Abstract
Introduction: Anti-tumor drug screening is the most popular technique in drug discovery. In this technique, various agents were tested for their cytotoxicity on cell lines, particularly cancer cell lines. Since most of these agents are weakly soluble in water, they can be normally dissolved in lipophilic solvents. To obtain accurate results, the requirement of these solvents are that they be biocompatible and non-toxic to cells. The aim of this study was to investigate the biological effects of the three agents most commonly used as drug vehicles, i.e. dimethyl sulfoxide (DMSO), ethanol and methanol, using cell proliferation measurement techniques.
Methods: To minimize the errors from the measurement techniques, this study used the xCelligence RTCA system which entails real-time monitoring of cell proliferation without dye labeling. Under the wide range concentration of solvents (from 0% to 100% of solvents by serial dilutions), the cell index (CI) and slope of cell proliferation curves of HepG2, MDA-MB-231, MCF-7 and VNBRCA1 cell lines were analyzed (by the software provided by the xCelligence system).
Results: The results showed that DMSO had a significant toxicity and inhibition of proliferation on 4 cell lines, at the concentrations of 10%, 5%, 2.5% and 1.25% (p<0.0001). Methanol and ethanol inhibited cellular proliferation at the concentrations of 10% and 5% (p<0.0001). The concentrations of ethanol and methanol ranging from 2.5% to 0.15% concentration were well-tolerated by cells with respect to proliferation. Depending on the extracts or agents, each should be diluted in the suitable vehicle at the proper concentrations.
Conclusion: Ethanol and methanol are good choices for solvents since they have low toxicity on HepG2, MDA-MB-231, MCF-7 and VNBRCA1 cell lines. However, in the case of agents only dissolvable in DMSO, low concentrations of DMSO from 0.6%-0.015% should be considered.
Introduction
In biological research, solvents for dilution of therapeutic drugs are necessary, especially for drugs that are weakly soluble in water. The most common solvents for use as vehicles for drug delivery purpose in biological studies are dimethyl sulfoxide (DMSO), ethanol and methanol. DMSO is an organic amphiphilic molecule that is widely used in cell biology; it exhibits a number of capabilities such as vasodilatory, diuretic, anti-inflammatory and bacteriostatic functions 1, 2.
In vitro, DMSO is routinely used for cryopreservation due to its high freeze temperature. However, its strong interaction with phospholipids makes it efficient in facilitating drug molecule delivery through the cell membrane, which is an area of study with great interest by many researchers. DMSO ranks high in terms of choice of solvent for drug delivery, despite its potential cytotoxicity on cells.
Alternative solvent that have been used in cell biology include ethanol and methanol. In drug screening, besides DMSO, crude plant extract could be dissolved in methanol or ethanol. However, ethanol could interfere with the structure of low cholesterol in the cell membrane and cause disorder of cellular physical activities 3. This damage in cell membrane could amplify the effect of therapeutic drugs, leading to non-reproducible results.
Furthermore, different cell types respond with different behavior to solvents 4. Although solvent control could be used to compare to the drug efficacy in experiment , nonetheless if the solvents are toxic to the cells over treatment, the effect of the drug could be wrongly evaluated. It is important to evaluate and define the maximum concentration of the solvents that could be used for dissolving drugs in biological assays. Hence, it is necessary to understand the optimal concentration of the solvents on specific cell lines to ensure accurate and reproducible experimental results.
Material - Methods
Cell lines
HepG2, MDA-MB-231 and MCF-7 cell lines was purchased from ATCC (Manassas, VA, USA). Cells was cultured in DMEM-F12 (Thermo Fisher Scientific, Waltham, MA, USA) complemented with 10% FBS (Thermo Fisher Scientific) and 1% antibiotic (Thermo Fisher Scientific), and cultured in 5% CO2 and 37oC incubator.
Solvents
Methanol, ethanol and DMSO was purchased from Merck (Darmstadt, Germany).
Evaluation of label-free real-time cellular proliferation
In brief, 2,000 cells were added into wells of E-plates (ACEA Biosciences, San Diego, CA, USA). After 24 hours of culture, cells were treated with DMSO, methanol and ethanol at the concentrations of 10%, 5%, 2.5%, 1.25%, 0.6%, 0.3%, 0.15%, and 0%. The E-plates were then connected into the xCelligence RTCA machine (ACEA Biosciences) in the incubator for the next 48 hours. Cell proliferation was measured by recording the change in the impedance of electron flow caused by adherent cells. The experiments were done in triplicate (n=3). The data were analyzed by the software attached to the xCelligence RTCA system (ACEA Biosciences).
Results
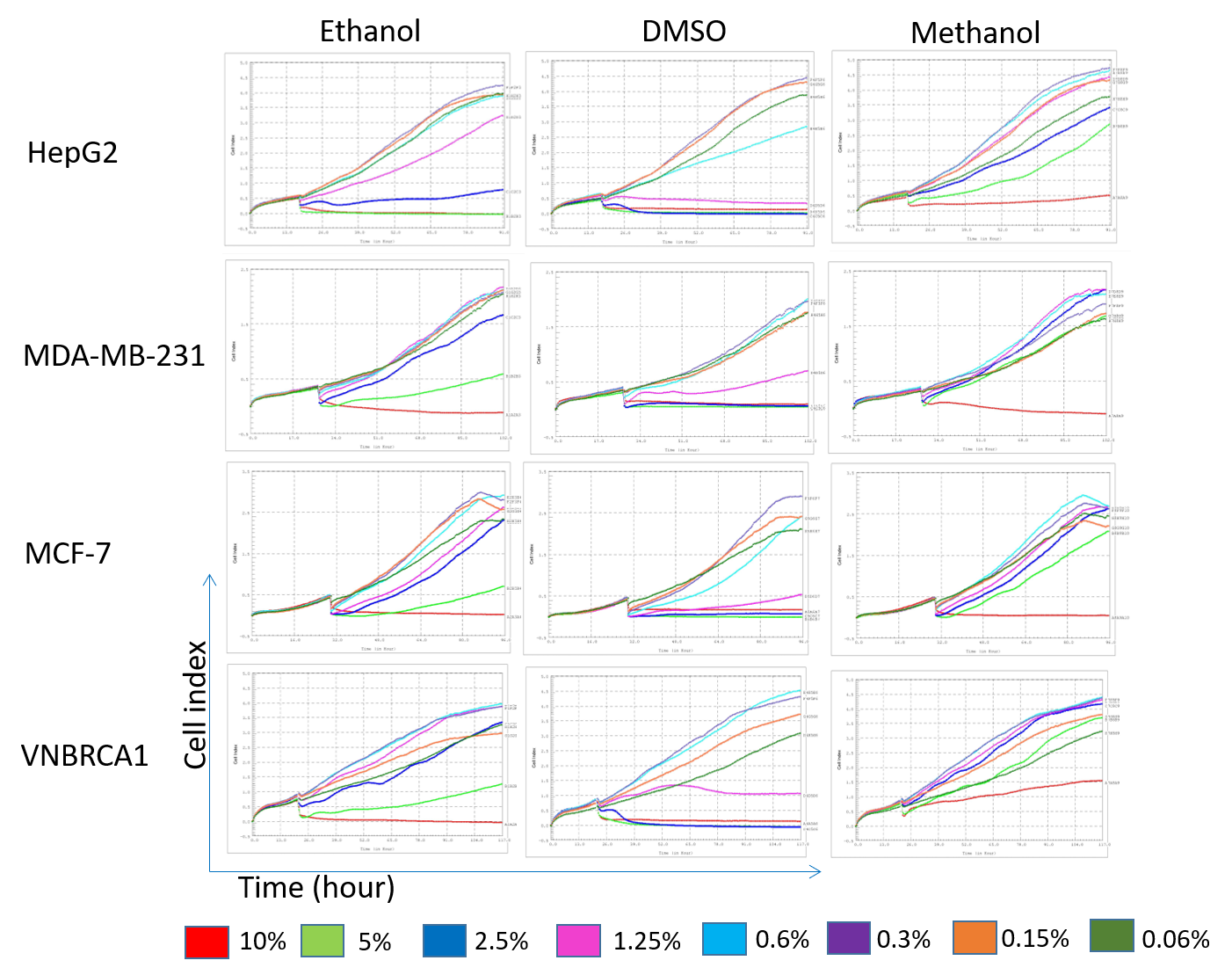
After cells were cultured for 24 hours for cell attachment, the solvents were added; the proliferation curves were slightly perturbed (Figure 1). From that time point onward, the cell proliferation curves dispersed. From observation of the proliferation curves, when treated with methanol as the solvent, the red curve (concentration of 10%) dipped down or became flattened in all the cell lines (Figure 1). Specially, the red curve (10% methanol) for the VNBRCA1 cell line increased upwards; this demonstrated that the proliferation of VNBRCA1 was less affected even at the high concentration of methanol.
In contrast to methanol, DMSO induced more than one flattened curve in the 4 cell lines. For example, the red curve (10% concentration), light green curve (5%), blue curve (2.5%), and pink curve (1.25%) all dipped downward.
Ethanol also appeared to induce divergent curves in the 4 cell lines. In HepG2, ethanol created 3 flattened curves compared to 2 curves in MDA-MB-231, MCF-7 and VNBRCA1. At the concentrations of 5% and 2.5%, ethanol inhibited the cell index (CI) values of the 3 cell lines MDA-MB-231, MCF-7 and VNBRCA1. However, the CI still showed upward increase at the concentration of 5% compared to that of 10% concentration for the MDA-MB-231, MCF-7 and VNBRCA1 cell lines. The concentrations of 1.25%, 0.6%, 0.3%, and 0.15% of ethanol induced upward curves in all 4 cell lines (Figure 1).
The results showed that DMSO significantly inhibited the proliferation of all the cell lines (HepG2, MDA-MD-231, MCF-7 and VNBRCA1) at the concentrations of 10%, 5%, 2.5% and 1.25%. The slope of the CI at these concentration were significantly different from that of the lower concentrations of 0.6%, 0.3% and 0.15% (P<0.0001) (Figure 2). Interestingly, DMSO at the concentration of 0.6% significantly affected cell growth of HepG2 and MCF-7, compared to the growth at the concentrations of 0.3% and 0.15%. However, 0.6% DMSO did not affect the CI slope of MDA-MB-231 or VNBRCA1.
Ethanol strongly affected the cell growth of HepG2 cell line at the concentrations of 10%, 5% and 2.5%. At the concentration of 2.5%, ethanol had less effect on MCF-7, MDA-MB-231 and VNBRCA1, compared to HepG2. The CI slope of MCF-7, MDA-MB-231 and VNBRCA1 were positive values, compared to the negative slope value of HepG2 (Figure 2).
Methanol seemed to have the least impact on the cell lines in this study. Indeed, 10% methanol showed a strong effect on all the cell lines while 5% methanol significantly affected HepG2 cells and MCF-7 cells but not MDA-MB-231 or VNBRCA1 cells. Lower concentrations of methanol, such as 2.5%-0.15%, showed no impact on any of the cell lines.
Discussion
Although almost all solvents are toxic to cells in vitro, they are still necessary for dissolving drug agents for biological assays. It is essential to keep the concentration of the solvents at the most suitable concentration for biological experimentation. To do this, in this study, we compared the effects of three commonly used solvents (methanol, ethanol and DMSO) on 4 cancer cell lines (HepG2, MDA-MD-231, MCF-7 and VNBRCA1).
Some studies from the literature have stated that the critical concentration of DMSO should be 1%, while others have used higher concentrations than that 4. The question is what the proper concentration of DMSO for use should be while still being non-toxic on experimental cells.
In our study, the results showed that at high concentration of DMSO, such as 10% and 5%, cell proliferation was strongly inhibited in all 4 cell lines. Interestingly, at the concentration 1.25% of DMSO, MDA-MB-231 and MCF-7 cancer cells were still alive while HepG2 cells showed a strong inhibition in proliferation. The sensibility to DMSO of different cell types may differ significantly. This also seems to be the case in other studies 4, 5. Therefore, it is essential to test the solvents at various concentrations if using different cell line models.
Previously, a study reported that lower concentrations of DMSO may lead to stimulation of proliferation of some cell types; for example, 0.05-0.2% DMSO significantly increased the proliferation of RPMI-8226 myeloma cells6. In the study herein, we did not recognize any significant difference in the doubling time of HepG2 and MDA-MB-231 at the concentrations of 0.3-0.15%.
In this study, ethanol and methanol were demonstrated to be good solvent for use on HepG2, MDA-MB-231, MCF-7 and VNBCRA1 cells. Ethanol is often used as a solvent for plant extracts and plant-derived components 7. There are toxicities to consider, but it is good for dissolution as long as it can dissolve the agents. Ethanol and methanol showed harmless effects on MDA-MB-231, MCF-7 and VNBRCA1 even at the high concentration of up to 2%. Similarly, though not using the same cell lines as in our study, a previous study also found that 2.8% ethanol did not affect the cell viability of RAW 264.7 8. In another report studying the effects of ethanol on HeLa cells, it was found that 5% ethanol (or higher concentrations) compromised cell viability 9.
Interestingly, 5% methanol showed less toxicity on the 4 cell lines, while 5% ethanol significantly compromised all cell lines. A previous study showed that butanol is even more toxic than ethanol 10; they observed some differences in toxicity between methanol, ethanol and butanol. The toxicity increase may be due to its longer carbon chain that can further intercalate into membranes and cause breaks in hydrogen bonds between lipid tails.
Ethanol is known to fluidize the cell membrane, leading to a disorder in trans-membrane protein flux such as that of Mg2+ 3, 11. Ethanol can also inactivate ATPase and glycolytic enzymes, causing the inhibition of proliferation 11, 12. At the concentration of 2%, 1.25%, 0.6% and 0.3%, the effects of ethanol on cells was not significantly different; methanol also exhibited the same behavior as ethanol at those concentrations. If only some of the material dissolve in ethanol or methanol, that could be an advantage.
Conclusion
In biological studies, solvents are essential for dissolving agents that cannot be dissolved in water. Particularly, in cancer research, there are a lot of agents which require evaluation and testing on cell models. However, the toxicity of solvents on cancer cells is a problem. Hence, it is necessary to determine the most suitable concentrations to use in biological assays. Here, in this study, we found that DMSO should be used as a solvent in the range of concentration of 0.6%-0.15% on HepG2, MDA-MB-231, MCF-7 and VNBRCA1 cells. Ethanol and methanol showed non-toxic effects on those cell lines at the concentrations of 1.25%-0.15%. Some toxicity was tolerable when a control sample with solvent alone was used in experiments.
Abbreviations
DMSO: Dimethyl sulfoxide
Acknowledgments
Not applicable.
Author’s contributions
All authors equally contributed in this study. All authors approved the final manuscript.
Funding
This work was supported by the Vietnam National University, Ho Chi Minh City, Vietnam, under grant A2015-18-01.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Horita
A.,
Weber
L.J.,
Skin Penetrating Property of Drugs Dissolved in Dimethylsulfoxide (Dmso) and Other Vehicles. Life Sci.
1962;
3
:
1389-1395
.
View Article Google Scholar -
Jacob
S.W.,
Bischel
M.,
Herschler
R.J.,
Dimethyl Sulfoxide (Dmso): A New Concept in Pharmacotherapy. Curr Ther Res Clin Exp.
1964;
6
:
134-135
.
-
Cartwright
C. P.,
Ethanol dissipates the proton-motive force across the plasma membrane of Saccharomyces cerevisiae. J. Gen. Microbiol.
1986;
132
:
369-377
.
View Article Google Scholar -
M.Timm
Considerations regarding use of solvents in in vitro cell based assays. Cytotechnology.
2013;
65
(5)
:
887-894
.
View Article PubMed Google Scholar -
Xing
L.,
Remick
D.G.,
Mechanisms of dimethyl sulfoxide augmentation of IL-1 beta production. J Immunol.
2005;
174
(10)
:
6195-6202
.
View Article PubMed Google Scholar -
Wen
J.,
Low Concentration DMSO Stimulates Cell Growth and In vitro Transformation of Human Multiple Myeloma Cells British. Journal of Medicine & Medical Research.
2014;
5
(1)
:
65-74
.
View Article Google Scholar -
Liu
Z.,
Preparation of botanical samples for biomedical research. Endocr Metab Immune Disord Drug Targets.
2008;
8
(2)
:
112-121
.
View Article PubMed Google Scholar -
Wakabayashi
I.,
Negoro
M.,
Mechanism of inhibitory action of ethanol on inducible nitric oxide synthesis in macrophages. Naunyn Schmiedebergs Arch Pharmacol.
2002;
366
(4)
:
299-306
.
View Article PubMed Google Scholar -
Forman
S.,
The effect of different solvents on the ATP/ADP content and growth properties of HeLa cells. . J Biochem Mol Toxicol.
1999;
13
(1)
:
11-15
.
View Article Google Scholar -
Ly
H.V.,
Longo
M.L.,
The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys J.
2004;
87
(2)
:
1013-1033
.
View Article PubMed Google Scholar -
O
I.L.,
Adaptation of membrane lipids to alcohols. J. Bacteriol.
1976;
125
:
670-687
.
View Article Google Scholar -
Ding
J.,
Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol.
2009;
85
(2)
:
253-263
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 7 (2020)
Page No.: 3855-3859
Published on: 2020-07-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 43946 times
- Download PDF downloaded - 7778 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



