Abstract
Background: Vascular endothelial growth factor (VEGF) is an angiopoetic factor; its variability in circulating levels is mediated by expression of specific VEGF-A gene variants. The aim of this study was to investigate the predictive role of VEGF-A gene polymorphism in clinical outcomes of STelevation myocardial infarction (STEMI) patients.
Methods: For the study, 135 patients with acute STEMI and 30 healthy volunteers were enrolled. The G634C polymorphism in VEGF-A gene was performed by real-time polymerase chain reaction at baseline. The 6-month combined clinical endpoint was then determined. Design: The study was an open prospective single-center cohort study.
Results: The entire patient population was distributed into two groups based on the G634G-genotype (n = 70) and combination of G634C and C634C-genotypes (n = 65). Unadjusted multivariate regressive logistic analysis showed peak troponin I levels at admission, Killip class of heart failure > 2, GC/CC polymorphisms in VEGF-A gene, and dynamic increase of NT-pro brain natriuretic peptide (BNP) and VEGF-A levels for 6 months, which were independent predictors for the combined clinical endpoint. After adjustment for dynamic changes of NT-proBNP and VEGF-A levels, we found that GC/CC polymorphisms in the VEGF-A gene was an independent predictor of clinical outcome. Kaplan-Meier curves demonstrated that STEMI patients with GG VEGF-A genotype had a lower frequency of clinical combined endpoint accumulation when compared to those who had GC/CC VEGF-A genotypes (Log-rank p = 0.02).
Conclusion: The G634C polymorphism in the VEGF-A gene was found to be an independent predictor for 6-month clinical combined endpoint in STEMI patients.
Introduction
Patients with acute ST-elevation myocardial infarction (STEMI) undergoing successful primary percutaneous coronary intervention (PCI) yield significant differences with respect to in-hospital mortality, hospital length of stay, cardiovascular (CV) events and complications, and late survival1, 2. Previous systematic network meta-analysis has shown that multi-vessel coronary artery disease (CAD) in STEMI patients is a much more powerful trigger than single-vessel CAD for survival and prognosis, regardless of revascularization strategy3. Additionally, complete revascularization based on comprehensive condition is disputed as the most suitable choice in actual clinical situations such as hemodynamic instability, newly onset heart failure, and high risk of severe short-term CV complications4, 5. However, staged revascularization strategy is recommended for complex non-infarct-related artery lesions under evaluation (fractional flow reserve, intravascular ultrasound, and optical coherent tomography)6, 7. In fact, long-term risk of CV events and severity of left ventricular post-MI remodeling remain to be uncertain and unpredictable following successful PCI with TIMI-III restoration of blood flow through culprit artery and even after complete revascularization8, 9. As a result, the complexity of STEMI patients undergoing primary successful PCI has not changed significantly.
Recent studies have revealed that effective angiogenesis and neovascularization play crucial roles in early restoration of microvascular perfusion in damaged myocardium and in prevention of late left ventricular remodeling for months after reperfused STEMI10, 11, 12. Vascular endothelial growth factor A (VEGF-A) is a key regulator of angiogenesis which mediates pro-angiopoetic, anti-inflammatory and anti-oxidative capacities by acting via appropriate receptors13, 14. VEGF-A synthesis and secretion are mediated by hypoxia through overexpressed hypoxia-induced factor-1 (HIF-1) and nuclear factor-kappa B (NF-kB)14. VEGF-A contributes to the development of collaterals by increasing vascular permeability, stimulating proliferation and migration of progenitor and mature endothelial cells, inhibiting apoptosis of endothelial precursors, inducing matrix metalloproteinases, and activating von Willebrand factor 15.
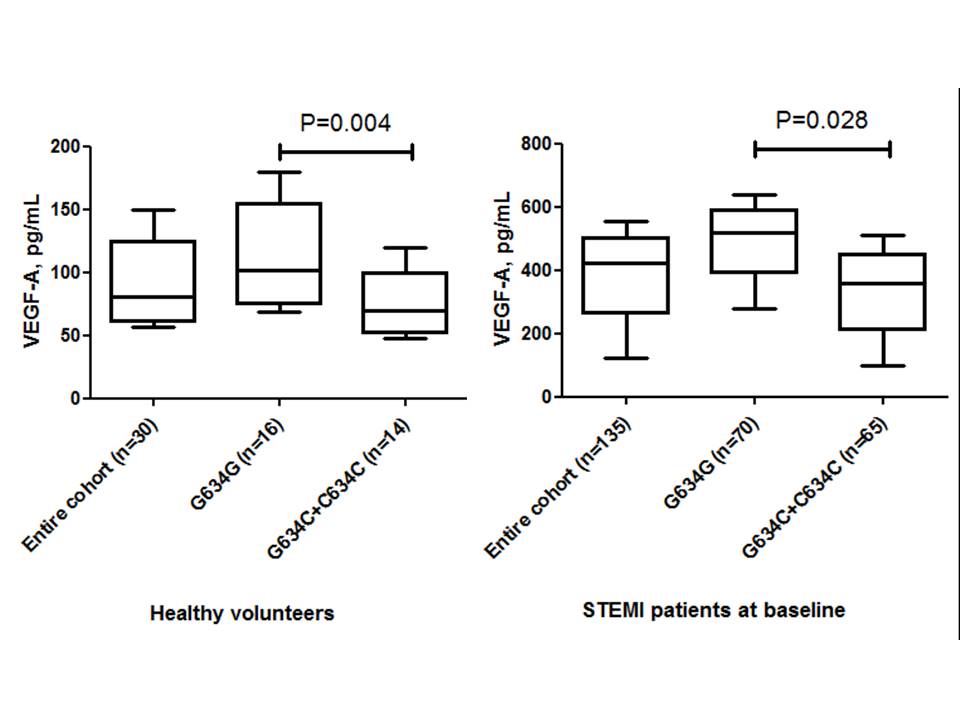
Previous studies have shown that the levels of VEGF-A in patients with acute STEMI have been found to be significantly higher than those of healthy volunteers16. After PCI, the concentration of biomarkers were seen to dramatically decrease by approximately 70%, with the most profound decline seen in individuals with the highest initial VEGF-A concentrations17. There is strong evidence regarding the negative predictive value of lowered circulating levels of VEGF-A and major cardiovascular events (MACEs) after STEMI in a one-year observation18. Interestingly, the circulating levels of VEGF-A showed profound variability in healthy volunteers as well as in patients with STEMI; these results were associated with a functional G634C (rs 2010963) polymorphism in the VEGF-A gene19, 20.
We hypothesized that the functional variant of the VEGF-A gene can determine dynamic changes of VEGF-A levels after PCI and potentiate the endogenous repair system activity to protect damaged myocardium, after STEMI, from microvascular obstruction and thereby improving long-term prognosis. The aim of this study was to investigate the predictive role of the VEGF-A gene polymorphism (rs 2010963) in clinical outcomes of STEMI patients after complete successful PCI.
Material and Methods
Patient population
A total of 190 patients with confirmed acute STEMI were evaluated for participation in the study (Figure 1). From the entire population of STEMI (n=190), according to inclusion and non-inclusion criteria we enrolled 140 individuals with acute STEMI who were admitted to intensive care unit of “L.T.Malaya TNI NAMSU” (Kharkiv, Ukraine) within a given period from 2016 January to 2019 June. Acute STEMI was diagnosed according to the European Cardiology Society (ECS) Guidelines (2017)21. Thirty healthy volunteers were enrolled as a control group.
Inclusion criteria included: established acute STEMI, age >18 years old, lack of contraindications to PCI, and written informed consent to participate in the study. Exclusion criteria included: previous myocardial infarction, established chronic heart failure, severe comorbidities (anemia, chronic obstructive lung disease, bronchial asthma, liver cirrhosis, chronic kidney disease with declined glomerular filtration rate < 35 mL/min × 1.73 m2, valvular heart disease, bleeding, etc.), known malignancy and pregnancy, and/or inability to understand or provide informed consent.
Primary PCI with bare-metal stent (COMMANDER, “Alvimedica”, Turkey) implantation was performed in 104 patients within 6-12 hours after initial acute STEMI confirmation in the V.T. Zaytsev Institute of General and Emergency Surgery NAMSU (Kharkiv, Ukraine). Systemic thrombolysis (tPA tenecteplase i.v. bolus per conventional protocol) was carried out in 31 STEMI patients prior to PCI. All acute STEMI patients received adjuvant treatment due to current ESC recommendations21. TIMI III blood flow restoring through culprit artery was determined for every reperfused patient with acute STEMI (Figure 2).
Ethical declaration
All procedures in the study involving human participants were performed in accordance with ethical standards according to the 1964 Helsinki declaration and its later amendments, or comparable ethical standards approved by the local ethics committee (Protocol №6, 30.05.2017). Written voluntary inform consent was obtained from each patient before entering the study.
Sample size calculation
The sample size was calculated through the effect size estimation (0.99), the type of present study, providing study power of 80% and type I error 5%, STEMI in-hospital mortality of 7.5%, and one-year mortality of 14%22. The sample size was 135 individuals.
Coronary angiography
Conventional coronary angiography was performed immediately after admission of the patients to the hospital using Digital X-Ray system “Integris Allura” (Philips Healthcare, Best, The Netherlands), and managed by radial or femoral vascular access. Coronary arteries were visualized with two-to-three orthogonal projections per conventional protocol. The number of views obtained was decided by the operator depending on coronary anatomy. The main coronary arteries were left main coronary artery, left anterior descending branch, left circumflex branch, right main coronary artery, and right coronary descending branch. In this study, the contrast “Ultravist-370” (Baier Pharma GmbH, Germany) and automatic contrast injector were used. The contrast amount used in coronary angiography in each injection was 8 - 10 mL at 4 mL/s for the left coronary artery and 6 mL at 3 mL/s for the right coronary artery (radiation exposure 20 to 35 mGycm). After coronary angiography, two experienced interventional cardiologists discussed the captures and filled in the final report of the results of the procedure after reaching consensus.
Determination of risk factors and comorbidities
Hypercholesterolemia (HCE) was diagnosed if total cholesterol (TC) level was > 5.2 mmol/L, and/or low density lipoprotein cholesterol (LDL) level was > 3.0 mmol/L, and/or level of triglycerides (ТG) was > 1.7 mmol/L, according to the ECS dyslipidemia guideline (2016)23. Hypertension was diagnosed if systolic blood pressure (SBP) was > 140 mm Hg and/or diastolic blood pressure (DBP) > 90 mm Hg, according to the European guideline on diagnostics and treatment of arterial hypertension (2018)24. Heart failure was diagnosed according to the ESC guidelines for the diagnosis and treatment of acute and chronic heart failure (2016)25. Positive smoking history was defined as having smoked daily or occasionally in the past.
Transthoracic Echocardiography and Doppler
Transthoracic echocardiography was performed on “Aplio 500” (TUS-A500; Toshiba Medical Systems Corporation), with usage of 3.5 MHz phase probe at discharge and at 6-month observation period. Left ventricular (LV) end diastolic volume (LVEDV), LV end systolic volume (LV ESV), and LV ejection fraction (LVEF) measurements were taken according to Simpson's method per contemporary recommendation. The left atrial diameter (LAD) and left atrial volume (LAV) were determined according to contemporary protocol26. LV myocardial mass (LVMM) was calculated in an automatic manner per protocol of echocardiogram evaluation. LV global longitudinal strain (e`) and early transmitral velocity (E) were measured by tissue Doppler imaging technique and impulse transmitral Doppler regime at baseline and at 6 months per the protocol.
Determination of STEMI prognosis
We used the TIMI score to validate the prognostic capacity after STEMI27.
SYNTAX score determination
SYNTAX score (SS) was used to assess the severity of coronary atherosclerotic lesions; it was calculated by experienced interventional cardiologists accordingly28.
Determination of endpoint
The endpoint was determined as combined events including CV death, recurrent angina, and newly diagnosed heart failure for 6 months after PCI. CV death was ascertained by personal or phone contact from the family doctor or the hospital where the patient died. The diagnosis of recurrent angina required the presence of clinical signs/symptoms or electrocardiographic changes. Hospitalization was ascertained by direct contact or phone call to the hospital where the patient was admitted. A discharge report or autopsy report was obligatorily reviewed before the endpoint determination.
Blood samples
Blood samples were drawn immediately before PCI and at 6 months following acute STEMI. Blood samples were centrifuged, serum was isolated within 30 min of sample acquisition, and the sera then frozen at -700C and stored in plastic tubes until they were shipped to the Laboratory of Immune-Chemical and Molecular Genetic Research of “L.T.Malaya TNI NAMSU” (Kharkiv, Ukraine).
The N-terminal brain natriuretic peptide (NT-proBNP) levels were measured using a commercial kit for ELISA manufactured by Vector-Best (Russia Federation).
The levels of troponin I (Tn I) and creatine kinase- isoenzyme-MB (CK-MB) were measured with chemiluminescent immunoassay on Humalyzer 2000 (Mannheim, Germany). The range of TnI and CK-MB levels were 0.5-50 ng/mL and 0-500 mmol/L, respectively. The intra-assay and inter-assay coefficients of variation were < 5%.
The levels of VEGF-A were measured using a commercial kit for ELISA produced by (IBL International GMBH, Germany). The VEGF-A level ranged from 0 to 1000 pg/mL. The intra-assay and inter-assay coefficients of variation were < 5%.
High-sensitive C-reactive protein (hs-CRP) levels in serum were measured with a commercially available standard kit (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). The hs-CRP level range was 0-100 mg/L.
Total cholesterol (TC), low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides (TG) were measured by direct enzymatic method (Roche P800 Analyzer, Basel, Switzerland). The intra-assay and inter-assay coefficients of variation were < 5%.
SNP G634G (rs 2010963) in VEGF-A gene determination
DNA extraction and analysis was performed from peripheral blood leukocytes per the commercial kit protocol «CFX96 Touch» (BioRad Laboratories Pte.Ltd., Сінгапур) and per the appropriate RT-PCR kit (Syntol, Russia Federation). Genotyping of the G634C VEGF-A gene polymorphism located in the promoter region was performed using primers and probes in real-time (RT) polymerase chain reaction (PCR). The primers used for VEGF-A (rs 2010963) polymorphism assay were: GAGAGAAGT CGAGGAAGAGAGAGA-3′ (forward primer), CCCAAAAGCAGGTCA CTCACTT-3′ (reverse primer), VEGF-A-FAM-5′- CCTGTCCCTTTCGC-3′, and VEGF-A-VIC-5′- CCTGTCGCTTTCGC-3′.
Statistical analysis
Statistical analyses were performed using SPSS for Windows v.23 (USA). Continuous variables were presented as mean ± standard deviation (SD) when normally distributed, or median and interquartile range (IQR) if otherwise. The categorical variables were presented as frequencies (n) and percentages (%). Mann-Whitney and Wald-Wolfowitz criteria were used for intergroup differences and quantitative values. The qualitative variables are expressed as percentages, and were compared with the χ2 test and exact F Fisher test. Allele frequencies were estimated, and all polymorphisms were tested for Hardy–Weinberg equilibrium. Correlations between G634G VEGF-A gene polymorphism, angiographic characteristics, hemodynamic performances, and biomarkers were assessed using rank-order correlation Spearman’s test. We performed univariate and multiple variate log-regression analysis to determine variables that predict endpoint and cardiac remodeling. Beta coefficient, standard errors (SE), odds ratio (OR), and 95% confidence interval (CI) for each factor were estimated. Factors for which P-values were calculated as > 0.5 were not included in the multiple variate log-regression analysis. Survival analysis for clinical outcomes was performed using Kaplan-Meier curves and the log-rank test. All differences were considered statistically significant with 2-tailed < 0.05.
Results
The observed frequencies of variants of G634C VEGF-A (rs 2010963) genotype among the entire STEMI patient population (n = 135) were GG = 51.9% (n = 70), GС = 47.4% (n = 64) and CC = 0.7% (n = 1), respectively. There was a deviation from the Hardy-Weinberg equilibrium due to an excess of heterozygosity (χ2 = 10.9, P = 0.00099). Healthy volunteers (n = 30) had GG VEGF-A (rs 2010963) genotype in 53% (n = 16), CT VEGF-A (rs 2010963) genotype in 43% (n = 13), and CC VEGF-A (rs 2010963) genotype in 4% (n = 1) of the patient population, without significant deviation from expected frequencies (χ2 = 0.726, P = 0.394). The circulating levels of VEGF-A in healthy volunteers and STEMI patients at baseline, based on G634C VEGF-A (rs 2010963) genotypes, are reported in Figure 3.
General baseline clinical and procedural characteristics of the patient study population are reported in Table 1. There were no significant differences between both STEMI patient cohorts with respect to age, sex, or CV risk factors (hypertension, dyslipidemia, abdominal obesity, type 2 diabetes mellitus, smoking, and premature CV events in family anamnesis). Therefore, we did not find sufficient differences between frequencies in atrial fibrillation, stable and unstable angina prior to STEMI, II-IV Killip classes of heart failure, or GRACE and TIMI score points in both patient cohorts. Additionally, baseline medications were similar in both STEMI patient cohorts. However, STEMI patients with GG variant of VEGF-A gene rarely presented anterior localization of myocardial infarction (MI) and frequently exhibited posterior localization of MI than individuals with GC/CC VEGF-A genotypes, while there was not difference in right main coronary artery injury between the cohorts (Table 2). On the contrary, STEMI patients with GC/CC VEGF-A genotypes had frequent left main coronary artery injury.
| Variables | Entire population(n = 135) | Patients with GG genotype(n = 70) | Patients with GC and СС genotypes (n = 65) | χ2, Р value |
| Age, years | 59.2 ± 8.92 | 59.30±8.50 | 59.69±8.85 | χ2= 0.03; p = 0.639 |
| Sex, male/female, n (%) | 109/26(80.7/19.3) | 57/13 (81.4/19.6) | 52/13 (80.0/20.0 ) | χ2= 0.04 p = 0,833 |
| Hypertension, n (%) | 110 (81.5) | 56 (80.0) | 54 (83.1) | χ2=0.21 p = 0.646 |
| Dyslipidemia, n (%) | 35 (25.9) | 19 (27.1) | 18 (27.7) | χ2=0.22 p = 0.688 |
| Abdominal obesity, n (%) | 28 (20.7) | 13 (18.5) | 15 (23.0) | χ2=0.82 p = 0.218 |
| Type 2 diabetes mellitus, n (%) | 33 (24.4) | 15 (21.4) | 18 (27.7) | χ2=0.72 p = 0.398 |
| Smoking, n (%) | 65 (48.1) | 33 (47.1) | 32 (49.2) | χ2=0.06 p = 0.808 |
| Premature CV events in family anamnesis, n (%) | 72 (53.3) | 38 (54.3) | 36 (55.4) | χ2=0.02 p = 0.898 |
| Stable angina prior to STEMI, n (%) | 49 (36.3) | 20 (28.6) | 29 (44.6) | χ2=3.75 p = 0.053 |
| Unstable angina prior to STEMI, n (%) | 46 (34.1) | 19 (27.1) | 27 (41.5) | χ2=3.11 p = 0.078 |
| Atrial fibrillation, n (%) | 10 (7.4) | 4 (5.7) | 6 (9.4) | χ2=0.59; p = 0.326 |
| II-III Killip classes of HF, n (%), | 26 (19.3) | 12 (17.1) | 14 (21.5) | χ2= 0.42 p = 0.518 |
| IV Killipclass of HF, n (%) | 11 (8.1) | 7 (10.0) | 4 (6.2) | χ2=0,67 p = 0.414 |
| Risk scores | ||||
| GRACE Score, points | 150 (120-172) | 143 (117-170) | 152 (119-176) | P = 0.294 |
| TIMI score, points | 6 (4-7) | 6 (4-7) | 7 (5-8) | P = 0.66 |
| Baseline medications | ||||
| Beta-blocker, n (%) | 125 (92.6) | 66 (94.3) | 59 (90.8) | χ2= 0.22; p = 0.762 |
| ACEI, n (%) | 61 (45.1) | 29 (41.4) | 32 (49.2) | χ2= 2.25 p = 0.068 |
| Ticagrelor, n (%) | 135 (100) | 70 (100) | 65 (100) | χ2= 0.016; p = 0.92 |
| Statins, n (%) | 135 (100) | 70 (100) | 65 (100) | χ2= 0.016; p = 0.92 |
| Variables | Entire population (n = 135) | Patients with GG genotype(n = 70) | Patients with GC and СС genotypes (n = 65) | χ2, Р value |
| STEMI localization | ||||
| Anterior wall, n (%) | 59 (43.7) | 24 (34.3) | 35 (53.8) | χ2= 5.24 p = 0.022 |
| Posterior wall, n (%) | 53 (39.3) | 34 (48.6) | 19 (29.2) | χ2= 5.29 p = 0.022 |
| Other, n (%) | 23 (17.0) | 12 (17.1) | 11 (16.9) | χ2= 0.001 p = 0.973 |
| Infarct-related coronary artery | ||||
| Left main coronary artery, n (%) | 7 (5.2) | 1 (1.4) | 6 (9.2) | χ2=4.17 p = 0.041 |
| Right main coronary artery, n (%) | 27 (20.0) | 10 (14,3) | 17 (26,2) | χ2= 2.97 p = 0.085 |
| Circumflex coronary artery, n (%) | 17 (12.6) | 9 (12.9) | 8 (12.3) | χ2= 0.01 p = 0,923 |
| Anterior interventricular artery, n (%) | 31 (23.0) | 12 (17.1) | 19 (29.2) | χ2= 2.78 p = 0.095 |
Table 3 shows the reported baseline cardio dynamic characteristics and biomarkers in the patient study population. STEMI patients with GC/CC VEGF-A genotypes had significantly increased LVEDV, LVESV, and E/e` ratio at baseline in comparison with patients who had GG VEGF-A genotype. We did not observe differences between the patient cohorts in terms of GFR, serum creatinine, peak TnI, CK-MB, NT-proBNP, lipid profile, or hs-CRP. However, circulating levels of VEGF-A were profoundly lower in STEMI patients who had the GC/CC VEGF-A genotypes.
| Variables | Entire population (n = 135) | Patients with GG genotype(n = 70) | Patients with GC and СС genotypes (n = 65) | Р value |
| Hemodynamics | ||||
| SBP, mm Hg | 135 ± 22 | 135 ± 24 | 136 ± 27 | 0.428 |
| DBP, mm Hg | 80 ± 12 | 79 ± 13 | 82 ± 14 | 0.154 |
| LVEDV, mL | 147 ± 25 | 138 ± 31 | 151 ± 33 | 0.044 |
| LVESV, mL | 68 ± 24 | 63 ± 23 | 73 ± 31 | 0.039 |
| LVEF, % | 50 ± 10 | 51 ± 13 | 49 ± 10 | 0.250 |
| E/e`, units | 12.50 ± 1.17 | 10.20 ± 1.21 | 13.90 ± 1.60 | 0.046 |
| LVMMI, g/m2 | 149.30 ± 44.32 | 148.78 ± 46.82 | 157.28 ± 47.10 | 0.607 |
| Biomarkers | ||||
| Creatinine, μmol/L | 98.13 [86.90 - 119.30] | 96.75 [86.30 - 113.20] | 104.40 [88.10 - 123.60] | 0.274 |
| GFR, ml/min | 69.67 [58.40 - 87.63] | 71.00 [61.00 - 89.00] | 67.50 [56.00 - 88.00] | 0.445 |
| hs-CRP, mg/L | 12.04 ± 4.77 | 11.82 ± 5.19 | 12.53 ± 5.03 | 0.531 |
| VEGF-A, pg/mL | 420.81 [123.76 – 553.19] | 314.01 [159.94 - 627.66] | 221.28 [77.58 - 440.82] | 0.045 |
| Peak TnI, ng/ml | 18.4 [5.44 - 77.3] | 17.70 [6.77 - 99.00] | 23.07 [4.07 - 75.50] | 0.914 |
| CK-MB, mmol/L | 106.80 [51.20 - 290.40] | 121.05 [42.30 - 275.05] | 87.00 [44.90 - 300.10] | 0.458 |
| NT-proBNP, pmol/L | 480.26 [116.81 – 1558.31] | 219.34 [75.70 - 440.82] | 515.56 [109.91 - 1727.77] | 0.821 |
| Lipid profile | ||||
| TC, mmol/l | 4.95 [3.97 - 5.71] | 4.97 [3.98 - 5.64] | 4.91 [4.10 - 5.63] | 0.85 |
| HDL, mmol/l | 1.09 [0.90 - 1.28] | 1.12 [0.90 - 1.31] | 1.14 [0.90 - 1.20] | 0.761 |
| LDL, mmol/l | 3.00 [2.11 - 3.71] | 2.97 [2.11 - 3.67] | 3.01 [2.07 - 3.99] | 0.303 |
| TG, mmol/l | 1.53 [1.17 - 2.02] | 1,90 [1.19 - 2.06] | 1.61 [1.13 - 1.91] | 0.023 |
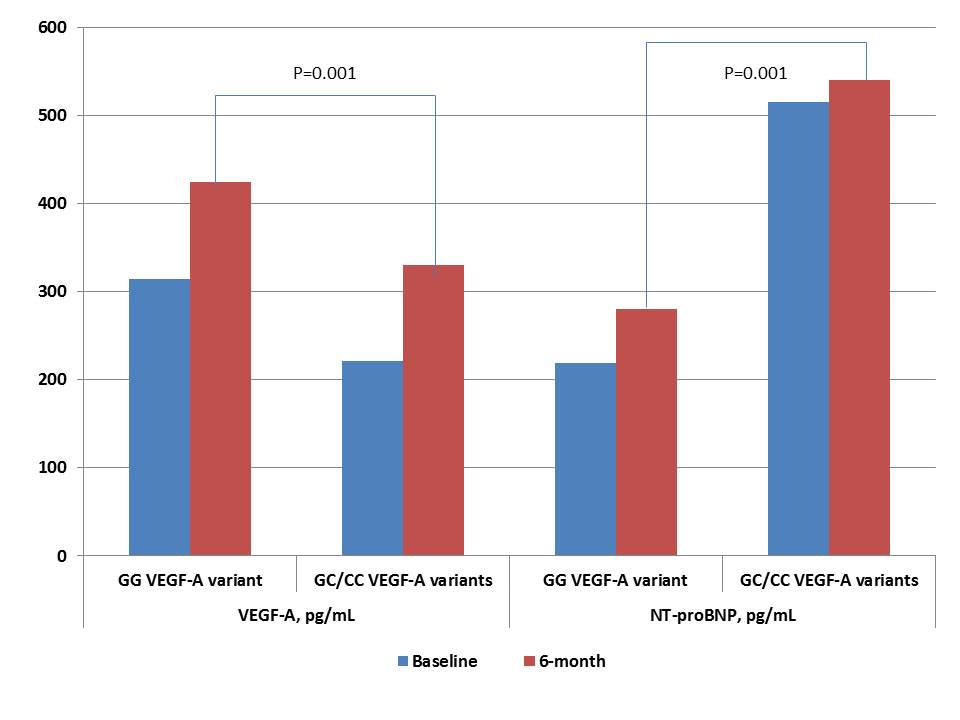
Six-month observation showed that cardiac hemodynamic performances and levels of cardiac biomarkers (hs-CRP, VEGF-A, NT-proBNP) were similar in both patient cohorts, while E/e` ratio was significantly increased in patients with GC/CC VEGF-A genotypes (Table 4). Dynamic changes of VEGF-A and NT-proBNP levels in STEMI patients with variants of G634C VEGF-A polymorphism for 6 months are reported in Figure 4. Patients with GC/CC VEGF-A genotypes demonstrated both lowered 6-month levels of VEGF-A and increased 6-month levels of NT-proBNP than individuals with GG VEGF-A genotype.
| Variables | Entire population (n = 135) | Patients with GG genotype(n = 70) | Patients with GC and СС genotypes (n = 65) | Р value |
| Hemodynamics | ||||
| SBP, mm Hg | 132 ± 15 | 136 ± 22 | 130 ± 14 | 0.186 |
| DBP, mm Hg | 82 ± 12 | 82 ± 14 | 82 ± 11 | 0.484 |
| LVEDV, mL | 155 ± 20 | 145 ± 39 | 158 ± 31 | 0.261 |
| LVESV, mL | 78 ± 24 | 70 ± 25 | 81 ± 39 | 0.308 |
| LVEF, % | 50 ± 8 | 52 ± 8 | 49 ± 12 | 0.220 |
| E/e`, units | 13.30 ± 1.17 | 10.90 ± 1.18 | 15.70 ± 1.25 | 0.038 |
| Biomarkers | ||||
| hs-CRP, mg/L | 9.82 ± 4.00 | 8.54 + 3.20 | 10.43 ± 4.10 | 0.662 |
| VEGF-A, pg/mL | 406.70[210.50 - 523.71] | 424.56[230.60 - 556.93] | 330.24[162.80 - 472.14] | 0.129 |
| NT-proBNP, pg/mL | 388.29[151.70 - 920.50] | 280.29[81.39 - 718.34] | 540.01[461.99 - 1217.31] | 0.074 |
| Clinical outcomes | ||||
| Admission due to HF, n (%) | 6 (4.4) | 1 (1.4) | 5 (7.7) | 0.088 |
| MI, n (%) | 14 (10.4) | 5 (7.1) | 9 (13.8) | 0.048 |
| Death, n (%) | 9 (6.7) | 3 (4.3) | 6 (9.2) | 0.160 |
| Total, n (%) | 29 (21.4) | 9 (12.9) | 20 (30.8) | 0.020 |
Therefore, we found that within the 6-month period, 29 cases of pre-specified clinical outcomes occurred (9 cases and 20 cases in GG VEGF-A genotype and GC/CC VEGF-A genotype cohorts, respectively). Interestingly, significant differences between the cohorts were observed in terms of frequencies of MI, but not in other aspects of clinical outcomes.
Correlations between G634G VEGF-A gene polymorphism, angiographic characteristics, hemodynamic performances, and biomarkers
There were positive correlations between GC/СС VEGF-A genotype and combined endpoint (r = 0.58; P = 0.0001), dynamic-increased NT-proBNP level for 6 months (r = 0.42; P = 0.001), SYNTAX score (r = 0.34; P = 0.001), anterior STEMI (r = 0.36; P = 0.003), LDL cholesterol (r = 0.22; P = 0.032), TIMI score (r = 0.26; P = 0.012), atrial fibrillation (r = 0.28; P = 0.001), unstable angina prior to STEMI (r = 0.25; P = 0.047), E/e` ratio (r = 0.23; P = 0.048), and multiple coronary vessel injury (r = 0.26; P = 0.002). There was inverted correlation between GC/СС VEGF-A genotype and dynamic-increased VEGF-A level for 6 months (r = -0.42; P = 0.001), and LV ejection fraction (r = -0.33; P = 0.001) in acute STEMI patients.
Therefore, we found a correlation between LV ejection fraction and dynamic-increased NT-proBNP (r = -0.48; P = 0.003), and VEGF-A level for 6 months (r = 0.46; P = 0.003) in STEMI patients with GC/CC VEGF-A genotypes. However, it was not confirmed in patients with the GG variant of the VEGF-A genotype. Yet, multiple coronary artery injury was found to correlate with LV ejection fraction at baseline (r = 0.33; P = 0.001), NT-proBNP (r = 0.32; P = 0.001), dyslipidemia (r = 0.30; P = 0.002), type 2 diabetes mellitus (r = 0.28; P = 0.001), hs-CRP (r = 0.26; P = 0.001), E/e` ratio at baseline (r = 0.24; P = 0.002), abdominal obesity (r = 0.23; P = 0.024), smoking (r = 0.22; P = 0.046), and male sex (r = 0.22; P = 0.048). There were no observed significant associations between the GG variant of the VEGF-A genotype and prevalence of traditional CV risk factors.
Determination of predictors for 6-month clinical endpoint
The univariate linear regression (stepwise) analysis allowed for verifying TnI eak at admission, TIMI score, abdominal obesity, Killip HF class > II at admission, anterior localization of STEMI, atrial fibrillation, GC/CC variants of VREGF-A gene, NT-proBNP at baseline, and dynamic changes in levels of NT-proBNP and VEGF-A in serial measures as predictors for combined clinical endpoint (Table 5). Other variables did not embed into multivariate regressive analysis due to P > 0.1.
Unadjusted multivariate regressive logistic analysis showed peak TnI at admission, Killip class of HF > 2, GC/CC polymorphisms in VEGF-A gene, and dynamic-increased NT-proBNP and VEGF-A levels for 6 months, which remained independent predictors for the combined endpoint (Table 5). After adjustment for dynamic changes of NT-proBNP and VEGF-A levels for 6 months, we found that GC/CC polymorphisms of the VEGF-A gene was an independent predictor of poor clinical outcome (β-coefficient = 1.6635; odds ratio = 2.8244; 95% confidence interval = 1.2649 - 11.2972; P = 0.0001).
| Variable | Depending variable: combined clinical end point | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate linear regressive analysis ( χ2= 61.293; Р < 0.0001) | Multivariate linear regressive analysis ( χ2=32.140; Р < 0.0001) | |||||||
| β-coefficient | OR | 95% CІ | Р | β-coefficient | OR | 95% CІ | Р | |
| Peak TnІ at admission | -1.8578 | 1.0111 | 1.0007 – 1.0215 | 0.0358 | -0.012610 | 1.0127 | 1.0016 – 1.0239 | 0.0247 |
| Peak CK-MB at admission | 0.47640 | 1.0254 | 1.0180 – 1.104 | 0.4820 | - | |||
| SYNTAX score | 0.98677 | 1.0028 | 1.0002 – 1.006 | 0.6884 | - | |||
| TIMI score | 1.37250 | 1.8970 | 0.9720 – 2.880 | 0.0410 | 1.17280 | 1.0940 | 1.010 – 1.3240 | 0.0520 |
| Killip class of HF > II | 0.9874 | 1.3725 | 0.0536 – 2.5869 | 0.3179 | 2.23331 | 9.3307 | 2.4408 – 35.6689 | 0.0011 |
| Smoking | -0.49898 | 0.6071 | 0.0328 – 11.2510 | 0.7376 | - | |||
| Dyslipidemia | 0.4582 | 0.8848 | 0.6638 – 1.1255 | 0.6388 | - | |||
| T2DM | 2.98372 | 19.7611 | 0.8341 – 46.1875 | 0.0647 | - | |||
| Abdominal obesity | 1.12320 | 2.1448 | 0.4607 – 3.8995 | 0.0383 | 1.02 | 1.9560 | 0.0774 – 3.4539 | 0.0526 |
| Stable angina prior to STEMI | 0.43968 | 1.5522 | 0.3988 – 6.0419 | 0.5260 | - | |||
| Unstable angina prior to STEMI | 1.55459 | 0.2113 | 0.0149 – 2.9884 | 0.2501 | - | |||
| Anterior STEMI | 1.98807 | 7.3014 | 1.1181 – 47.6782 | 0.0378 | 1.85810 | 5.2064 | 0.0122 – 21.4412 | 0.0586 |
| Atrial fibrillation | 1.00347 | 0.1349 | 0.0157 – 1.1565 | 0.0476 | 1.00254 | 0.1104 | 0.0120 – 1.1433 | 0.0662 |
| Multiple coronary vessel injury | 1.37022 | 3.9362 | 0.8228 – 18.8312 | 0.0862 | - | |||
| GC/CC polymorphisms in VEGF-A gene | 5.89420 | 2.9263 | 1.5366 – 5.3407 | 0.0199 | 1.72401 | 5.6070 | 1.4777 – 21.2745 | 0.0113 |
| E/e` at baseline | 0.35360 | 0.9160 | 1.0136 – 1.1630 | 0.0870 | - | |||
| NT-proBNP at baseline | 1.18440 | 1.7044 | 1.0633 – 2.954 | 0.03420 | 1.17230 | 1.0144 | 1.0330 – 1.1422 | 0.0620 |
| Dynamic increased NT-proBNP level for 6 month | 1.21370 | 1.8692 | 1.1354 – 4.8264 | 0.02427 | 1.09377 | 1.2177 | 1.0464 – 3.5569 | 0.0357 |
| Dynamic increased VEGF-A level for 6 month | -0.005467 | 1.0055 | 1.0007 – 1.0103 | 0.0241 | -0.0015896 | 1.0016 | 1.0002 – 1.0029 | 0.0201 |
Kaplan-Meier analysis for endpoint accumulation trends in STEMI patients with variants of VEGF-A gene
Kaplan-Meier curves have demonstrated that STEMI patients with GG VEGF-A genotype had a lower frequency of clinical combined endpoint accumulation when compared to those who had GC/CC VEGF-A genotypes (Log-rank p = 0.02) (Figure 5).
Discussion
The results of our study maintain the hypothesis that the GG variant of the VEGF-A gene is able to mediate myocardial protection via higher levels of VEGF-A in peripheral blood, prior to STEMI as well as for 6 months after the event. Consequently, GC/CC variants of the VEGF-A gene, which were associated with lowered basic and post-event levels of VEGF-A, have demonstrated close relation to accumulation of combined clinical endpoint for 6 months. We first revealed that complete revascularization in STEMI patients having the GC/CC variants of the VEGF-A gene can draw unexpectedly worse clinical results for follow-up than those patients with the GG variant of the VEGF-A gene. Interestingly, at baseline, both STEMI patient cohorts did not significantly differ from each other with regards to traditional CV risk factors, several biomarkers levels (including CRP and NT-proBNP), and severity of coronary atherosclerosis Meanwhile left main coronary artery injury was determined frequently in patients with GC/CC variants of VEGF-A gene.
Previous animal and clinical studies have shown that VEGF-A exhibited powerful cardiac reparative effect, protected from ischemia/reperfusion injury, and reduced myocardial edema and MI size. Indeed, the cardiac macrophages recruited by pro-inflammatory cytokines are certainly conductors of these protective impacts29, 30, 31. Moreover, VEGF-A levels were independently associated with microvascular obstruction during STEMI31. However, our results may indirectly confirm evidence of VEGF-A acting as a protector of endovascular edema and microvascular obstruction, preventing adverse LV remodeling. Indeed, Ferraro B et al. (2019)29 reported that the VEGF-A levels were significantly and inversely correlated with LVEF at 6-month follow-up. Our results corroborate with this evidence, although we did not find strong correlation between the VEGF-A levels at baseline and LVEF at baseline. However, there were strong interrelations between GG VEGF-A genotype and circulating levels of VEGF-A. Probably, the role of variants of VEGF-A gene has become crucial for post-event periods and correspond to maintaining higher VEGF-A levels in peripheral blood to prevent adverse LV remodeling through lowered risk of distal coronary obstruction32. However, angiographic parameters and coronary anatomy in STEMI patients who were effectively treated with primary PCI (with TIMI-III restoring blood flow and complete reperfusion) did not strongly correlate to a risk of late microvascular obstruction, which remained an independent predictor of LV remodeling, mortality following STEMI, and all-cause mortality even after further adjustment for infarct size33, 34. In this context, a prediction of follow-up survival during STEMI treated effectively with PCI seems to be a credible tool for risk stratification of STEMI patients, and GC/CC VEGF-A genotypes could be discussed as a prognosticator for poor clinical outcomes. However, there is no optimal methodology that best predicts the surrogate outcome marker of LV function and survival in post-STEMI patients35, 36.
The strength of our investigation is the enrollment in the study of STEMI patients with normal and preserved LVEF who were candidates for complete reperfusion therapy with PCI. There are limiting data which were able to predict CV events and late LV remodeling in post-STEMI individuals without clinical significance and declining of LV pump function and symptomatic HF36. The results of our study have revealed that presentation of GC/CC variants of VEGF-A gene predicted well the tendency for LFEF and LF diastolic filling to worsen. Note that these results may be interpreted in the context of late microvascular obstruction that frequently follows complete PCI. Indeed, our results showed that combined the clinical endpoint occurred predominantly due to new MI, but not any other reasons. Probably, GC/CC variants of VEGF-A gene were not able to support vascular protection with adequate VEGF-A production after STEMI and could not protect microvascular obstruction, which led to MI in the follow-up period37.
It has been revealed that STEMI patients who might have microvascular obstruction had higher peak TnT and lower LVEF because of an increased LVEDV38. Although an association between GC/CC variants of the VEGF-A gene exists, the risk of CAD and quality of life in the general population has been previously determined39, 40 There were not close associations between severity of atherosclerosis, number of damaged coronary artery, and GC/CC VEGF-A genotype in the STEMI patient population41. On contrary, there are other findings (which are clarified positive associations) between the culprit artery lesion localization, MI size, and microvascular obstruction42, 43. Because of there are close interrelationships between VEGF-A tissue expression and serum levels, microvessel density, ROS production, and expression of vascular endothelial cadherin in re-perfused myocardium44, the role of variants of VEGF-A genotypes in tissue protection in STEMI has been suggested.
In this context, it has remained largely unclear as to the exact molecular mechanisms which mediate the causative relation of VEGF-A to vascular and myocardium protection in post-STEMI patients. It has been postulated that elevated levels of VEGF-A in post-STEMI patients is a result of myocardial ischemia/hypoxia induced by microvascular dysfunction after distal coronary artery embolization and vascular inflammation. In fact, early microvascular obstruction is accompanied with decline of LVEF, whereas late microvascular dysfunction in post-STEMI individuals can associate with LV diastolic dysfunction without reduced LVEF45, 46. Large clinical studies are required to clearly understand the role of genetic polymorphisms of the VEGF-A gene in mediating endogenous reparation and tissue protection. However, we believe that determination of the GC/CC VEGF-A genotype could provide relevant prognostic insights, leading to improved short-term and long-term risk stratification in STEMI patients treated with complete revascularization.
There are several study limitations. First, the small sample size does not allow for the analysis to be conducted in detail in greater subgroups. Additionally, it does not allow for fully interpreting and understanding the causes that lead to the appearance of MACEs directly related to the new MI. Second, it would be optimal to use late gadolinium-enhanced magnetic resonance images to determine an interrelationship between microvascular obstruction and increased risk of adverse CV events in post-STEMI patients. Third, it has still not fully understand whether attenuated plaques and well-positioned stents were causes for adverse outcomes. Yet, we have measured total VEGF-A levels, but we did not determine fractions of VEGF-A which were found to mediate different impacts on myocardium and vasculature. Finally, we believe that these study limitations will not be able to sufficiently diminish the value of the results of our investigation.
Conclusion
The G634C polymorphism of the VEGF-A gene was found to be an independent predictor for 6-month clinical combined endpoints in STEMI patients after successful primary PCI.
List of abbreviations
ADA: American Diabetic Association
CAD: coronary artery disease
CI: 95% confidence interval
CK-MB: creatinine kinase isoenzyme-MB
CV: cardiovascular
DBP: diastolic blood pressure
E: early transmitral velocity
e`: global longitudinal LV strain
ECS: European Cardiology Society
EDV: end diastolic volume
EF: ejection fraction
ESV: end systolic volume
HDL: high density lipoprotein
HF: heart failure
IQR: interquartile range
LDL: low density lipoprotein
LV: left ventricular
MACE: major cardiovascular events
NT-proBNP: NT-fragment pro-natriuretic peptide
PCI: percutaneous coronary intervention
ROS: reactive oxygen species
SBP: systolic blood pressure
SD: standard deviation
STEMI: ST segment elevation myocardial infarction
T2DM: type 2 diabetes mellitus
TC: total cholesterol
TG: triglycerides
Acknowledgments
There are no previous presentations of the information reported in the article.
Author’s contributions
Study Design: Inna M. Kutia, Mykola P. Kopytsya, Yaroslava V. Hilova, Alexander E. Berezin. Data Collection: Inna M. Kutia, Mykola P. Kopytsya, Yaroslava V. Hilova; Olga V Petyunina. Statistical Analysis: Inna M. Kutia, Alexander E. Berezin. Data Interpretation: Inna M. Kutia, Mykola P. Kopytsya, Yaroslava V. Hilova, Olga V Petyunina; Alexander E. Berezin. Manuscript Preparation: Inna M. Kutia, Mykola P. Kopytsya, Yaroslava V. Hilova, Olga V Petyunina; Alexander E. Berezin. Literature Search: Inna M. Kutia; Alexander E. Berezin. All authors read and approved the final manuscript.
Funding
The study is a fragment of the research project: “To study the biochemical, genetic mechanisms of reperfusion damage of the myocardium and to assess the cardioprotective effect of antiplatelet therapy in acute myocardial infarction”, State Registration No. 0117U003028 / Ukraine
No commercial funds were collected for this study.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study (Protocol №6, 30.05.2017), and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Thomas
M.P.,
Bates
E. R.,
Update on primary PCI for patients with STEMI. Trends Cardiovasc Med.
2017;
27
(2)
:
95-102
.
View Article PubMed Google Scholar -
Banga
S.,
Gumm
D.C.,
Kizhakekuttu
T.J.,
Kizhakekuttu
T.J.,
Emani
V.K.,
Singh
S.,
Singh
S.,
Left Ventricular Ejection Fraction along with Zwolle Risk Score for Risk Stratification to Enhance Safe and Early Discharge in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention: A Retrospective Observational Study. Cureus.
2019;
11
(7)
:
e5272
.
View Article Google Scholar -
Tarantini
G.,
D'Amico
G.,
Brener
S.J.,
Tellaroli
P.,
Basile
M.,
Schiavo
A.,
Survival After Varying Revascularization Strategies in Patients With ST-Segment Elevation Myocardial Infarction and Multivessel Coronary Artery Disease: A Pairwise and Network Meta-Analysis. JACC Cardiovasc Interv.
2016;
9
(17)
:
1765-1776
.
View Article PubMed Google Scholar -
Song
F.,
Wu
Q.,
Chen
X.P.,
New debate of revascularization strategy of non-infarct-related artery lesions in patients with ST-segment elevation myocardial infarction and cardiogenic shock: decoding the CULPRIT-SHOCK trial, not enough to challenge the current guidelines. Chin Med J (Engl).
2019;
132
(5)
:
613-615
.
View Article PubMed Google Scholar -
Thiele
H.,
Akin
I.,
Sandri
M.,
Fuernau
G.,
Waha
S.,
Meyer-Saraei
R.,
PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med.
2017;
377
:
2419-2432
.
View Article PubMed Google Scholar -
Windecker
S.,
Kolh
P.,
Alfonso
F.,
Collet
J.P.,
Cremer
J.,
Authors/Task Force members. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J.
2014;
35
:
2541-2619
.
View Article PubMed Google Scholar -
Park
D.W.,
Clare
R.M.,
Schulte
P.J.,
Pieper
K.S.,
Shaw
L.K.,
Califf
R.M.,
Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA.
2014;
312
:
2019-2027
.
View Article PubMed Google Scholar -
Kosmidou
I.,
McAndrew
T.,
Redfors
B.,
Embacher
M.,
Dizon
J.M.,
Mehran
R.,
Correlation of Admission Heart Rate With Angiographic and Clinical Outcomes in Patients With Right Coronary Artery ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: HORIZONS-AMI (The Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) Trial. J Am Heart Assoc.
2017;
6
(7)
.
View Article PubMed Google Scholar -
Gajanana
D.,
Weintraub
W.S.,
Kolm
P.,
Rogers
T.,
Iantorno
M.,
Ben-Dor
I.,
Trends in Death Rate 2009 to 2018 Following Percutaneous Coronary Intervention Stratified by Acuteness of Presentation. Am J Cardiol..
2019;
9149
(19)
:
30890-30892
.
View Article PubMed Google Scholar -
Hueso
L.,
Rios-Navarro
C.,
Ruiz-Sauri
A.,
Chorro
F.J.,
Nunez
J.,
Sanz
M.J.,
Dynamics and implications of circulating anti-angiogenic VEGF-A165b isoform in patients with ST-elevation myocardial infarction. Sci Rep.
2017;
7
(1)
:
9962
.
View Article PubMed Google Scholar -
Grąbczewska
Z.,
Dębski
R.,
Góralczyk
K.,
Sukiennik
A.,
Świątkiewicz
I.,
Kubica
J.,
Associations between selected angiographic parameters and the number of CD34⁺ cells and plasma levels of vascular endothelial growth factor and angiogenin in patients with ST-segment elevation myocardial infarction. Pol Arch Med Wewn.
2015;
125
(3)
:
132-140
.
View Article PubMed Google Scholar -
Kim
B.H.,
Ko
Y.G.,
Her
A.Y.,
Kim
J.S.,
Hwang
K.C.,
Shin
D.H.,
Serial plasma levels of angiogenic factors in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J.
2012;
42
(7)
:
464-470
.
View Article PubMed Google Scholar -
Olsson
A.K.,
Dimberg
A.,
Kreuger
J.,
Claesson-Welsh
L.,
VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol.
2006;
7
:
359-371
.
View Article PubMed Google Scholar -
Ferrara
N.,
Gerber
H.P.,
LeCouter
J.,
The biology of VEGF and its receptors. Nat Med.
2003;
9
:
669-676
.
View Article PubMed Google Scholar -
Sato
A.,
Yoshihisa
A.,
Yokokawa
T.,
Shimizu
T.,
Nakamura
Y.,
Misaka
T.,
The association between circulating anti-angiogenic isoform of vascular endothelial growth factor and clinical profiles in patients with peripheral artery disease. Int J Cardiol.
2016;
207
:
368-369
.
View Article PubMed Google Scholar -
Cochain
C.,
Channon
K.M.,
Silvestre
J.S.,
Angiogenesis in the infarcted myocardium. Antioxid Redox Signal.
2013;
18
:
1100-1113
.
View Article PubMed Google Scholar -
Pyda
M.,
Korybalska
K.,
Ksiazek
K.,
Grajek
S.,
Lanocha
M.,
Lesiak
M.,
Effect of heparin on blood vascular endothelial growth factor levels in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol.
2006;
98
(7)
:
902-905
.
View Article PubMed Google Scholar -
Liu
K.L.,
Lin
S.M.,
Chang
C.H.,
Chen
Y.C.,
Chu
P.H.,
Plasma angiopoietin-1 level, left ventricular ejection fraction, and multivessel disease predict development of 1-year major adverse cardiovascular events in patients with acute ST elevation myocardial infarction - a pilot study. Int J Cardiol.
2015;
182
:
155-160
.
View Article PubMed Google Scholar -
Al-Habboubi
H.H.,
Sater
M.S.,
Almawi
A.W.,
Al-Khateeb
G.M.,
Almawi
W.Y.,
Contribution of VEGF polymorphisms to variation in VEGF serum levels in a healthy population. Eur. Cytokine Netw.
2011;
22
:
154-158
.
View Article PubMed Google Scholar -
Watson
C.J.,
Webb
N.J.,
Bottomley
M.J.,
Brenchley
P.E.,
Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine.
2000;
12
(8)
:
1232-1235
.
View Article PubMed Google Scholar -
Ibanez
B.,
James
S.,
Agewall
S.,
Antunes
M.J.,
Bucciarelli-Ducci
C.,
Bueno
H.,
ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J..
2018;
39
(2)
:
119-177
.
View Article PubMed Google Scholar -
Kirby
A.,
Gebski
V.,
Keech
A.C.,
Determining the sample size in a clinical trial. Med J Aust. 2002.
https://doi.org/10.5694/j.1326-5377.2002.tb04759.x;
177
(5)
:
256-257
.
PubMed Google Scholar -
Catapano
A.L.,
Graham
I.,
Backer
G.D.,
Wiklund
O.,
Chapman
M.J.,
Drexel
H. ,
ESC Scientific Document Group. 2016 ESC/EAS Guidelines for the Management of Dyslipidemias: The Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis.
2016;
253
:
281-344
.
View Article PubMed Google Scholar -
Williams
B.,
Mancia
G.,
Spiering
W.,
Rosei
E. Agabiti,
Azizi
M. ,
Burnier
M.,
ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J.
2018;
39
(33)
:
3021-3104
.
View Article PubMed Google Scholar -
Ponikowski
P.,
Voors
A.A.,
Anker
S.D.,
Bueno
H.,
Cleland
J.G.F.,
Coats
A.J.S.,
ESC Scientific Document Group 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail.
2016;
18
:
891-975
.
View Article PubMed Google Scholar -
Jessup
M.,
Drazner
M.H.,
Book
W.,
Cleveland
J.C.J.,
Dauber
I.,
Farkas
S.,
2017 ACC/AHA/HFSA/ISHLT/ACP Advanced Training Statement on Advanced Heart Failure and Transplant Cardiology (Revision of the ACCF/AHA/ACP/HFSA/ISHLT 2010 Clinical Competence Statement on Management of Patients With Advanced Heart Failure and Cardiac Transplant): A Report of the ACC Competency Management Committee. J Card Fail.
2017;
23
(6)
:
492-511
.
View Article PubMed Google Scholar -
Morrow
D.A.,
Antman
E.M.,
Charlesworth
A.,
Cairns
R.,
Murphy
S.A.,
Lemos
J.A. de,
TIMI Risk Score for ST-Elevation Myocardial Infarction: A Convenient, Bedside, Clinical Score for Risk Assessment at Presentation. An Intravenous nPA for Treatment of Infarcting Myocardium Early II Trial Substudy. Circulation.
2000;
102
:
2031-2037
.
View Article PubMed Google Scholar -
Kappetein
A.P.,
Dawkins
K.D.,
Mohr
F.W.,
Morice
M.C.,
Mack
M.J.,
Russell
M.E.,
Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. Eur J Cardiothorac Surg.
2006;
29
(4)
:
486-491
.
View Article PubMed Google Scholar -
Ferraro
B.,
Leoni
G.,
Hinkel
R.,
Ormanns
S.,
N
N. Paulin,
Ortega-Gomez
A.,
Pro-Angiogenic Macrophage Phenotype to Promote Myocardial Repair. J Am Coll Cardiol.
2019;
73
(23)
:
2990-3002
.
View Article PubMed Google Scholar -
Dong
J.,
Xu
M.,
Zhang
W.,
Che
X.,
Effects of Sevoflurane Pretreatment on Myocardial Ischemia-Reperfusion Injury Through the Akt/Hypoxia-Inducible Factor 1-alpha (HIF-1α)/Vascular Endothelial Growth Factor (VEGF) Signaling Pathway. Med Sci Monit.
2019;
25
:
3100-3107
.
View Article PubMed Google Scholar -
Garcia
R.,
Bouleti
C.,
Sirol
M.,
Logeart
D.,
Monnot
C.,
Ardidie-Robouant
C.,
VEGF-A plasma levels are associated with microvascular obstruction in patients with ST-segment elevation myocardial infarction. Int J Cardiol.
2019;
291
:
19-24
.
View Article PubMed Google Scholar -
Berezin
A.E.,
Endogenous vascular repair system in cardiovascular disease: The role of endothelial progenitor cells . Australasian Medical J.
2019;
12
(2)
:
42-48.
.
View Article Google Scholar -
Wong
D.T.,
Leung
M.C.,
Richardson
J.D.,
Puri
R.,
Bertaso
A.G.,
Williams
K.,
Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: a comparison of angiographic and cardiac magnetic resonance derived measurements. Int J Cardiovasc Imaging.
2012;
28
(8)
:
1971-1981
.
View Article PubMed Google Scholar -
Waha
S.,
Patel
M.R.,
Granger
C.B.,
Ohman
E.M.,
Maehara
A.,
Eitel
I.,
Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J.
2017;
38
(47)
:
3502-3510
.
View Article PubMed Google Scholar -
Berezin
A.E.,
Berezin
A.A.,
Dynamic Changes of Circulating Vascular Endothelial Growth Factor Levels in ST-Segment Elevation Myocardial Infarction: Controversies in Clinical Interpretation. Clin Trial Cardiol.
2019;
6
(1)
:
1-4
.
View Article Google Scholar -
Galea
N.,
Dacquino
G.M.,
Ammendola
R.M.,
Coco
S.,
Agati
L.,
Luca
L.D.,
Microvascular obstruction extent predicts major adverse cardiovascular events in patients with acute myocardial infarction and preserved ejection fraction. Eur Radiol.
2019;
29
(5)
:
2369-2377
.
View Article PubMed Google Scholar -
Shiono
Y.,
Kubo
T.,
Tanaka
A.,
Tanimoto
T.,
Ota
S.,
Ino
Y.,
Impact of attenuated plaque as detected by intravascular ultrasound on the occurrence of microvascular obstruction after percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv.
2013;
6
(8)
:
847-853
.
View Article PubMed Google Scholar -
Durante
A.,
Laricchia
A.,
Benedetti
G.,
Esposito
A.,
Margonato
A.,
Rimoldi
O.,
Identification of High-Risk Patients After ST-Segment-Elevation Myocardial Infarction: Comparison Between Angiographic and Magnetic Resonance Parameters. Circ Cardiovasc Imaging.
2017;
10
(6)
.
View Article Google Scholar -
Han
X.,
Liu
L.,
Niu
J.,
Yan
J.,
Zhang
Z.,
Association between VEGF polymorphisms (936c/t, -460t/c and -634g/c) with haplotypes and coronary heart disease susceptibility. Int J Clin Exp Pathol.
2015;
8
(1)
:
922-927
.
-
Li
L.,
Pan
Y.,
Zhang
D.,
Association of Genetic Polymorphisms on Vascular Endothelial Growth Factor and its Receptor Genes with Susceptibility to Coronary Heart Disease. Med Sci Monit.
2016;
22
:
31-40
.
View Article PubMed Google Scholar -
Nia
S.K.,
Ziaee
S.,
Boroumand
M.A.,
Anvari
M.S.,
Pourgholi
L.,
Jalali
A.,
The impact of vascular endothelial growth factor +405 C/G polymorphism on long-term outcome and severity of coronary artery disease. J Clin Lab Analysis.
2017;
31
(4)
:
e22066
.
View Article PubMed Google Scholar -
Joost
A.,
Stiermaier
T.,
Eitel
C.,
Fuernau
G.,
Waha
S.,
Desch
S.,
Impact of Initial Culprit Vessel Flow on Infarct Size, Microvascular Obstruction, and Myocardial Salvage in Acute Reperfused ST-Elevation Myocardial Infarction. Am J Cardiol.
2016;
118
(9)
:
1316-1322
.
View Article PubMed Google Scholar -
Ahn
S.G.,
Hung
O.Y.,
Lee
J.W.,
Lee
J.H.,
Youn
Y.J.,
Ahn
M.S.,
Combination of the Thermodilution-Derived Index of Microcirculatory Resistance and Coronary Flow Reserve Is Highly Predictive of Microvascular Obstruction on Cardiac Magnetic Resonance Imaging After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv.
2016;
9
(8)
:
793-801
.
View Article PubMed Google Scholar -
Zou
J.,
Fei
Q.,
Xiao
H.,
Wang
H.,
Liu
K.,
Liu
M.,
VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol.
2019;
234
(10)
:
17690-17703
.
View Article PubMed Google Scholar -
Douvaras
P.,
Antonatos
D.G.,
Kekou
K.,
Patsilinakos
S.,
Chouliaras
G.,
Association of VEGF gene polymorphisms with the development of heart failure in patients after myocardial infarction. Cardiology.
2009;
114
(1)
:
11-18
.
View Article PubMed Google Scholar -
Chin
B.S.,
Chung
N.A.,
Gibbs
C.R.,
Blann
A.D.,
Lip
G.Y.,
Vascular endothelial growth factor and soluble P-selectin in acute and chronic congestive heart failure. Am J Cardiol.
;
2002
(90)
:
1258-1260
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 5 (2020)
Page No.: 3744-3759
Published on: 2020-05-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6255 times
- Download PDF downloaded - 1743 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress