacy and Experimental Medicine.
2008;
8
:
47-52
.
-
J.-P.
Rauha,
S.
Remes,
M.
Heinonen,
A.
Hopia,
M.
Kähkönen,
T.
Kujala,
K.
Pihlaja,
H.
Vuorela,
P.
Vuorela.
Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology.
2000;
56
:
3-12
.
-
C.
Sanchez-Moreno.
Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Science and Technology International.
2002;
8
:
121-137
.
-
F.
Shahidi,
P.K.
Janitha,
P.D.
Wanasundara.
Phenolic antioxidants. Critical Reviews in Food Science and Nutrition.
1992;
32
:
67-103
.
-
R.K.
Singh,
G.
Nath.
Antimicrobial activity of Elaeocarpus sphaericus. Phytother Res.
1999;
13
:
448-450
.
-
D.
Tarle,
I.
Dvorzak.
Antimicrobial activity of the plant Cirsium oleraceum (L.) Scop. Acta Pharm Jugosl.
1990;
40
:
569-571
.
-
M.a.L.
Tereschuk,
M.V.Q.
Riera,
G.R.
Castro,
L.R.
Abdala.
Antimicrobial activity of flavonoids from leaves of Tagetes minuta. Journal of Ethnopharmacology.
1997;
56
:
227-232
.
-
W.A.R.
Thomson.
Medicines from the Earth (UK: McGraw- HillBook).Thurman, R. (1998). II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. American Journal of Physiology-Gastrointestinal and Liver Physiology.
1978;
275
:
G605-G611
.
-
S.
Toda,
M.
Kimura,
M.
Ohnishi.
Effects of Phenolcarboxylic Acids on Superoxide Anion and Lipid Peroxidation Induced by Superoxide Anion. Planta Med.
1991;
57
:
8-10
.
-
R.D.
Torrenegra,
A.A.
Ricardo,
J.P.
Pedrozo,
O.C.
Fuentes.
Flavonoids from Gnaphalium gracile H.B.K.. Pharmaceutical Biology.
1989;
27
:
22-24
.
-
H.
Tsukamoto,
Y.
Takei,
C.J.
McClain,
S.
Joshi-Barve,
D.
Hill,
J.
Schmidt,
I.
Deaciuc,
S.
Barve,
A.
Colell,
C.
Garcia-Ruiz.
How Is the Liver Primed or Sensitized for Alcoholic Liver Disease?. Alcoholism: Clinical and Experimental Research.
2001;
25
:
171S-181S
.
-
T.
Vani,
M.
Rajani,
S.
Sarkar,
C.J.
Shishoo.
A NTIOXIDANT P ROPERTIES OF THE A YURVEDIC F ORMULATION T RIPHALA AND ITS C ONSTITUENTS. Pharmaceutical Biology.
1997;
35
:
313-317
.
-
F.
Nussbaum,
M.
Brands,
B.
Hinzen,
S.
Weigand,
D.
Häbich.
Antibacterial Natural Products in Medicinal Chemistry—Exodus or Revival?. Angewandte Chemie International Edition.
2006;
45
:
5072-5129
.
-
S.A.
Waksman.
What Is an Antibiotic or an Antibiotic Substance?. Mycologia.
1947;
39
:
565
.
-
C. G.S.H.
Ya,
T.H.
Lilley,
E.
Haslam.
Carbohydrate-Polyphenol Complexation. In Chemistry and Significance of condensed tannins, K.J. Hemingway RW, ed.. New York: Plenum Press.
1988;
:
553
.
-
B.P.
Yu.
Cellular defenses against damage from reactive oxygen species. Physiological reviews.
1994;
74
:
139-162
.
-
S.
Zakhari,
T.-K.
Li.
Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology.
2007;
46
:
2032-2039
.
-
Z.
Zhou,
L.
Wang,
Z.
Song,
J.C.
Lambert,
C.J.
McClain,
Y.J.
Kang.
A Critical Involvement of Oxidative Stress in Acute Alcohol-Induced Hepatic TNF-α Production. The American Journal of Pathology.
2003;
163
:
1137-1146
.
Comments

Downloads
Article Details
Volume & Issue : Vol 2 No 07 (2015)
Page No.: 324-332
Published on: 2015-07-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6747 times
- Download PDF downloaded - 1964 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress