Abstract
Introduction: Diabetes mellitus (DM) is well-known metabolic disorder, which causes serious effects on human health with its complications. Many mechanisms has been postulated to cause DM related complications. One of the main complications is neuronal damage, for which no proper preventive and curative therapies are available.
Methods: In this study the effects of Ginger, Nigella sativa, Punica granatum and Metformin were seen on the prevention and treatment of brain damage caused by diabetes mellitus in streptozotocin (STZ)- induced diabetes in rats. 50 adult Wistar albino male rats were used in the study, the rats were divided in 10 study groups. The body weight, serum glucose levels were measured, and histopathological examination was performed.
Results: In comparison to the diabetic control rats, significant increase in weight was found in animals of all the studied groups. Serum glucose levels reduced significantly in comparison to the STZ induced diabetic rats in all the animals. Histopathological examination showed prevention from brain damage and repair of the neuronal tissues by Ginger, Nigella sativa, Punica granatum and Metformin.
Conclusion: The studied substances were observed to possess preventive and curative effects on the brain damage caused by diabetes mellitus.
Introduction
Diabetes mellitus (DM) is a metabolic disorder that is characterized by an increase in blood glucose (BG) and excretion of glucose in urine. DM is one of the major global health hazards of the recent years and also an emerging threat to public health in Kingdom of Saudi Arabia. The endemic Saudi population appears to possess a special genetic trend to develop Type II DM, that is complicated by an increment in obesity statistics, and the presence of other causes of the insulin resistance1.
The main pathology associated with Type II DM is insulin resistance, which causes insulin deficiency in the hepatic and peripheral tissues causing hyperglycemia 2,3.
In type1 and type II diabetes mellitus, glucose uptake gets disturbed, especially in muscle and adipose tissues, resulting in hyperglycemia 4. The neurons start taking up glucose without insulin, therefore, in hyperglycemic diabetes mellitus, hyperglycemia causes an up to four-fold increase in neuronal cell glucose uptake. Increased cytosolic glucose concentration and metabolism leads to neuron damage 2,5.
In previous human and animal studies, DM has been found to be associated with pathological changes in the central nervous system, causing cognitive function decline, behavior disorders and an increased risk of vascular abnormalities in the brain2,6,7.
It has been suggested that long-term DM augments the risk of brain shrinkage, lacunar infarcts and white matter (WM) abrasions. The functional and behavioral consequences of diabetic brain abnormalities include cognitive decline and movement disorders. Many structural brain changes have been described such as increased hippocampal astrocyte reactivity, abnormal synaptic plasticity, vascular alterations, decreased dendritic complexity and disturbed neurotransmission8,9,10.
In the pathogenesis of diabetes related brain damage, disturbed coupled electron transport systems are caused by damaged mitochondria, which is the main foci for reactive oxygen species (ROS) in the neurons. Therefore, oxidative stress is recognized as a main mediating process in the pathogenesis of diabetic complications, due to increased production of free radicals and abnormal antioxidant defenses11,12.
Many of herbal substances have been found to possess antioxidants and protect brain from damages. Nigella Sativa is known to possess antioxidants and anti-hyperglycemic effects and prevents neuronal damage13,14.
Because of the chronic symptoms, the development of new treatment strategies is requiredto improve the effectiveness in treatments10.
Ginger has also been found to possess anti-oxidative properties and has been shown to perfrom neuroprotection in diabetic rats and protective and therapeutic effect on Alzheimer’s disease 15,16,17.
Pomegranate or Punica granatum, is a small tree or shrub found in the Mediterranean region18. It possess several biological effects such as antitumor and antibacterial functions, which have been reported with the extracts from different parts of P. Granatum 19,20. It has been shown that Punica granatum can alleviate brain oxidative stress in diabetic rats via the regulation of antioxidant defense mechanisms, which can ameliorate the abnormality in learning and memory performances in diabetic rats21,22.
Metformin is a standard antidiabetic drug, belonging to the Biguanide group, which was shown to attenuate stroke-induced nitrative signaling in GK rats23. Previous studies showed that metformin can significantly reduce Neuro-inflammation, decrease the loss of neurons in the hippocampus of diabetic animals, and prevent diabetes-induced memory loss in rats24,25.
The main objective of this study was to ascertain the prophylactic and therapeutic potential of Zingeber officinale, Nigella sativa, Punica granatum, and Metformin in diabetes mellitus associated brain damage and to discover cost effective treatment of the ailment.
Materials and Methods
Plant materials
Seeds of Nigella sativa, Ginger (Zingiber officinale), and Punica granatum were acquired from the regional market.
Preparation of Ginger ( Zingiber Officinale ) Extracts
Fresh ginger root was purchased from the local market in Rafha Municipal Council, Northern Border Region of Saudi Arabia. The roots were identified and authenticated by the Department of Pharmacognosy, in the Faculty of Pharmacy, Northern Border University, Rafha, Saudi Arabia.
Preparation was done according to the modified method used by (Kebe et al., 2015)26. Cleaning of 2.5 kg of fresh ginger rhizome was done with running tap water. It was shredded into small pieces and air dried for 2 weeks, then crushed into powder form with electric blender. 2000 g of this powdered Zingiber officinale was soaked in 5000 ml of 99.9% ethanol and rattled vigorously. It was left for 48 hours at room temperature and was stirred at different times. The dissolved ginger in ethanol was filtered with a mesh with small pores after 48 hours. Then, it was filtered using No1 Whatmann paper (filter paper) and funnel. The percolate was assembled in a tray and was air dried for 5 days to ensure complete evaporation of the ethanol used. The ginger paste was collected from the tray with a spatula into a container and was weighed using an electric scale. 50g of ginger paste was collected and then dissolved in extra virgin olive oil 100ml (to serve as a vehicle). The extract was then kept in a dry place at 37⸰C.
Preparation of Black Seed ( Nigella sativa) Extract
This method was adopted from Shahraki et al.27. To obtain a hydroalcholic extract, powder was made from 100 g of dried N. sativa seeds. After that, it was macerated in a solution of 70% alcohol and 30% DW for 72 hours. To prepare the fractions, 10 g of the extract was mixed with 100 ml of ethanol and decanted by funnel. The n-hexane solvent was added to the funnel, and the n-hexane fraction was then extracted. Then, the remaining solvent in the decanter funnel was mixed with dichloromethane solvent, followed by extraction of the dichloromethane fraction. Finally, the remaining solvent from the previous steps was mixed with ethyl acetate, and the ethyl acetate fraction was taken out. The total extract, n-hexane and ethyl acetate fractions were prepared after the removal of the solvent.
Preparation of Punica granatum peels extract
The preparation was performed as done by Anibal et al.28. Fresh fruit, separated into skin, coverings and seeds, pericarp, and the whole fruit was submitted to ethanolic extraction (70% ethanol) at 37⸰C by maceration. The extract was filtered, the solvents was eliminated under vacuum and lyophilized to get the crude extract. Crude extracts from all parts of the fruit were monitored by Thin Layer Chromatography.
Animals
50 adults Wistar albino male rats, of 8 weeks old and weighing 250±10g, were obtained from the animal facility of faculty of pharmacy, Northern Border University. The experimental animals were kept in temperature controlled rooms (25°C), with uniform humidity (40–70%) and 12h/light-12h/dark cycle before the experiment. All the animals were treated in consensus with the Principles of Laboratory Animal Care.
The research protocol approval was taken from Deanship of Scientific Research at Northern Border University, in conformity with the guidelines for the care and use of experimental animals.
All rats were fed a proper feed and aqua. The daily intake of animal water was checked once a week before beginning of treatments to determine the amount of water needed per experimental rat.
Induction of diabetes with STZ
DM was induced by a single intra-peritoneal injection of STZ, (Sigma-Aldrich, St Louis, MO, USA) in 0.1 M citrate buffer (pH 4.0), 55 mg/kg body weight29. Serum glucose concentration and alterations in body weight were checked regularly. Male Wistar rats were divided into ten groups, each group comprised of five rats as follows:
G1: Control rats were given only 5cc saline (0.9% NaCl).
G2: Control rats were given Zingeber officianale (ginger) (100mg/kg/rat) daily).
G3: Control rats were given Nigella sativa (Black seed) (80 mg/kg).
G4: Rats were given Punica granatum (Pomegranate) 400 mg/kg/day.
G5: Rats were given Metformin 150 mg/kg/day
G6: Diabetic control (55 mg/kg, single I/p injection of STZ).
G7: Diabetic group (55 mg/kg, single I/p injection of STZ) received 100mg/kg/day ginger.
G8: Diabetic group (55 mg/kg, single I/p injection of STZ) received Nigella sativa 80 mg/kg/day.
G9: Diabetic group (55 mg/kg, single I/p injection of STZ) received (Pomegranate) 400 mg/rat/day.
G10: Diabetic group (55 mg/kg, single I/p injection of STZ) received Metformin 150 mg/kg/day.
Histological examination
Anesthetized rats were perfused trans-cardially with normal saline and 4% paraformaldehyde in phosphate-buffered solution. The brains of the sacrificed animals were removed immediately and post fixed in the same fixative at 4 °C, until being sectioned on a cryostat (Leica, Germany). Coronal brain sections of 10 μm measurement were obtained and stored at -20 °C until used.
Assessment of neuronal damage in the cortex was done with Nissl staining. Incubation of the brain sections was done with a 5% toluidine blue solution at room temperature for fifteen minutes. The brain sections were dehydrated and mounted following rinses water.
The axons and neutrophil, morphology and integrity were assessed with Bielschowsky's sliver (BS) staining.
Statistical Analysis
To analyze the data, Statistical Package for Social Science (SPSS) version 20 was applied. The data were expressed as means +/- standard deviation (SD). Comparison of variables between groups were performed using One Way ANOVA test (LSD). Statistical significance was considered at P-value ≤ 0.05.
Results
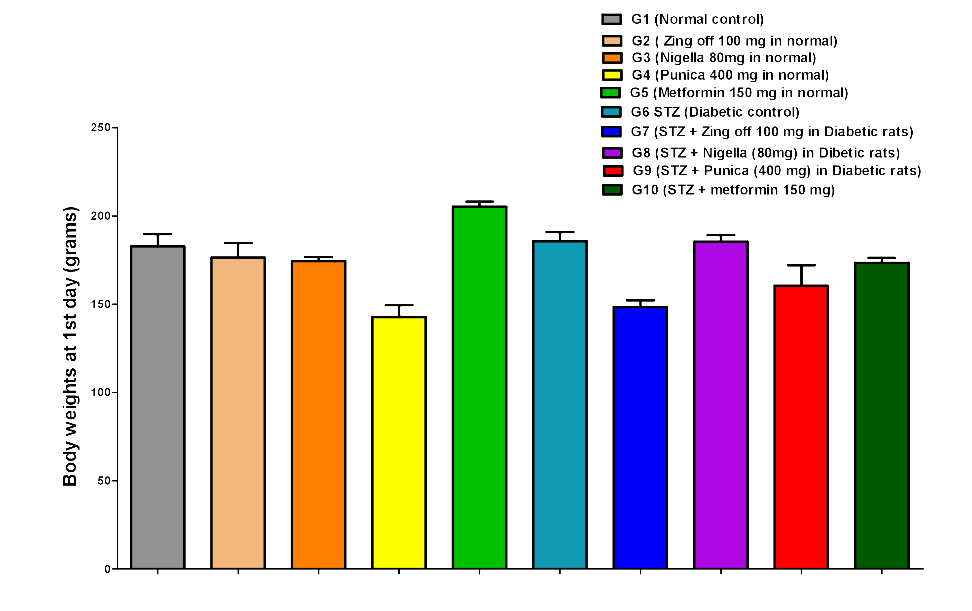
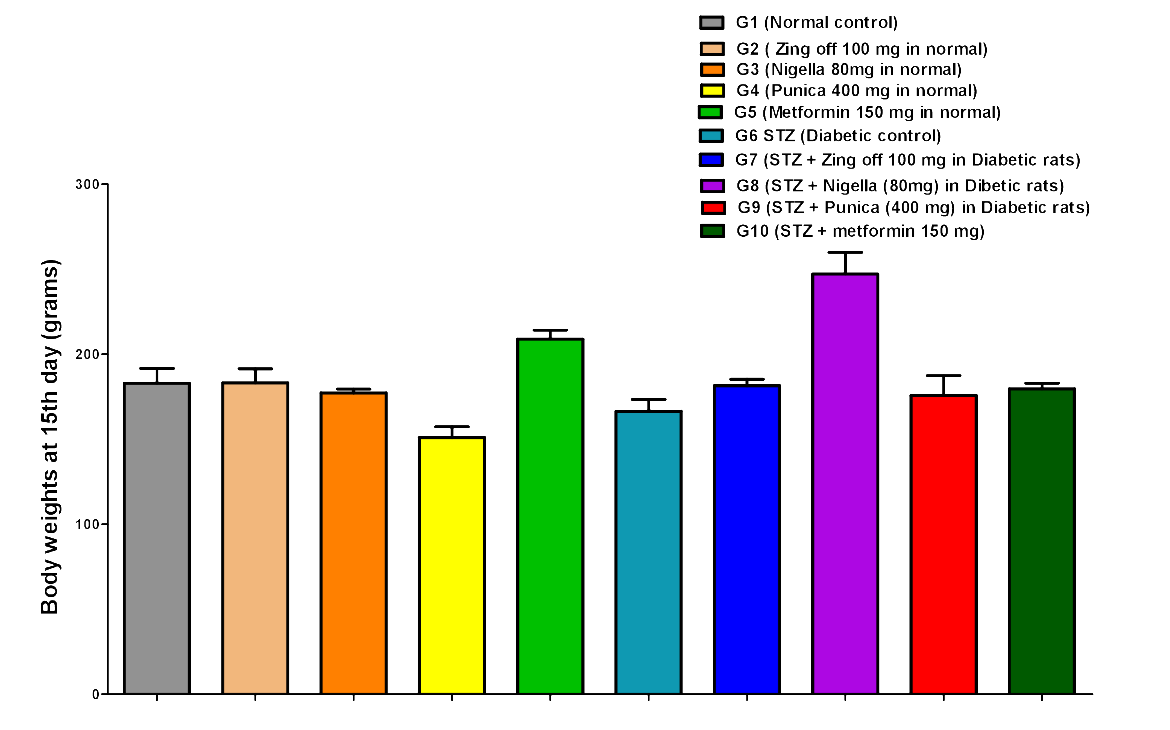
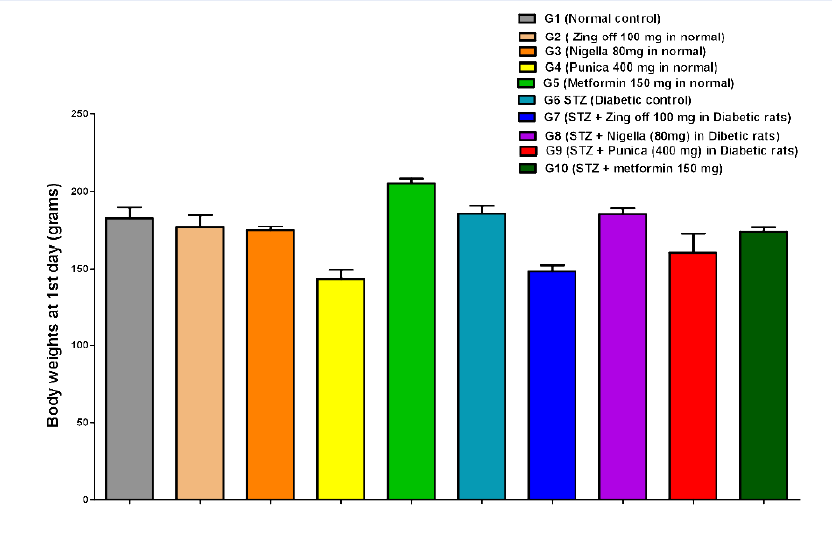
The body weight in G4 significantly decreased compared to G1, G2, and G3 on the 1st day (142.83±16.36 versus 182.83±16.81, 176.50±20.34 and 174.50±5.68; P =0.001 for all groups), day 15th (151.17±15.47 versus 183.00±21.23, 183.33±19.97 and 177.33±5.65; P =0.005, P =0.004 and P =0.019, respectively) and on 28th day (158.33±15.24 versus 190.00±20.36, 195.50±16.53 and 183.83±9.62; P =0.004, P =0.001 and P =0.018, respectively). Body weight in G5 significantly increased compared to G1, G2, G3 and G4 at 1st (205.33±6.98 versus 182.83±16.81, 176.50±20.34 174.50±5.68 and 142.83±16.36; P =0.011, P =0.001, P =0.001 and P =0.001, respectively), on day 15th (209.00±12.82 versus 183.00±21.23, 183.33±19.97, 177.33±5.65 and 151.17±15.47; P =0.020, P =0.021, P =0.005 and P =0.001, respectively) and on 28th day (218.00±16.46 versus 190.00±20.36, 195.50±16.53, 183.83±9.62 and 158.33±15.24; P =0.010, P =0.035, P =0.002 and P =0.001, respectively). In G6, on 1st day, body weight significantly increased compared to G4 (185.80±11.41 versus 142.83±16.36; P =0.001), but significantly decreased compared to G5 (185.80±11.41 versus 205.33±6.98; P =0.035). While on 15th day, BW significantly decreased compared to G5 (166.60±15.53 versus 209.00±12.82, P =0.001); on 28th day, BW significantly decreased compared to G1, G2, G3 and G5 (142.5±8.54 versus 190.00±20.36, 195.50±16.53, 183.83±9.62 and 218.00±16.46; P = 0.001 for all).
In G7, on 1st day, BW significantly decreased compared to G1, G2, G3, G5 and G6 (148.50±9.03 versus 182.83±16.81, 176.50±20.34, 174.50±5.68, 205.33±6.98, 185.80±11.41; P= 0.001. P =0.00. P =0.004, P =0.001 and P =0.001) at 15th day, BW significantly increased compared to G4 (181.67±9.07 versus 151.17±15.47; P =0.007) but significantly decreased compared to G5 (181.67±9.07 versus 209.00±12.82; P =0.014); on 28th day, BW significantly increased compared to G4 and G6 (158.33±15.24 and 194.83±8.08 versus 142.5±8.54; P =0.001 for both) but significantly decreased compared to G5 (194.83±8.08 versus 218.00±16.46; P =0.030). In G8, on 1st day BW significantly increased than G4 and G7 (185.50±8.89 versus 142.83±16.36 and 148.50±9.03; P =0.001 for both) but significantly decreased than G5 (185.50±8.89 versus 205.33±6.98; P =0.025); on 15th day, BW significantly increased than G1-7 (247.33±31.19 versus 183.00±21.23, 183.33±19.97, 177.33±5.65, 151.17±15.47, 209.00±12.82, 166.60±15.53, 181.67±9.07; P =0.001 for all); on 28th day, BW significantly increased than G1, G2, G3, G4, G5, G6, and G7 (263.00±33.93 versus 190.00±20.36, 195.50±16.53, 183.83±9.62, 158.33±15.24, 218.00±16.46, 142.5±8.54, 194.83±8.08; P =0.001 for all).
In G9, on 1st day, BW significantly decreased compared to G1, G5, G6 and G8 (160.50±28.31 versus 182.83±16.81, 205.33±6.98, 185.80±11.41, 185.50±8.89; P = 0.012, P =0.001, P =0.007 and P =0.005); on 15th day, BW significantly increased than G4 (175.83±28.58 versus 151.17±15.47; P =0.026) but significantly decreased than G5 (175.83±28.58 versus 209.00±12.82; P =0.003); on 28 days, BW significantly increased than G4, G6 (195.33±25.56 versus 158.33±15.24 and 142.5±8.54; P =0.001 for both) but significantly decreased than G5 and G8 (195.33±25.56 versus 218.00±16.46 and 263.00±33.93; P =0.034 and P =0.001). In G10, BW increased than G4 (173.50±6.80 versus 142.83±16.36; P =0.001) but significantly decreased than G5and G7 173.50±6.80 versus 205.33±6.98 and 148.50±9.03; P =0.001 and P =0.005); on 15th day, BW significantly increased than G4 (179.83±7.83 versus 151.17±15.47; P =0.010) but significantly decreased than G5 and G8 (179.83±7.83 versus 209.00±12.82 and 247.33±31.19; P =0.009 and P =0.001); on 28th day, BW significantly increased than G4 and G6 (186.00±8.60 versus 158.33±15.24 and 142.5±8.54; P =0.011 and P =0.001) but significantly decreased than G5 and G8 (186.00±8.60 versus 218.00±16.46 and 263.00±33.93; P =0.003 and P =0.001)Figure 1,Figure 2,Figure 3.
Blood glucose levels in G6 significantly increased compared to G1, G2, G3, G4 and G5 on 1st (234.80±13.03 versus 130.17±6.46, 126.50±17.00, 116.33±9.05, 121.67±6.95, 122.83±7.33; P =0.001 for all), on 15th (237.20±12.72 versus 119.17±5.04, 124.83±5.27, 125.83±5.42, 124.33±4.03 and 122.67±9.37; P =0.001 for all) and on 28th day (227.00±8.37 versus 125.17±3.76, 123.67±6.15, 122.67±2.80, 125.83±8.64 and 124.33±5.47; P =0.001 for all). Blood glucose levels in G7 significantly increased compared to G1, G2, G3, G4 and G5 but significantly decreased compared to G6 on 1st (195.83±10.03 versus 130.17±6.46, 126.50±17.00, 116.33±9.05, 121.67±6.95, 122.83±7.33; P =0.001 for all and 195.83±10.03 versus 234.80±13.03; P =0.001), on 15th day (157.00±8.00 versus 119.17±5.04, 124.83±5.27, 125.83±5.42, 124.33±4.03 and 122.67±9.37 and 157.00±8.00 versus 237.20±12.72; P =0.001) and on 28th day (155.67±7.03 versus 125.17±3.76, 123.67±6.15, 122.67±2.80, 125.83±8.64 and 124.33±5.47; P =0.001 for all; and 155.67±7.03 versus 227.00±8.37, P =0.001).
Blood glucose levels in G8 significantly increased compared to G1, G2, G3, G4 and G5 but significantly decreased compared to G6 on 1st day (190.67±5.13 versus 130.17±6.46, 126.50±17.00, 116.33±9.05, 121.67±6.95, 122.83±7.33; P =0.001 for all and 190.67±5.13 versus 234.80±13.03; P =0.001), on 15th day (156.40±6.88 versus 119.17±5.04, 124.83±5.27, 125.83±5.42, 124.33±4.03 and 122.67±9.37 and 156.40±6.88 versus 237.20±12.72; P =0.001) and on 28th day 143.00±4.00 versus 125.17±3.76, 123.67±6.15, 122.67±2.80, 125.83±8.64 and 124.33±5.47; P =0.001 for all; and 143.00±4.00 versus 227.00±8.37, P =0.001) and significantly decreased compared to G7 on 28th day (143.00±4.00 versus 155.67±7.03; P =0.009).
Blood glucose levels in G9, on 1st day significantly increased compared to G1, G2, G3, G4, G5 and G8 (208.67±18.93 versus 130.17±6.46, 126.50±17.00, 116.33±9.05, 121.67±6.95, 122.83±7.33, 190.67±5.13; P =0.001, P =0.001, P =0.001, P =0.001, P =0.001 and P =0.008, respectively) but significantly decreased compared to G6 (208.67±18.93 versus 234.80±13.03; P =0.001), on 15th day (174.83±12.84 versus 119.17±5.04, 124.83±5.27, 125.83±5.42, 124.33±4.03, 122.67±9.37 and 156.40±6.88; P =0.001 for all) but significantly decreased compared to G6 (174.83±12.84 versus 237.20±12.72; P =0.001) and 28th days blood glucose levels significantly increased compared to G1, G2, G3, G4 and G5 (144.67±4.97 versus 125.17±3.76, 123.67±6.15, 122.67±2.80, 125.83±8.64 and 124.33±5.47; P =0.001 for all) but significantly decreased compared to G6 and G7 (144.67±4.97 versus 227.00±8.37 and 155.67±7.03; P =0.001 and P =0.017).
Blood glucose levels in G10, on 1st day significantly increased when compared to G1, G2, G3, G4, G5, G7 and G8 (209.17±10.26 versus 130.17±6.46, 126.50±17.00, 116.33±9.05, 121.67±6.95, 122.83±7.33, 195.83±10.03 and 190.67±5.13; P = 0.001, P =0.001, P =0.001, P =0.001, P =0.001, P =0.046 and P =0.006) but significantly decreased when compared to G6 (209.17±10.26 versus 234.80±13.03; P = 0.001); on 15th day, blood glucose levels significantly increased compared to G1, G2, G3, G4 and G5 (158.00±10.37 versus 119.17±5.04, 124.83±5.27, 125.83±5.42, 124.33±4.03, 122.67±9.37; P =0.001 for all) but significantly decreased compared to G6 and G9 (158.00±10.37 versus; 237.20±12.72 and 174.83±12.84; P =0.001 for all); on 28th day, blood glucose levels significantly decreased compared to G6, G7, G8 and G9 (131.17±16.30 versus 227.00±8.37, 155.67±7.03, 143.00±4.00, 144.67±4.97; P= 0.001, P =0.001, P =0.015 and P =0.004, respectively)Figure 4,Figure 5,Figure 6.
The control group showed that most vital neurons (cortical, hippocampal and cerebellar Purkinje cells) have active large vesicular lightly stained nuclei figure 7. STZ induced Type 2 diabetic rat showed an increase in dark degenerated neurons compared to cells with highly active stained nuclei, the cortex and striatum of the diabetic animals were characterized by demyelination and axonal degradation (Figure 7,Figure 8).
In all the treated groups, brain tissue sections show cerebral cortex with distinct gray and white matter areas. Cortex shows normal neuronal cells with scattered glial cells and neutrophils in background (Figure 9).
Discussion
Diabetes mellitus type II is a common prevailing metabolic disorder emerging as global health hazard and it is associated with various complications such as micro-angiopathy, nephropathy, retinopathy, and neuropathy. Dating back to 1922, it has been noticed that diabetes mellitus may even lead to central nervous system disorder30.
Long-standing hyperglycemia DM affects the brain and manifests anatomical, structural, neurophysiological, and neuropsychological changes. Various pathophysiological factors are found to be involved in the development of the cerebral dysfunction in diabetes mellitus, such as the hypoglycemic bouts, cerebrovascular changes, insulin's role in the brain and associated structures, and the mechanisms of hyperglycemia induced alterations31.
Diabetes mellitus type II is known to cause a decrease in different areas of cognitive functioning. The risk of cognitive dysfunction is higher for the patients, who suffer from diabetes mellitus, prediabetes and from metabolic syndrome, characterized by dyslipidemia, central abdominal obesity and hyperglycemia32,33.
Hyperglycemia decreases antioxidant levels and at the same time, causes an increase in the production of free radicals. These effects add to the deleterious effects on tissues, facilitating the complications/tissue damage in DM, leading to changes in the redox potential of the cells with consequent activation of redox-sensitive genes34.
Neurons are especially sensitive to oxidative stress, and because of that, reactive oxygen species (ROS) cause several neural degenerative processes in diabetes35,36,37.
In the current study, it was discovered that long-standing hyperglycemia in rats causes significant damage in different areas of the brain. Treatment with different substances containing antioxidants prevented the harmful effects of diabetes mellitus on neurological tissues.
In the previous studies38,39,40, it has been found that metformin prevents the brain damage by reducing oxidative stress. The results of our study are in line with these studies, and significant prevention of the neuronal tissue damage was found in all areas of the brain of the animals.
Studies conducted by41,42,43 revealed that Nigella sativa, which is rich in antioxidants, reduces neuro-inflammatory damage and improves cognitive functions. The results in this study are in conformity with the previous studies regarding these findings.
The previous research suggests that ginger shows a neuroprotective effect by speeding up the processes of brain antioxidant defense and down-regulating the N-MDA levels to the normal range in diabetic rats44,45,46. The current research results also verify the observations found in previous studies regarding the decrease, prevention and improvement by Ginger in the damage caused by DM in the brain.
In previous studies, Pomegranate (Punica granatum), has been found to be effective in reducing oxidative stress and damage caused by oxidative stress. It has also been found effective in reducing the blood glucose levels47,48,49. In the current study, it was found to possess neuro-protective effects. The results of the current study show promising preventive and repairing effects of brain damage caused by DM.
Conclusion
DM is notorious to cause irreversible complications including peripheral neuropathy and brain damage, leading to the early occurrence of dementia (Alzheimer's disease). The substances used in the study, including Metformin, Nigella sativa, Ginger and Punica granatum, effectively prevented the brain damage and repaired the damaged neurons. These substances can be used as adjuvant therapy to prevent DM related complications. It is highly suggested that the active ingredients of these substances should be studied for their effects on oxidative stress parameters, and composition to pinpoint exact neuro-protective mechanism.
Competing Interests
The authors declare that there is no conflict of interest.
Authors' Contributions
Author 1 responsible for research /experimental design, histopathological study and interpretation of results. Author 2 responsible for literature review, compilation of results.
Acknowledgments
The author wishes to acknowledge the approval and the support of this research study by the grant No (7267-PHM-2017-1-8-F) from the Deanship of Scientific Research in Northern Border University (N.B.U.), Arar, KSA.
References
-
Khlid Al
A.A.,
Nisha
S.,
Risk Factors Associated with Diabete s Mellitus in a Saudi Community: A Cross-Sectional Study. Prim Health Care.
2017;
7
(270)
:
2167-1079
.
-
Biessels
G.J.,
Gispen
W.H.,
The impact of diabetes on cognition: what can be learned from rodent models?. Neurobiol Aging.
2005;
26
(1)
:
36-41
.
View Article PubMed Google Scholar -
Kahn
S.E.,
Cooper
M.E.,
Del Prato
S.,
Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet.
2014;
383
(9922)
:
1068-83
.
View Article PubMed Google Scholar -
Association
American Diabetes,
Diagnosis and classification of diabetes mellitus. Diabetes Care.
2014;
37
:
81-90
.
View Article PubMed Google Scholar -
Tomlinson
D.R.,
Gardiner
N.J.,
Glucose neurotoxicity. Nat Rev Neurosci.
2008;
9
(1)
:
36-45
.
View Article PubMed Google Scholar -
Moheet
A.,
Mangia
S.,
Seaquist
E.R.,
Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci.
2015;
1353
(1)
:
60-71
.
View Article PubMed Google Scholar -
Munshi
M.N.,
Cognitive dysfunction in older adults with diabetes: what a clinician needs to know. Diabetes Care.
2017;
40
(4)
:
461-7
.
View Article PubMed Google Scholar -
Magariños
A.M.,
McEwen
B.S.,
Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci USA.
2000;
97
(20)
:
11056-61
.
View Article PubMed Google Scholar -
van Elderen
S.G.,
de Roos
A.,
de Craen
A.J.,
Westendorp
R.G.,
Blauw
G.J.,
Jukema
J.W.,
Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology.
2010;
75
(11)
:
997-1002
.
View Article PubMed Google Scholar -
Moran
C.,
Phan
T.G.,
Chen
J.,
Blizzard
L.,
Beare
R.,
Venn
A.,
Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care.
2013;
36
(12)
:
4036-42
.
View Article PubMed Google Scholar -
Ceriello
A.,
New insights on oxidative stress and diabetic complications may lead to a causal antioxidant therapy. Diabetes Care.
2003;
26
(5)
:
1589-96
.
View Article PubMed Google Scholar -
Oswald
M.C.,
Garnham
N.,
Sweeney
S.T.,
Landgraf
M.,
Regulation of neuronal development and function by ROS. FEBS Lett.
2018;
592
(5)
:
679-91
.
View Article PubMed Google Scholar -
Sen
N.,
Kar
Y.,
Tekeli
Y.,
Antioxidant activities of black cumin (Nigella sativa L.) seeds cultivating in different regions of Turkey. J Food Biochem.
2010;
34
:
105-19
.
View Article Google Scholar -
Sangi
S.M.,
Sulaiman
M.I.,
El-Wahab
M.F.,
Ahmedani
E.I.,
Ali
S.S.,
Antihyperglycemic effect of thymoquinone and oleuropein, on streptozotocin-induced diabetes mellitus in experimental animals. Pharmacogn Mag.
2015;
11
(44)
:
251-7
.
View Article PubMed Google Scholar -
El-Akabawy
G.,
El-Kholy
W.,
Neuroprotective effect of ginger in the brain of streptozotocin-induced diabetic rats. Ann Anat.
2014;
196
(2-3)
:
119-28
.
View Article PubMed Google Scholar -
Karam
A.,
Nadia
A.,
Abd
E.F.,
Nemat
A.,
Siham
M.A.,
Protective effect of ginger (Zingiber officinale) on Alzheimer's disease induced in rats. J Neuroinfect Dis.
2014;
5
(159)
:
2
.
-
Kota
N.,
Krishna
P.,
Polasa
K.,
Alterations in antioxidant status of rats following intake of ginger through diet. Food Chem.
2008;
106
(3)
:
991-6
.
View Article Google Scholar -
Kaur
G.,
Jabbar
Z.,
Athar
M.,
Alam
M.S.,
Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol.
2006;
44
(7)
:
984-93
.
View Article PubMed Google Scholar -
Afaq
F.,
Saleem
M.,
Krueger
C.G.,
Reed
J.D.,
Mukhtar
H.,
Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer.
2005;
113
(3)
:
423-33
.
View Article PubMed Google Scholar -
Prashanth
D.,
Asha
M.K.,
Amit
A.,
Antibacterial activity of Punica granatum. Fitoterapia.
2001;
72
(2)
:
171-3
.
View Article PubMed Google Scholar -
Cambay
Z.,
Baydas
G.,
Tuzcu
M.,
Bal
R.,
Pomegranate (Punica granatum L.) flower improves learning and memory performances impaired by diabetes mellitus in rats. Acta Physiol Hung.
2011;
98
(4)
:
409-20
.
View Article PubMed Google Scholar -
Middha
S.K.,
Usha
T.,
RaviKiran
T.,
RaviKiran T. Influence of Punica granatum L. on region specific responses in rat brain during Alloxan-Induced diabetes. Asian Pac J Trop Biomed.
2012;
2
(2)
:
905-9
.
View Article Google Scholar -
Abdelsaid
M.,
Prakash
R.,
Li
W.,
Coucha
M.,
Hafez
S.,
Johnson
M.H.,
Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes.
2015;
64
(5)
:
1804-17
.
View Article PubMed Google Scholar -
Oliveira
W.H.,
Nunes
A.K.,
França
M.E.,
Santos
L.A.,
Lós
D.B.,
Rocha
S.W.,
Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res.
2016;
1644
:
149-60
.
View Article PubMed Google Scholar -
Mousavi
S.M.,
Niazmand
S.,
Hosseini
M.,
Hassanzadeh
Z.,
Sadeghnia
H.R.,
Vafaee
F.,
Keshavarzi
Z.,
Beneficial effects of Teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. International Journal of Alzheimer’s Disease.
2015;
2015
.
-
Kebe
E.O.,
Ede
P.O.,
Isaac
V.N.,
Obasee
P.P.,
Ethanolic Extract of Ginger on the Histology of the Pancrease in Adult Wistar Rats. International Journal of Medical and Health Sciences Research..
2015;
2
(2)
:
25-35
.
View Article Google Scholar -
Shahraki
S.,
Khajavirad
A.,
Shafei
M.N.,
Mahmoudi
M.,
Tabasi
N.S.,
Effect of total hydroalcholic extract of Nigella sativa and its n-hexane and ethyl acetate fractions on ACHN and GP-293 cell lines. J Tradit Complement Med.
2015;
6
(1)
:
89-96
.
View Article PubMed Google Scholar -
Anibal
P.C.,
Peixoto
I.T.,
Foglio
M.A.,
Höfling
J.F.,
Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz J Microbiol.
2013;
44
(3)
:
839-48
.
View Article PubMed Google Scholar -
Mahesh
T.,
Menon
V.P.,
Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res.
2004;
18
(2)
:
123-7
.
View Article PubMed Google Scholar -
Miles
W.R.,
Root
H.F.,
Psychologic tests applied to diabetic patients. Arch Intern Med (Chic).
1922;
30
(6)
:
767-77
.
View Article Google Scholar -
Brands
M.W.,
Bell
T.D.,
Gibson
B.,
Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension.
2004;
43
(1)
:
57-63
.
View Article PubMed Google Scholar -
Feinkohl
I.,
Price
J.F.,
Strachan
M.W.,
Frier
B.M.,
The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther.
2015;
7
(1)
:
46
.
View Article PubMed Google Scholar -
Tamaddonfard
E.,
Farshid
A.A.,
Asri-Rezaee
S.,
Javadi
S.,
Khosravi
V.,
Rahman
B.,
Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci.
2013;
16
(1)
:
91-100
.
PubMed Google Scholar -
Bonnefont-Rousselot
D.,
Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care.
2002;
5
(5)
:
561-8
.
View Article PubMed Google Scholar -
Jackson
G.R.,
Werrbach-Perez
K.,
Pan
Z.,
Sampath
D.,
Perez-Polo
J.R.,
Neurotrophin regulation of energy homeostasis in the central nervous system. Dev Neurosci.
1994;
16
(5-6)
:
285-90
.
View Article PubMed Google Scholar -
Dugan
L.L.,
Sensi
S.L.,
Canzoniero
L.M.,
Handran
S.D.,
Rothman
S.M.,
Lin
T.S.,
Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci.
1995;
15
(10)
:
6377-88
.
View Article PubMed Google Scholar -
Yuan
J.,
Yankner
B.A.,
Apoptosis in the nervous system. Nature.
2000;
407
(6805)
:
802-9
.
View Article PubMed Google Scholar -
Patil
S.P.,
Jain
P.D.,
Ghumatkar
P.J.,
Tambe
R.,
Sathaye
S.,
Neuroprotective effect of metformin in MPTP-induced Parkinson's disease in mice. Neuroscience.
2014;
277
:
747-54
.
View Article PubMed Google Scholar -
Tang
G.,
Yang
H.,
Chen
J.,
Shi
M.,
Ge
L.,
Ge
X.,
Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget.
2017;
8
(58)
:
97977-89
.
View Article PubMed Google Scholar -
Akinola
O.,
Gabriel
M.,
Suleiman
A.A.,
Olorunsogbon
F.,
Treatment of alloxan-induced diabetic rats with metformin or glitazones is associated with amelioration of hyperglycaemia and neuroprotection. Open Diabetes J.
2012;
5
(1)
:
8-12
.
View Article Google Scholar -
Sheikh
T.,
Joshi
D.,
Patel
B.,
Modi
C.,
Protective role of Nigella sativa against experimentally induced type-II diabetic nuclear damage in Wistar rats. Vet World.
2013;
6
(9)
:
698-702
.
View Article Google Scholar -
Farkhondeh
T.,
Samarghandian
S.,
Shahri
A.M.,
Samini
F.,
The neuroprotective effects of thymoquinone: A review. Dose Response.
2018;
16
(2)
:
1559325818761455
.
View Article PubMed Google Scholar -
Khazdair
M.R.,
The protective effects of Nigella sativa and its constituents on induced neurotoxicity. Journal of toxicology.
2015;
2015
.
-
Shanmugam
K.R.,
Mallikarjuna
K.,
Kesireddy
N.,
Sathyavelu Reddy
K.,
Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol.
2011;
49
(4)
:
893-7
.
View Article PubMed Google Scholar -
Wattanathorn
J.,
Jittiwat
J.,
Tongun
T.,
Muchimapura
S.,
Ingkaninan
K.,
Zingiber officinale mitigates brain damage and improves memory impairment in focal cerebral ischemic rat. Evidence-Based Complementary and Alternative Medicine.
2011;
2011
.
-
Sangi
S.M.,
Elwahab
M.F.,
Experimental evaluations of the nephroprotective properties of ginger (Zingiber officinale), Cinnamomum verum and Nigella sativa in STZ induced diabetic rats. International Journal of Biology, Pharmacy and Allied Sciences.
2017;
6
(6)
:
1195-1209
.
-
Cambay
Z.,
Baydas
G.,
Tuzcu
M.,
Bal
R.,
Pomegranate (Punica granatum L.) flower improves learning and memory performances impaired by diabetes mellitus in rats. Acta Physiol Hung.
2011;
98
(4)
:
409-20
.
View Article PubMed Google Scholar -
Mollazadeh
H.,
Sadeghnia
H.R.,
Hoseini
A.,
Farzadnia
M.,
Boroushaki
M.T.,
Effects of pomegranate seed oil on oxidative stress markers, serum biochemical parameters and pathological findings in kidney and heart of streptozotocin-induced diabetic rats. Ren Fail.
2016;
38
(8)
:
1256-66
.
View Article PubMed Google Scholar -
Aboonabi
A.,
Rahmat
A.,
Othman
F.,
Antioxidant effect of pomegranate against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Toxicol Rep.
2014;
1
:
915-22
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 7 (2019)
Page No.: 3274-3285
Published on: 2019-07-05
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 9931 times
- Download PDF downloaded - 2891 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress