Abstract
Introduction: Aqueous leave extract of Tridax procumbens (AETPL) is reported to improve erectile functions; however, the mechanism is unclear. This study investigates the mechanism involved in the contractile activity of the corpus cavernosum after AETPL treatment of paroxetine-induced erectile dysfunctional adult male Wistar rats.
Methods: A total of 20 male Wistar rats were categorized into four groups of five and treated orally for four weeks: Group 1 (distilled water), Group 2 (paroxetine 10 mg/kg), Group 3 (paroxetine + AETPL 100 mg/kg), and Group 4 (paroxetine + Viagra 0.5 mg/kg). Contractile responses of excised corpus cavernosum strips (CS) were determined in response to acetylcholine (ACh), phenylephrine (PHE), potassium chloride (KCl), and calcium chloride (CaCl2), and after incubation in L-NAME, indomethacin, nifedipine, adenosine, caffeine, nicorandil, and acetovanillone.
Results: The relaxation response (%) of CS to ACh was significantly inhibited in the paroxetine group compared to the AETPL- and the Viagra-co-treated group. Pre-incubation in L-NAME considerably enhanced the percentage relaxation in groups co-treated with AETPL and Viagra. Groups co-treated with AETPL and Viagra significantly inhibited contraction in response to cumulative doses of CaCl2. Contractile responses of CS to cumulative doses of PHE after incubation in caffeine and adenosine were considerably inhibited in groups co-treated with AETPL and Viagra. Similarly, nicorandil (10-4 M) enhanced the percentage relaxation to cumulative doses of ACh (10-9 — 10-5 M) in groups co-treated with AETPL and Viagra. The pre-incubation of CS with acetovanillone (10-4 M) enhanced the percentage relaxation to ACh across groups.
Conclusion: Erectile dysfunction was reversed by AETPL-induced antioxidant/NADPH oxidase inhibitor activity, reduced calcium sensitivity, activation of ATP-sensitive K+ channel, and endothelial Nitric Oxide (NO) release.
Introduction
The Tridax procumbens leaf contains several bioactive phytochemicals that include a rich presence of flavonoids, alkaloids, hydroxycinnamates, tannins, phytosterols, as well as a modest amount of lignans carotenoids1, 2.
There have been extensive reports on the vasorelaxant activities of the Tridax procumbens aqueous leaf extract (AETPL). In normotensive animals, AETPL reduced arterial blood pressure and heart rate in a dose-dependent manner3. AETPL also caused both endothelium-dependent and endothelium-independent vasorelaxant effects in the aortic ring of normotensive rats4. Furthermore, Salahdeen et al.5 reported that calcium-dependent mechanisms are involved in the vasorelaxant activities of AETPL; specifically, non-specific and non-competitive inhibition of calcium influx and mobilization from stores were reported5.
Furthermore, a mechanistic study by Salahdeen et al.6 observed that the stimulation of prostacyclin production and opening of small conductance calcium-activated potassium channels are involved in the vasorelaxant activities of AETPL in normotensive rats. In hypertensive rats, treatment with AETPL significantly reduced arterial blood pressure in Nitric Oxide (NO) synthase inhibitor (L-NAME)-induced hypertensive male rats7.
The ability of the Tridax procumbens leaf extract to significantly reduce arterial blood pressure in hypertensive rats opened a vista in the mechanistic study of the vasorelaxant activities of AETPL. The prospect of Tridax procumbens leaf extract as an antihypertensive agent prompted us to investigate its effect on erectile tissues and reproductive functions. The relationship between hypertension/endothelial dysfunction and erectile dysfunction is well-established8. A previous study had reported a concentration-dependent relaxant effect by the ethanol extract of Tridax procumbens leaf in rats’ corpus cavernosum9. The relaxation was proposed to be mediated by NO release9. NO release was suggested in the study because pretreatment with L-NAME did not completely inhibit the relaxant effect of Tridax procumbens.
Recently, we reported10 that AETPL treatment in L-NAME-induced hypertensive male rats reduced blood pressure and attenuated reproductive function impairments. Fertility success was 100%, and sperm count, motility, viability, and serum testosterone were enhanced. Furthermore, AETPL treatment in L-NAME-induced hypertensive male rats promoted the relaxant effect on isolated corpus cavernosum strips (CS)11. It was observed that phenylephrine (PHE), potassium chloride (KCl), and calcium chloride (CaCl2)-mediated contraction were substantially inhibited in the corpus cavernosum. Relaxant responses to sodium nitroprusside and acetylcholine (ACh) were also much improved. It was suggested in the study that the relaxant activity of AETPL may act via NO donor and its strong antioxidant properties. Drugs that could increase NO donor/synthesis in cavernosal bodies are of interest in erectile dysfunction management and treatment12.
The preliminary prospect shown by AETPL in attenuating impaired contractile functions in hypertensive rats provided us with the impetus to presently investigate the ability of AETPL to reverse erectile dysfunction (and what the probable mechanisms involved in this could be) specifically. This study analyzes the mechanism of AETPL-induced contractile activity of the corpus cavernosum in paroxetine-induced erectile dysfunctional male rats. Nicorandil (mediator of corpus cavernosum relaxation by KATP channel opening action and NO donor activity), adenosine (endogenous vasodilator in penile erection), acetovanillone (NADPH oxidase inhibitor), nifedipine (L-type calcium channel inhibitor), indomethacin (non-specific COX inhibitors), and caffeine (non-selective inhibitor of phosphodiesterase five inhibitor [PDE]) were used. This was at further elucidating the relaxant mechanisms of AETPL. Paroxetine induces erectile dysfunction13 by inhibiting NO synthase (NOS) activity and NO synthesis. NO is a key mediator in penile erection14. Viagra (sildenafil citrate), used as a reference drug in this study, is a PDE used in the treatment of ED15. PDEs are predominantly expressed in the corpus cavernosum. They (through the NO/cGMP signaling pathway) restrain smooth muscle cell relaxation and subsequently inhibit erectile processes15.
Methods
Animals, acclimatization, and ethics approval
A total of 20 adult male Wistar albino rats (150 – 200 g) were used. The animals were acclimatized for two weeks under conventional laboratory conditions (12-hour light–dark cycle at 18 – 26 ℃ and relative humidity of 30% — 70%) and allowed free access to a standard pellet diet (Ladokun Feeds Nigeria Ltd.) and water. Standard procedure (NIH guide) for the safety and use of laboratory animals was adhered to throughout the study. The procedures for animal use were also certified by the Lagos State University College of Medicine Animal Ethics Committee.
Collection and aqueous extraction from Tridax procumbens leaves
Tridax procumbens leaves were collected from Lagos State University College of Medicine Ikeja, Lagos State, Nigeria, between September and October 2019. The sample of the plant was authenticated by a certified taxonomist. Leaves of Tridax procumbens were air-dried and then blended into a fine powder, then 1,000 g of this powder was placed in a conical flask containing 1,000 mL of distilled water. The mixture was shaken thoroughly and then allowed to sit for 72 hours. The filtrate was then obtained using Whatman filter paper and concentrated by evaporation in a water bath (35 – 40 ℃)8. The yield for the extraction was 35% of a light-brown powdery extract.
Experimental design and treatment
There were four groups of five male rats each for this study. Group 1 (control) received distilled water, while groups 2, 3, and 4 received 10 mg/kg paroxetine16. However, groups 3 and 4 were co-treated with 100 mg/kg AETPL10 and 0.5 mg/kg Viagra17, respectively. All treatments were performed daily via oral gavage for four weeks. The body weights of the animals were monitored using an HCB 1002 scale (Adams Equipment, UK).
Drugs and chemicals for experiments
The paroxetine was manufactured by Medreich PLC, UK, and the Viagra was manufactured by Zim Laboratories Ltd., Nagpur, India. PHE and CaCl2 were purchased from Tocris, UK. N-nitro-l-arginine methyl ester (L-NAME), acetovanillone, and nicorandil were purchased from AK Scientific, Inc., CA, USA. Adenosine, ACh, KCl, and caffeine were purchased from Research Chemicals Ltd., Heysham, Lancs, UK. Nifedipine was bought from Unicure Pharmaceutical Co. Ltd., Lagos, Nigeria, and indomethacin from Jiangxi Pharmaceutical Co., Ltd., Jiangxi, China.
Animal sacrifice and preparation of corpus cavernosum strips
Animals were anesthetized with sodium pentobarbital (30 mg/kg) before being sacrificed by cervical dislocation. The penis was surgically removed as a whole and carefully placed in a petri-dish containing physiological salt solution (PSS). The corpus cavernosum tissue was then isolated and carefully dissected from the surrounding tunica albuginea as previously described11. The CSs were suspended in a 50-ml chamber of organ bath. The chamber contains PSS consisting of the following in m/mol: KCl (0.35); KH2PO4 (0.16), MgSO47H2O (0.29), NaCl (6.96), CaCl2 (0.24), NaHCO3 (2.1), and D-Glucose (2.1). One end of the cavernosal strips were anchored in the organ chamber and the other end to a force isometric transducer (model 7004; Ugo-Basile Varese, Italy), which was, in turn, connected to a Data Capsule Acquisition System Model 17,400 for recording isometric contractions.
Contractile study on cavernous tissue strips
Contractile responses of Corpus cavernosum strips to cumulative doses of PHE, KCl, ACh, and CaCl2
The tissue was allowed to stabilize in the physiological solution for 90 minutes, during which it was stimulated three times at 30-minute intervals with PHE (10−7). Contractile responses of the CS from each group to cumulative doses of ACh (10−9 — 10−5 M), PHE (10−9 — 10−5 M), and KCl were determined. Contractile responses to CaCl2 were determined by adding CaCl2 cumulatively (10 – 50 LogM) to the calcium-free solution in the organ chamber. The responses were at a steady level before the addition of another dose. Tissues were washed three times between each drug administration.
Contractile responses of corpus cavernosum strips after pre-incubation
Statistical analysis and data representation
Data were analyzed using Prism GraphPad (version 5) statistical software. Data were presented as mean ± standard error of the mean (SEM). One-way ANOVA with Neuman-Keuls posthoc-test was determined, and statistical significance was taken at p < 0.05.
Results
Contractile responses of cavernosal strips to cumulative doses of PHE, KCl, ACh, and CaCl2
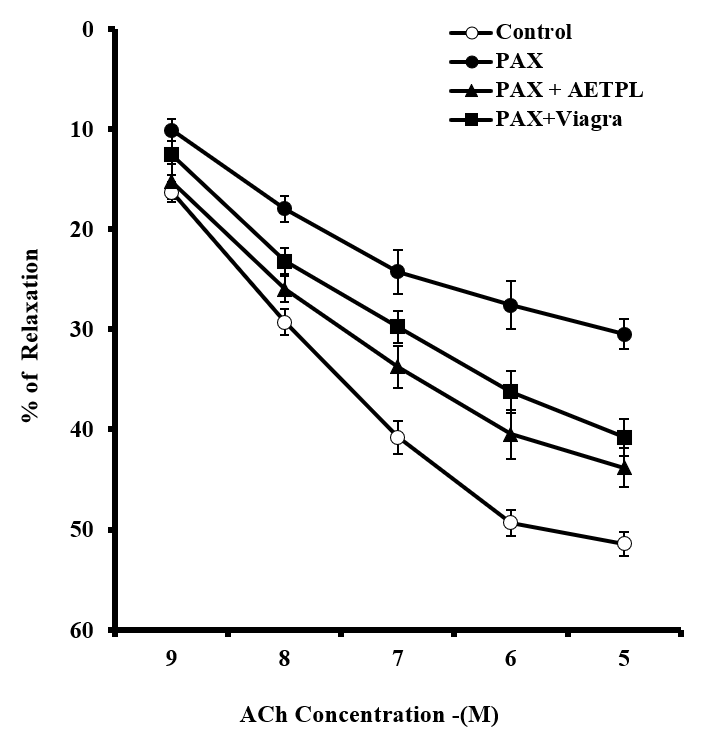
Group co-treated with AETPL showed a significant and dose-dependent increase in contractile responses to PHE (18.7%, 39.7%, 56.2%, 79.1%, 92.8%) and KCl (20%, 40.2%, 61.4%, 76%, 86.4%, 89.4%) (Figure 1). However, contraction responses were significantly inhibited in the paroxetine-only treated group (7.7%, 15.6%, 24.9%, 33.2%, 37.7% for PHE and 8.4%, 9.6%, 10.6%, 12.8%, 15.4%,17.6% for KCl). Furthermore, the percentage relaxation responses to ACh were considerably inhibited in the paroxetine-only group (7.7%,15.6%,24.9%,33.2%, 37.7%) compared to the AETPL-co-treated (9.5%, 22.8%, 34.2%, 44.1%, 46.8%) and the Viagra-co-treated groups (10.5%, 28%, 38%, 45%, 50%) (Figure 2A). Contractile responses to cumulative doses of CaCl2 (10 – 50 M) were significantly inhibited in groups co-treated with AETPL (6.2%, 9.6%, 13.1%, 15.3%, 21.2%) and Viagra (11.3%, 19.1%, 28.1%, 34.7%, 36.5%) compared to the one treated with paroxetine alone (13.1%, 27.1%, 39.7%, 54.3%, 65%) (Figure 2B).
Contractile responses of corpus cavernosum strips after pre-incubation
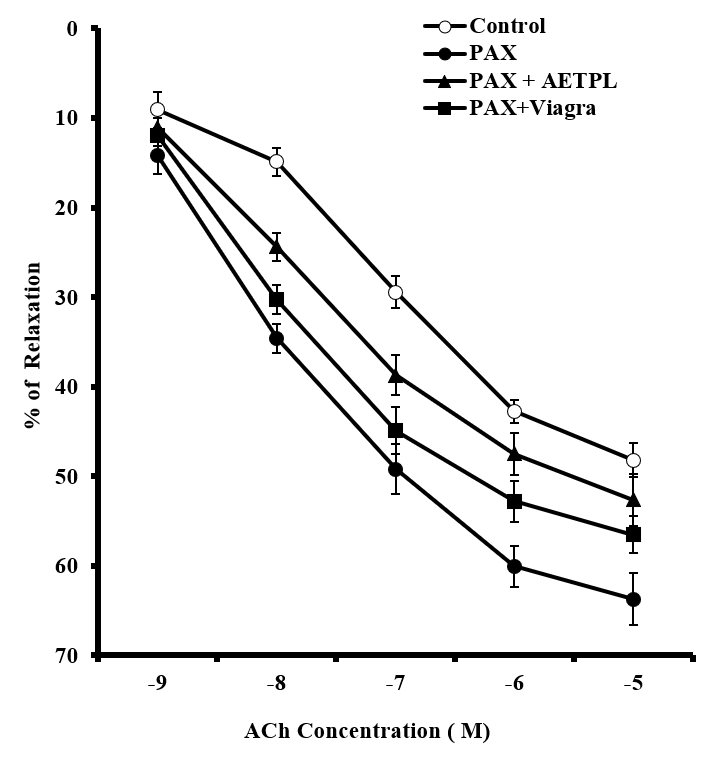
Pre-incubation in L-NAME (Figure 3) and indomethacin (Figure 4) significantly enhanced percentage relaxation in groups co-treated with AETPL (25.5% and 43.8% in L-NAME and indomethacin, respectively) and Viagra (30.1% and 40.8% for L-NAME and indomethacin, respectively), compared to the paroxetine-only treated group (9.6% and 30.5% for L-NAME and indomethacin, respectively). Nevertheless, pre-incubation with nifedipine failed to enhance the percentage relaxation in groups co-treated with AETPL (1.2%, 6%, 10.2%, 13.2%, 15.3%) and Viagra (2.5%,11.1%, 15.5%, 18.9%, 21.1%) (Figure 5).
The incubation of cavernosal strips in caffeine (Figure 6) and adenosine (Figure 7) resulted in significant inhibition of contractile activity of the strips to cumulative doses of PHE (10−9 — 10−5 M) in AETPL-co-treated (5.7%, 11%, 14.5%, 20.3%, 22%, and 8%, 14.2%, 25.4%, 31.2%, 40.6% for caffeine and adenosine, respectively) and the Viagra co-treated groups (6.3%, 17.2%, 21.5%, 26%, 28.8% and 10.2%, 19.2%, 34%, 45.4%, 50.8% for caffeine and adenosine, respectively) compared to the paroxetine-only group (17.5%, 40.5%, 69.7%, 83%, 91% and 19.2%, 48.6%, 67.8%, 75.2%, 80.2% for caffeine and adenosine, respectively).
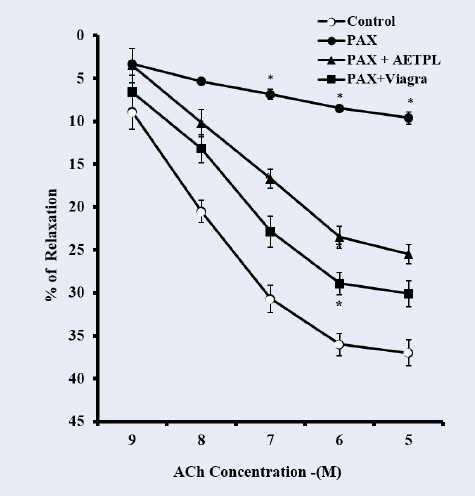
As shown in Figure 8, the pre-incubation of cavernosal strips with nicorandil (10−4 M) enhanced the percentage relaxation to cumulative doses of ACh (10−9 — 10−5 M) in groups co-treated with AETPL (9.4%, 20.4%, 29.2%, 39.4%, 43.1%) and Viagra (8.7%, 23.5%, 34.5%, 48.4%, 57.7%). However, this was significantly reduced in the paroxetine-only treated group (9.3%, 17.2%, 23.2%, 30.3%, 35.6%) (Figure 8). Pre-incubation of cavernosal strips with acetovanillone (10−4 M) enhanced the percentage relaxation to cumulative doses of ACh (10−9 — 10−5 M) across groups (paroxetine-only 63.7%, AETPL-co-treated 52.6%, and Viagra-co-treated 56.5%) (Figure 9).
Discussion
The thrust of this study was to elucidate the mechanisms involved in the reversal of erectile dysfunction by AETPL treatment in adult male rats. This study revealed key mechanisms involved in the reversal of paroxetine-induced erectile dysfunction of the corpus cavernosum by AETPL.
KCl, PHE, ACh, and NADPH oxidase inhibitor activity
Inhibited/impaired contractile activity to PHE and KCl was observed in the cavernous strips of the group treated with paroxetine alone. Co-treatment with AETPL was able to reverse the inhibition to contractile activity in response to PHE and KCl (Figure 1). Likewise, ACh-induced relaxation was most repressed in paroxetine-only treated rats compared to groups co-treated with AETPL (Figure 2 A). Nonetheless, the incubation of cavernous strips in apocynin/acetovanillone, which is a free radical scavenger and NADPH oxidase inhibitor18, was able to enhance the cavernous strips’ relaxation responses to cumulative doses of ACh in all groups (Figure 9). Ordinarily, alpha-adrenergic receptor agonist (PHE) and high K+19 are known to enhance contractile activities in the corpus cavernosum. However, the damage that was done by the paroxetine treatment impaired this function as observed in this study, and co-treatment with AETPL was able to reverse the inhibition and significantly enhance the cavernous strips’ contractile activity in response to these agonists. Earlier, we observed a similar protective role where AETPL treatment was able to attenuate impaired cavernous strips contractile activity in L-NAME-induced hypertensive male rats11.
The key pathophysiology in erectile dysfunction is the reported role of oxidative stress in causing endothelial dysfunction in erectile tissues20. Paroxetine is reported to cause erectile dysfunction by inhibiting the activity of NOS. Results from this study highlight paroxetine-induced damage in endothelial, smooth muscle functions by the generation of reactive oxygen species (ROS). However, we suggest this was mopped up in the CS during the incubation with apocynin and may be responsible for the comparable relaxation responses in the paroxetine-only, the AETPL-co-treated, and the Viagra co-treated groups after incubation in apocynin (Figure 9). Viagra can prevent erectile tissue damage as shown in this study. It has been earlier reported that Viagra prevented endothelial dysfunction in cavernous tissues21 and vardenafil; an analog was reported to reverse erectile dysfunction induced by paroxetine in rats22. In the AETPL-co-treated group, however, we speculate that the strong antioxidant properties of AETPL offered protection against erectile tissue damage. Substantial antioxidants, such as quercetin, Vitamins E & C, flavonoids, and linoleic acid, have been reported present in AETPL1, 2.
L-type calcium channel, NOS, and COX inhibitors activity
The incubation of cavernous strips in nifedipine (Figure 5) failed to enhance the relaxation of cavernous strips to cumulative doses of ACh, which suggests that L-type calcium channel inhibitor activity only partly contributes to the relaxant activity of AETPL. Nevertheless, contraction in response to extracellular calcium influx was significantly inhibited in the AETPL-co-treated group (Figure 2 B). The ability of AETPL to stimulate non-competitive and non-specific inhibition of calcium influx and mobilization from stores has been reported previously for rats’ aorta5. Preliminary results from this study indicate that a similar mechanism may be at play in the corpus cavernosum. This suggests a definitive yet fully unclear role for calcium in the relaxant activity of AETPL in the corpus cavernosum.
The incubation of cavernous strips in NOS inhibitor (L-NAME) did not adversely impair the percentage of relaxation response to ACh in groups co-treated with AETPL and Viagra (Figure 3). Relaxation to ACh was significantly inhibited in the paroxetine-only treated group after L-NAME incubation. Defective NO activity is reported to play a role in the pathogenesis of erectile dysfunction23. The reduced relaxation in the paroxetine-only treated group is obvious due to reported inhibition to NOS and expression of nNOS by paroxetine14. The ability of the AETPL-co-treated group to a show comparable relaxation response, like the Viagra-co-treated group, suggests that it could have enhanced the activity of NOS as reported for Viagra21. It is also rational to posit that AETPL acted as a NO donor as previously suggested9. In the recent past, drugs that increase NO synthesis in cavernosal bodies were of great interest for ED treatment. One of such ruthenium, was reported to mediate relaxation in rabbit corpus cavernosum smooth muscle by its NO release activity24.
The incubation of cavernous strips from all the groups in cyclooxygenase inhibitor (indomethacin) resulted in a relaxation of 43% in the AETPL-co-treated group compared to 30% in the paroxetine-only treated group (Figure 4). Indomethacin is a non-steroidal anti-inflammatory drug. Tridax procumbens leaf has also recently bee reported to reduce the expression of inflammatory mediators25. It can be safely assumed that treatment with AETPL coupled with incubation in indomethacin synergistically inhibited inflammation and the production of inflammatory markers. Although inflammatory markers were not determined in this study, it will be worthwhile to investigate their role in AETPL-mediated activity in cavernous tissues.
KATP channel and non-selective phosphodiesterase activity
In this study, cavernous strips from the Viagra-co-treated and the AETPL-co-treated groups showed comparably enhanced relaxation to cumulative doses of ACh after incubation in nicorandil. However, this was absent for the paroxetine-only treated group (Figure 8). The comparable relaxation effects shown in the AETPL-co-treated and the Viagra-co-treated groups indicate the likelihood that they both share relaxation mechanisms in the corpus cavernosum. Another plausible postulate is that AETPL treatment will not interfere, but rather, potentiate relaxant activity as mediated by nicorandil. Besides, nicorandil is reported to effectively relax the corpus cavernous by stimulating KATP channels and soluble guanylyl cyclase26. Recently, nicorandil is also suggested to be a NO donor27. Viagra-induced prevention of endothelial dysfunction by activation of ATP-sensitive K+ channels, similar to what nicorandil does in cavernous tissue, is reported by Gori et al.21. Furthermore, Viagra is known to activate NOS to produce NO and guanylyl cyclase to enhance relaxation.
It was also observed in this study that pre-incubation in caffeine was able to significantly inhibit PHE-induced contractile activity in the corpus cavernosum of AETPL and Viagra co-treated groups (Figure 6). Caffeine is a non-selective inhibitor of phosphodiesterases (PDEs)28. It was also reported to upregulate cGMP29 and decrease Ca2+ influx and sensitivity in the corpus cavernosum. Adeniyi and Adaikan19 had earlier suggested a caffeine-induced decrease in Ca2+ influx and sensitivity in the cavernous contractile activity after infusion of noradrenaline. The particular caffeine-mediated mechanism potentiated by AETPL is currently unknown. However, the inhibition of calcium influx and reduced calcium sensitivity had earlier been reported for AETPL relaxant action in rats’ aorta5. We also reported in this study that increasing the concentration of Ca2+ in the calcium-free solution inhibited contractile activity in the corpus cavernosum of the AETPL-co-treated group (Figure 2 B).
Adenosine mediated and hyperpolarization activity
Incubation of corpus cavernosum strips in adenosine significantly inhibited contraction in response to cumulative doses of PHE in the AETPL-co-treated group (Figure 7). Adenosine via adenosine receptor signaling is known to decrease cAMP, which leads to hyperpolarization and K+ efflux, which, in turn, inhibit the Ca2+ current, resulting in relaxation29. According to the results of this study, AETPL treatment did not impair the adenosine-mediated mechanism involved in erectile tissue relaxation. Moreover, the adenosine pathway may have contributed to the AETPL-induced reversal of erectile dysfunction in the AETPL-co-treated group. Phatarkar et al.30 observed that erectile dysfunction is mostly present in impaired adenosine signaling, and Wen and Xia31 have reported that adenosine is a key endogenous vasodilator in the maintenance of an erection.
The fact that we were not able to identify the specific constituent/constituents in AETPL that is/are responsible for the reversal of erectile dysfunction in this study is a limitation. We earlier reported the presence of octadecatrienoic acid (65.57%), octadecadienal (9.47%), chloropropionic acid, octadecyl ester (9.22%), and butyl 9,12-octadecadienoate (8.76%) in AETPL after gas chromatography and molecular spectrometry (GC-MS) analysis6. The octadecatrienoic acid in AETPL is linolenic acid, for which dilatory properties have been reported in the literature6. We believe its rich presence in AETPL contributed to the relaxant activities of AETPL in cavernosal tissues in this study. Furthermore, preliminary observations from this study are indicative of AETPL’s potential in reversing erectile dysfunction. It is instructive to note that Tridax procumbens plant belongs to the Asteraceae family, and the Asteraceae family has long been used in Persian medicine Nimrouzi et al.,32 in treating impotence and erectile dysfunction.
Conclusions
This study found that AETPL was able to reverse paroxetine-induced erectile dysfunction in the corpus cavernosum of rats. This could be mediated by AETPL-induced antioxidant/NADPH oxidase inhibitor activity, reduced sensitivity to calcium, activation of ATP-sensitive K+ channel, and endothelial NO release.
Abbreviations
Ach: Acetylcholine
AETPL: Aqueous extract of Tridax procumbens leaf
CaCl2: Calcium chloride
cAMP: cyclic adenosine monophosphate
cGMP: cyclic guanylyl monophosphate
CS: cavernous strip
ED: Erectile dysfunction
eNO: endothelia nitric oxide
KATP: adenosine triphosphate sensitive potassium channel
KCl: Potassium chloride
L-NAME: N-nitro-L-arginine methyl ester
NADPH: Nicotinamide adenine dinucleotide phosphate hydrogenase
NIH: National Institute of Health
nNO: neuronal nitric oxide
NO: Nitric oxide
NOS: nitric oxide synthase
PDE: Phosphodiesterase
PHE: Phenylephrine
Acknowledgments
Authors acknowledge the assistance of Shuaib and Segun.
Author’s contributions
Conceptualization: SAS, Methodology: SAS & HMS, Software: SAS, HMS & BAM, Validation: SAS, HMS, Formal Analysis: SAS, HMS & BAM, Investigation: ZAB, BAM, Resources: SAS, HMS, ZAB, Data Curation: SAS,HMS,ZAB,BAM,YR, Writing – Original Draft: SAS, Writing – Review &, Editing: SAS,HMS,YR,BAM, Supervision: SAS. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Petchi
R.R.,
Vijaya
C.,
Parasuraman
S.,
Antidiabetic activity of polyherbal formulation in streptozotocin - nicotinamide induced diabetic wistar rats. J Tradit Complement Med.
2014;
4
(2)
:
108-17
.
View Article PubMed Google Scholar -
Ikewuchi
C.C.,
Ikewuchi
J.C.,
Ifenacho
M.O.,
Phytochemical composition of Tridax procumbens Linn leaves: potential as a functional food. Food Nutr Sci.
2015;
6
(11)
:
992-1004
.
View Article Google Scholar -
Salahdeen
H.M.,
Yemitan
O.K.,
Alada
A.R.,
Effect of aqueous leaf extract of Tridax procumbens on blood pressure and heart rate in rats. Afr J Biomed Res.
2004;
7
:
27-39
.
-
Salahdeen
H.M.,
Idowu
G.O.,
Murtala
B.A.,
Endothelium-dependent and independent vasorelaxant effects of aqueous extract of Tridax procumbens Lin. leaf in rat aortic rings. Indian J Exp Biol.
2012;
50
(12)
:
883-8
.
PubMed Google Scholar -
Salahdeen
H.M.,
Idowu
G.O.,
Yemitan
O.K.,
Murtala
B.A.,
Alada
A.R.,
Calcium-dependent mechanisms mediate the vasorelaxant effects of Tridax procumbens (Lin) aqueous leaf extract in rat aortic ring. J Basic Clin Physiol Pharmacol.
2014;
25
(2)
:
161-6
.
View Article PubMed Google Scholar -
Salahdeen
H.M.,
Adebari
A.O.,
Murtala
B.A.,
Alada
A.R.,
Potassium channels and prostacyclin contribute to vasorelaxant activities of Tridax procumbens crude aqueous leaf extract in rat superior mesenteric arteries. Afr J Med Med Sci.
2015;
44
(1)
:
5-19
.
PubMed Google Scholar -
Salahdeen
H.M.,
Salami
S.A.,
Paul
C.O.,
Murtala
B.A.,
Alada
A.A.,
Biochemical parameters as indicators of antihypertensive efficacy of leaf aqueous extract of Tridax procumbens (Lin) in L-NAME induced hypertensive rats. Journal of Molecular Pathophysiology.
2017;
6
(2)
:
30-37
.
View Article Google Scholar -
Maas
R.,
Schwedhelm
E.,
Albsmeier
J.,
Böger
R.H.,
The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med.
2002;
7
(3)
:
213-25
.
View Article PubMed Google Scholar -
Salahdeen
H.M.,
Idowu
G.O.,
Yemitan
O.K.,
Murtala
B.A.,
Alada
A.R.,
The relaxant actions of ethanolic extract of Tridax procumbens (Linn.) on rat corpus cavernosum smooth muscle contraction. J Basic Clin Physiol Pharmacol.
2015;
26
(2)
:
211-6
.
PubMed Google Scholar -
Salami
S.A.,
Salahdeen
H.M.,
Rahman
O.C.,
Murtala
B.A.,
Raji
Y.,
Oral administration of Tridax procumbens aqueous leaf extract attenuates reproductive function impairments in L-NAME-induced hypertensive male rats. Middle East Fertil Soc J.
2017;
22
(1)
:
219-25
.
View Article Google Scholar -
Salami
S.A.,
Salahdeen
H.M.,
Ugbebor
E.C.,
Murtala
B.A.,
Raji
Y.,
Effects of aqueous leaf extract of Tridax procumbens on contractile activity of corpus cavernosum in N-nitro-l-arginine methyl ester-induced hypertensive male rats. J Integr Med.
2018;
16
(1)
:
51-6
.
View Article PubMed Google Scholar -
Kalsi
J.S.,
Kell
P.D.,
Cellek
S.,
Ralph
D.J.,
NCX-911, a novel nitric oxide-releasing PDE5 inhibitor relaxes rabbit corpus cavernosum in the absence of endogenous nitric oxide. Int J Impot Res.
2004;
16
(2)
:
195-200
.
View Article PubMed Google Scholar -
Ahn
G.J.,
Kang
K.K.,
Kim
D.S.,
Ahn
B.O.,
Kim
W.B.,
Kang
S.K.,
DA-8159 reverses selective serotonin reuptake inhibitor-induced erectile dysfunction in rats. Urology.
2005;
65
(1)
:
202-7
.
View Article PubMed Google Scholar -
Angulo
J.,
Peiró
C.,
Sanchez-Ferrer
C.F.,
Gabancho
S.,
Cuevas
P.,
Gupta
S.,
Differential effects of serotonin reuptake inhibitors on erectile responses, NO-production, and neuronal NO synthase expression in rat corpus cavernosum tissue. Br J Pharmacol.
2001;
134
(6)
:
1190-4
.
View Article PubMed Google Scholar -
Andersson
K.E.,
Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev.
2011;
63
(4)
:
811-59
.
View Article PubMed Google Scholar -
Yakubu
M.T.,
Jimoh
R.O.,
Carpolobia lutea roots restore sexual arousal and performance in paroxetine-induced sexually impaired male rats. Rev Int Androl.
2014;
12
(3)
:
90-9
.
View Article Google Scholar -
Dare
A.,
Salami
S.A.,
Kunle-Alabi
O.T.,
Akindele
O.O.,
Raji
Y.,
Comparative evaluation of the aphrodisiac efficacy of sildenafil and Carpolobia lutea root extract in male rabbits. J Intercult Ethnopharmacol.
2015;
4
(4)
:
302-7
.
View Article PubMed Google Scholar -
Li
M.,
Zhuan
L.,
Wang
T.,
Rao
K.,
Yang
J.,
Yang
J.,
Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression. J Sex Med.
2012;
9
(12)
:
3041-50
.
View Article PubMed Google Scholar -
Adebiyi
A.,
Adaikan
P.G.,
Effect of caffeine on response of rabbit isolated corpus cavernosum to high K+ solution, noradrenaline and transmural electrical stimulation. Clin Exp Pharmacol Physiol.
2004;
31
(1-2)
:
82-5
.
View Article PubMed Google Scholar -
Jeremy
J.Y.,
Jones
R.A.,
Koupparis
A.J.,
Hotston
M.,
Persad
R.,
Angelini
G.D.,
Reactive oxygen species and erectile dysfunction: possible role of NADPH oxidase. Int J Impot Res.
2007;
19
(3)
:
265-80
.
View Article PubMed Google Scholar -
Gori
T.,
Sicuro
S.,
Dragoni
S.,
Donati
G.,
Forconi
S.,
Parker
J.D.,
Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: a human in vivo study. Circulation.
2005;
111
(6)
:
742-6
.
View Article PubMed Google Scholar -
Angulo
J.,
Bischoff
E.,
Gabancho
S.,
Cuevas
P.,
Sáenz de Tejada
I.,
Vardenafil reverses erectile dysfunction induced by paroxetine in rats. Int J Impot Res.
2003;
15
(2)
:
90-3
.
View Article PubMed Google Scholar -
Sullivan
M.E.,
Thompson
C.S.,
Dashwood
M.R.,
Khan
M.A.,
Jeremy
J.Y.,
Morgan
R.J.,
Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease?. Cardiovasc Res.
1999;
43
(3)
:
658-65
.
View Article PubMed Google Scholar -
Cerqueira
J.B.,
Gonzaga-Silva
L.F.,
Silva
F.O.,
Cerqueira
J.V.,
Oliveira
R.R.,
Moraes
M.E.,
Identification of mechanisms involved in the relaxation of rabbit cavernous smooth muscle by a new nitric oxide donor ruthenium compound. Int Braz J Urol.
2012;
38
(5)
:
687-94
.
View Article PubMed Google Scholar -
Grace
V.M. Berlin,
Viswanathan
S.,
Wilson
D.D.,
Kumar
S.J.,
Sahana
K.,
Arbin
E.F. Maria,
Significant action of Tridax procumbens L. leaf extract on reducing the TNF‑α and COX‑2 gene expressions in induced inflammation site in Swiss albino mice. Inflammopharmacology.
2019;
28
:
929-938
.
View Article PubMed Google Scholar -
Hsieh
G.C.,
Kolasa
T.,
Sullivan
J.P.,
Brioni
J.D.,
Dual mechanism of action of nicorandil on rabbit corpus cavernosal smooth muscle tone. Int J Impot Res.
2001;
13
(4)
:
240-6
.
View Article PubMed Google Scholar -
Tarkin
J.M.,
Kaski
J.C.,
Nicorandil and Long-acting Nitrates: Vasodilator Therapies for the Management of Chronic Stable Angina Pectoris. Eur Cardiol.
2018;
13
(1)
:
23-8
.
View Article PubMed Google Scholar -
Corbin
J.D.,
Francis
S.H.,
Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction. J Androl.
2003;
24
(6)
:
38-41
.
View Article PubMed Google Scholar -
Yang
R.,
Wang
J.,
Chen
Y.,
Sun
Z.,
Wang
R.,
Dai
Y.,
Effect of caffeine on erectile function via up-regulating cavernous cyclic guanosine monophosphate in diabetic rats. J Androl.
2008;
29
(5)
:
586-91
.
View Article PubMed Google Scholar -
Phatarpekar
P.V.,
Wen
J.,
Xia
Y.,
Role of adenosine signaling in penile erection and erectile disorders. J Sex Med.
2010;
7
(11)
:
3553-64
.
View Article PubMed Google Scholar -
Wen
J.,
Xia
Y.,
Adenosine signaling: good or bad in erectile function?. Arterioscler Thromb Vasc Biol.
2012;
32
(4)
:
845-50
.
View Article PubMed Google Scholar -
Nimrouzi
M.,
Jaladat
A.M.,
Zarshenas
M.M.,
A panoramic view of medicinal plants traditionally applied for impotence and erectile dysfunction in Persian medicine. J Tradit Complement Med.
2018;
10
(1)
:
7-12
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 8 No 6 (2021)
Page No.: 4405-4416
Published on: 2021-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6273 times
- Download downloaded - 1795 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress