Abstract
The hepatitis E virus (HEV) is an important public health concern and a significant cause of enterically-transmitted viral hepatitis infections. HEV infection remains a serious threat to life, especially in immunocompromised individuals and pregnant women. Globally, vaccines have had a massive impact on public health and saved millions of lives. Vaccination can reduce the healthcare expenditure, decrease the mortality rate, and increase life expectancy. The availability of commercially effective vaccines is the most effective means for the prevention of HEV. However, the development of classic inactive or attenuated HEV vaccines is not feasible due to the lack of an efficient cell culture system for HEV. In recent years, recombinant HEV vaccine approaches have been explored. Many vaccine candidates have showed potential efficacy against HEV infection. Currently, the only licensed vaccine is Hecolin®, a recombinant vaccine developed by Xiamen Innovax Biotech Co., Ltd. It is available in China. However, there are many hindrances when it comes to the acrossthe- board application of Hecolin® and other vaccines worldwide. Large-scale efforts are needed to further evaluate the efficacy and safety of Hecolin® in at-risk populations and to pass the World Health Organization prequalification for licensing outside of China.
Introduction
Hepatitis E infection, caused by the hepatitis E virus (HEV), is the fifth known type of human viral hepatitis and it is considered to be the most common cause of jaundice, acute liver failure, and acute viral hepatitis1, 2, 3, 4, 5, 6. Despite being an important viral pathogen, HEV and its origin remain involves many unanswered questions7, 8. The mechanisms of HEV pathogenesis and its replication are poorly understudied, mostly due to the lack of reliable diagnostic methods7, 8. HEV is common in developing countries, causing small- and large-scale outbreaks. In developed countries, human infections occur mainly through zoonotic transmission1.
Although most HEV infections cause mild hepatitis, infection is usually more severe in pregnant women1. Furthermore, HEV infection during pregnancy often leads to premature births, a low birth weight, and infant death9, 10, 11. In addition, chronic HEV infection has been reported in immunocompromised patients, transplant recipients, and patients receiving chemotherapy12, 13, 14. According to the World Health Organization (WHO), there are an estimated 20 million HEV infections every year globally. Of these cases, approximately 3.3 million develop into symptomatic cases. In 2015, HEV caused an estimated 44,000 deaths worldwide, accounting for 3.3% of the mortality due to viral hepatitis15.
Vaccines have had an enormous impact on public health around the world, saving millions of lives. Vaccination has the potential to reduce healthcare costs, lower death rates, and extend life expectancy16, 17. The critical property of vaccines is to stimulate the immune system against diseases. Some vaccines also protect against infection. Since the 1990s, the immunization/vaccination coverage has increased substantially. As a result, millions of lives have been saved. According to the WHO statistics, vaccines save more than 2.5 million deaths per year18, 19. At present, vaccines are available for more than 30 life-threatening viral and bacterial diseases20, 21. Vaccinations prevent illnesses and death associated with infectious diseases such as diphtheria, influenza, measles, pneumonia, polio, and pertussis/whooping cough22. Several HEV vaccine candidates have progressed into the clinical development stage, and one of them has been approved and licensed only in China23.
This review aims to summarize the current development of HEV vaccines and the key challenges that are a part of vaccine development and deployment.
Transmission
In countries with limited resources, HEV is most frequently transmitted through drinking water contaminated with fecal matter. In Southeast Asia, the number of HEV cases increases during the rainy season24. Nevertheless, case numbers have been increasing in the dry season when the drinking water supplies are not flooded or contaminated25. Furthermore, the low levels of household sanitation may also increase the risk of HEV infection26.
In addition, HEV transmission has been reported via blood and nosocomial routes27, 28. It is also well documented that a pregnant woman can transmit HEV to her unborn fetus25, 26, 27, 28, 29. No HEV transmission through sexual intercourse has been documented25, 26, 27, 28, 29.
HEV taxonomy and geographical distribution
HEV is a small (27–34 nm) single-stranded positive-sense RNA virus from the family Hepeviridae and genus Orthohepevirus (Figure 1 A)31, 32. The virus has a genome of about 7.2 kb in length with three open reading frames (ORF1, ORF2, and ORF3) (Figure 1 B)4, 31, 32, 33.
HEV causes infections worldwide but it is more common in low- and middle-income countries with limited access to clean drinking water, acceptable hygiene practices, proper sanitation, and health services. Both sporadic cases and outbreaks have been documented15, 34, 35. HEV cases are commonly observed to have two different patterns: (i) resource-limited regions with frequent water contamination and (ii) regions with safe drinking water supplies. The HEV infections in developed regions are generally triggered by zoonotic transmission, mainly through undercooked pork products15, 36, 37, 38.
A recent study reported that the seroprevalence of HEV among countries in Southeast Asia ranged from 2% (Malaysia) to 77.7% (Lao People's Democratic Republic)39.
Another recent meta-analysis including 1,099,717 subjects from various countries worldwide reported that the HEV IgG and IgM antibody seropositivity was 12.47% (95% CI: 10.42–14.67) and 1.47% (95% CI: 1.14–1.85), respectively30. The highest HEV IgG seropositivity was reported in Africa, followed by Asia, Europe, North America, South America, and Oceania, as presented in Figure 2. In total, the HEV RNA seropositivity in the general population was 0.20% (95% CI: 0.15–0.25)30. The potential key risk factors for HEV IgG antibody positivity were living in rural areas, exposure to soil, the consumption of raw meat, traveling to high disease burden regions, blood transfusion, contact with dogs, and a low level of education30.
HEV vaccine development
After the discovery of HEV in 1981, attempts have been made to develop effective HEV vaccines40, 41. Currently, the advancement of an attenuated or inactivated vaccine is not feasible because HEV cannot be grown reproducibly in cell culture systems. Instead, the development of recombinant protein or nucleic acid-based vaccines has seen significant progress42, 43.
Meanwhile, human papillomavirus and HEV virus-like particle (VLP) vaccines consist of nanoparticles of 30 and 60 nm in diameter that contain only the viral capsid protein and no lipids. For HEV VLPs, the central building block is the pORF2 E2 dimer44, 45.
At least 11 experimental HEV vaccines have been assessed in non-human primates with a viral challenge46. Numerous vaccines have been discovered using a cell culture system for HEV47, 48. The results obtained from a study on avian HEV in chickens suggested that oral vaccination using L. lactis expressing a part of the avian HEV-ORF2 protein can counteract hepatitis and liver injury caused by a HEV infection49. On the other hand, an alternative cell culture system, the A549 cell line that does not require RNA transfection, was found to support the partial replication of genotype-1 HEV from a patient blood serum sample50. The preclinical studies on HEV vaccines are presented in Table 1. The registered clinical trials on HEV vaccines are presented in Table 2.
| Name | ORF2 range | Stage | Expression | Study country | Subjects studied | References |
|---|---|---|---|---|---|---|
| TrpE-C2 | 221–660 | Preclinical study | Prokaryotes | USA | Cynomolgus Macaques | Purdy et al . 51 |
| Bacmid-HEV ORF2 | 126-621 | Preclinical study | Baculovirus-infected | China | Sf9 cells, BALB/c mice | Qi et al . 52 |
| HP/HEV2.3 | 112-607 | Preclinical study | Yeast | China | - | Su et al. 53 |
| HEV ORF2 | 69-600 | Preclinical study | Yeast | China | BALB/c mice | Tong et al . 54 |
| HEV truncated4(aa1-111)-ORF2 | 112-660 | Preclinical study | Vectored vaccine | Tunisia, Spain | Sf9 cells, BHK-21 cells. mice | Trabelsi et al . 55 ; Jiménez et al . 56 |
| tPAsp-PADRE-truncated ORF2 | 112-660 | Preclinical study | Vectored vaccine | Iran | CHO & HEK293 cells | Farshadpour et al . 57 |
| Hepatitis A & E vaccine (HA+ E vaccine) | 439-617 | Preclinical study | Combined and recombinant chimeric vaccine | China | 180 Balb/c mice | Dong et al . 58 |
| HAV-HEp148 | 459-606 | Preclinical study | Combined and recombinant chimeric vaccine | China, Germany | 24 Balb/c mice | Xiang et al . 59 |
| HE-ORF2, HA-VP1, | 368-607 | Preclinical study | Combined and recombinant chimeric vaccine | China | 40 Balb/c mice | Gao et al . 60 |
| HEV-HBsAg | 551-607 | Preclinical study | Combined and recombinant chimeric vaccine | China | Li et al . 61 | |
| GST-NoV P(-)-HEV P | 452-617, | Preclinical study | Combined and recombinant chimeric vaccine | USA | HepG2/3A cells, Balb/c mice | Wang et al . 62 |
| HEV-RV-AstV | 112-607 | Preclinical study | Combined and recombinant chimeric vaccine | Iran | sf9 cell | Makvandi et al . 63 |
| rHEV VLPs | 112-660 | Preclinical study | Oral immunization HEV vaccine | Japan, India | Balb/c mice, Cynomolgus monkeys | Li et al . 64 65 |
| HEV p179 | 439-617 | Phase Ib clinical trial | Prokaryotes | China | Human, mice & monkey | Meng et al . 66 ; Wen et al . 67 ; Dong et al . 68 |
| GSK candidate vaccine [vAc-ORF2(D111/DTM)] | 112-607 | Phase II clinical trial | Baculovirus-infected | India, USA, Nepal | Cynomolgus Macaques, Human, Sf9 cells | Sehgal et al . 69 ; Robinson et al . 70 ; Zhang et al . 71 ; Shrestha et al . 72 |
| Clinical trial title | Clinical trial No. | Country | Status |
|---|---|---|---|
| A clinical trial to evaluate a recombinant hepatitis E vaccine in healthy adults | NCT02603055 | China | Completed |
| A Study on the recombinant hepatitis E vaccine (Escherichia coli) (accelerated vaccination schedule) | NCT03168412 | China | Completed |
| A phase Ⅳ clinical trial of the recombinant hepatitis E vaccine (Escherichia coli) (the lot consistency trial) | NCT03365921 | China | Completed |
| A phase Ⅳ clinical trial of the recombinant hepatitis E vaccine (Escherichia coli) (the chronic hepatitis B patients) | NCT02964910 | China | Completed |
| Clinical trial of recombinant hepatitis E vaccine | NCT01014845 | China | Completed |
| Phase Ⅳ clinical trial of recombinant hepatitis E vaccine (Hecolin®) | NCT02189603 | China | Completed |
| Effectiveness trial to evaluate protection of pregnant women by hepatitis E vaccine in Bangladesh | NCT02759991 | Bangladesh | Completed |
| A safety and efficacy study of the hepatitis E vaccine in Nepal | NCT00287469 | Nepal | Completed |
| A phase Ⅳ clinical trial of the recombinant hepatitis E vaccine (Escherichia coli) (Coadministration with recombinant hepatitis B vaccine) | NCT02584543 | China | Completed |
| Safety study of hepatitis E vaccine (HEV239) | NCT03827395 | USA | Completed |
| Immunogenicity study of the recombinant human Papillomavirus virus type 6/11 bivalent vaccine | NCT02710851 | China | Active |
| Vaccine | Company | Clinical trials | Efficacy | Reference |
|---|---|---|---|---|
| HEV vaccine p239 Hecolin® | Xiamen Innovax Biotech Co., Ltd, China | Phase III | 100% received all 3 doses 86.8% received at least one dose | Zhu et al . 73 |
| Recombinant HEV (rHEV) vaccine | GlaxoSmithKline Biologicals, Rixensart, Belgium | Phase II | 95.5 % efficacy | Shrestha et al . 72 |
| Hepatitis E virus (HEV) p179 | Changchun Institute of Biological Products Co., Ltd, China | Phase I | Not mentioned. [Deemed safe and well tolerated] | Cao et al . 74 |
HEV vaccine efficacy
Several studies have been conducted to develop HEV vaccines and assess their efficacy, of which only three studies have registered for clinical trials as shown in Table 3. In China, a phase III clinical trial was conducted among participants (n = 112,604) aged 16 – 65 years old. After receiving the complete 3 doses, the Hecolin® HEV p239 vaccine showed 100% (95% CI, 72–100) efficacy. Additionally, the efficacy was 96% (95% CI, 66–99) among the individuals who received at least one dose73. The HEV p239 vaccine is immunogenic and well-tolerated. It induces immunity against HEV infection in the age group older than 65 years74. Another study conducted in Bangladesh among pregnant women showed that the HEV p239 vaccination induces immunogenicity against HEV infection and prevented maternal and neonatal deaths due to HEV infection75.
In Nepal, another clinical trial (898 in the vaccine group and 896 in the placebo group that received three vaccine doses) of a recombinant HEV protein (rHEV) vaccine showed 95.5% (95% CI, 85.6–98.6) efficacy72. The intention-to-treat analysis also strengthens the promise of this vaccine. After administering the first dose of the rHEV vaccine, the estimated efficacy was 88.5 to 89.9%72.
The HEV vaccine p179 showed good safety and tolerance after a phase I clinical trial conducted among participants (n = 120, 16 – 65 years) in China. Three different dosages, 20, 30, and 40 μg of HEV p179 vaccines, were received by the experimental groups with 30 μg HEV vaccine p239 Hecolin® used as a control. Vaccination occurred at 0-, 1-, and 6-month intervals. The incidence of solicited local adverse reactions (ARs) in the experimental groups was significantly lower than in the control group (P = 0.027). However, no significant difference was reported between the incidence of solicited total and systemic ARs in the experimental and control groups. Thus, the tested dosages of the HEV p179 vaccine are well tolerated and safe with no serious adverse reactions76.
The use of two truncated ORF2 proteins (54KDa and 26KDa) as a vaccine showed that they have immunogenicity. It acts as a nanoparticle against HEV infection77. A study carried out in China reported that antibody loss is significantly lower for innate immunity compared to immunity acquired from vaccination after 10 years78. The recombinant VLP-based Hecolin® HEV vaccine available in China acts as a trivalent vaccine. This vaccine produces an immune response against HEV infection, blocks the activity of novavirus binding to histo-blood group antigens, and inhibits astrovirus infection79, 80. The Hecolin® vaccine completed its phase III clinical trial. It has been licensed in China but is not yet available commercially. Furthermore, to launch the HEV vaccine globally, the safety and efficacy data of HEV p239 is needed for high-risk and immunocompromised groups, including pregnant women and individuals with chronic liver disease, HIV, and immune disorders81, 82, 83, 84.
Key challenges in vaccine distribution
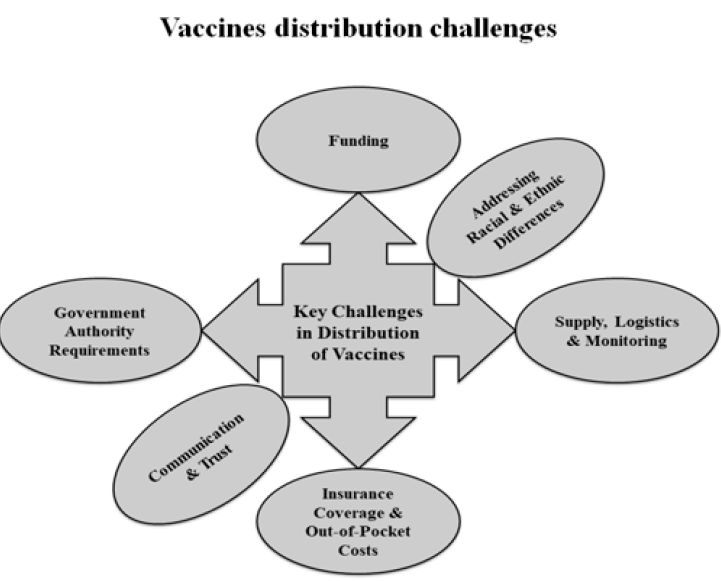
Developing a vaccine is a tremendous challenge, but a considerable challenge will still exist even when one becomes available, specifically getting enough people vaccinated. Misinformation and fear are two of the leading causes of low vaccination coverage. Some of the challenges in the distribution of vaccines are shown in Figure 3. A crucial factor when distributing vaccines is the resource constraints faced by the state or local health departments. Funding issues become severe in developing and undeveloped countries85. Providing a complete number of doses according to the need is itself a challenge. Other logistical issues include monitoring, tracking vaccine safety, identifying a broad network of sites for administration, and ensuring that the cold chain requirements are met85.
Differing rules and regulations across the jurisdictions can also influence the success of vaccine distribution and availability. Covering the vaccine's expenses with insurance enhances the availability of vaccines to individuals. Despite this, limitations remain and some individuals face difficulties accessing the vaccine. Addressing racial and ethnic differences is an unprecedented challenge and has a significant impact on communities. Ensuring the equal and easy availability of a vaccine regardless of any ethnic disparities could improve the success of the vaccine overall. Finally, achieving a high rate of vaccination directly depends on the people’s trust in and willingness to receive the vaccine. To some extent, all vaccines must face the public's confidence and this issue must be overcome through robust communication and trust-building efforts.
Adverse reactions to vaccines
Some common local and systematic reactions such as swelling, pain, irritability, drowsiness, rashes, or fever have been detected after vaccination85. Most commonly, erythema at the injection site is reported, but using a longer needle (25 mm vs. 16 mm) may decrease the prevalence of injection-site reactions85, 86. Some collaborations have been established to monitor the adverse reactions of vaccines. Vaccine Safety Datalink, a collaboration between the Centers for Disease Control and Prevention and nine health care organizations, was established in 1990 to investigate rare and serious adverse effects of vaccines. The Vaccine Adverse Event Reporting System also monitors vaccine safety for newly approved or recommended vaccines87, 88. When considering recommended childhood vaccines, many parents are more concerned about the theoretical risks and real effects89.
Some vaccines, such as the measles vaccine, are associated with allergic reactions90. The varicella vaccine and MMR vaccine are associated with rashes, which appear between 9 to 16 days after vaccination91.
Conclusions
There is a pressing need for a globally available HEV vaccine. Routine vaccination should be implemented in countries with endemic HEV with a special emphasis on pregnant women and immunocompromised individuals. Improving access to clean drinking water and the sanitary disposal of human waste are the two most critical strategies to prevent HEV infections. Further understanding of the exact HEV burden in endemic areas would assist in developing a vaccination policy if a commercially HEV licensed vaccine becomes available worldwide. However, in most underdeveloped countries where HEV is a leading cause of acute viral hepatitis, there is a lack of epidemiological data. The endemic countries need to begin monitoring viral hepatitis and stress etiological rather than syndrome diagnosis to benefit from the vaccine optimally.
HIGHLIGHTS
ABBREVIATIONS
CDC: Centers for Disease Control and Prevention
FDA: Food and Drug Administration
HEV: Hepatitis E virus
HBGAs: Histo-blood group antigens
HPV: Human papillomavirus
ORF: Open reading frame
rHEV: HEV recombinant protein
VAERS: Vaccine Adverse Event Reporting System
VLPs: Virus-like particles
US: United States
WHO: World Health Organization
ACKNOWLEDGMENTS
The authors acknowledge their respective universities.
AUTHOR’S CONTRIBUTIONS
Tauseef Ahmad: Conceptualization, data collection and writing-original draft preparation. All the authors potentially contributed, and approved the final version for publication.
FUNDING
This review received no financial support from any government or private sector.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
References
-
Goel
A.,
Aggarwal
R.,
Hepatitis
E.,
Hepatitis E: Epidemiology, Clinical Course, Prevention, and Treatment. Gastroenterol Clin North Am.
2020;
49
(2)
:
315-30
.
View Article PubMed Google Scholar -
Krawczynski
K.,
Foreword. Hepatitis E virus. Semin Liver Dis.
2013;
33
(1)
:
1-2
.
View Article PubMed Google Scholar -
Kamar
N.,
Bendall
R.,
Legrand-Abravanel
F.,
Xia
N.S.,
Ijaz
S.,
Izopet
J.,
Hepatitis E. Lancet.
2012;
379
(9835)
:
2477-88
.
View Article PubMed Google Scholar -
Hoofnagle
J.H.,
Nelson
K.E.,
Purcell
R.H.,
Hepatitis
E.,
Hepatitis E. N Engl J Med.
2012;
367
(13)
:
1237-44
.
View Article PubMed Google Scholar -
Chandra
N.S.,
Sharma
A.,
Malhotra
B.,
Rai
R.R.,
Dynamics of HEV viremia, fecal shedding and its relationship with transaminases and antibody response in patients with sporadic acute hepatitis E. Virol J.
2010;
7
(1)
:
213
.
View Article PubMed Google Scholar -
Purcell
R.H.,
Emerson
S.U.,
Hepatitis E: an emerging awareness of an old disease. J Hepatol.
2008;
48
(3)
:
494-503
.
View Article PubMed Google Scholar -
Meng
X.J.,
From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res.
2011;
161
(1)
:
23-30
.
View Article PubMed Google Scholar -
Guerra
J.A.,
Kampa
K.C.,
Morsoletto
D.G.,
Junior
A.P.,
Ivantes
C.A.,
Hepatitis E: a literature review. J Clin Transl Hepatol.
2017;
5
(4)
:
376-83
.
View Article PubMed Google Scholar -
Bergl∅v
A.,
Hallager
S.,
Weis
N.,
Hepatitis E during pregnancy: maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J Viral Hepat.
2019;
26
(11)
:
1240-8
.
View Article PubMed Google Scholar -
Chaudhry
S.A.,
Verma
N.,
Koren
G.,
Hepatitis E infection during pregnancy. Can Fam Physician.
2015;
61
(7)
:
607-8
.
PubMed Google Scholar -
Berkane
N.,
Liere
P.,
Oudinet
J.P.,
Hertig
A.,
Lefèvre
G.,
Pluchino
N.,
From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr Rev.
2017;
38
(2)
:
123-44
.
View Article PubMed Google Scholar -
Dalton
H.R.,
Bendall
R.P.,
Keane
F.E.,
Tedder
R.S.,
Ijaz
S.,
Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med.
2009;
361
(10)
:
1025-7
.
View Article PubMed Google Scholar -
Kamar
N.,
Selves
J.,
Mansuy
J.M.,
Ouezzani
L.,
Péron
J.M.,
Guitard
J.,
Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med.
2008;
358
(8)
:
811-7
.
View Article PubMed Google Scholar -
Geng
Y.,
Zhang
H.,
Huang
W.,
J Harrison
T.,
Geng
K.,
Li
Z.,
J Harrison T, Geng K, Li Z, Wang Y. Persistent hepatitis E virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat Mon.
2014;
14
(1)
:
e15618
.
View Article PubMed Google Scholar -
World health Organization (WHO). Hepatitis E. WHO. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on February 3, 2021). .
.
-
Andre
F.E.,
Booy
R.,
Bock
H.L.,
Clemens
J.,
Datta
S.K.,
John
T.J.,
Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ.
2008;
86
(2)
:
140-6
.
View Article PubMed Google Scholar -
Wang
Y.B.,
Wang
L.P.,
Li
P.,
Perspectives on novel vaccine development. Pol J Vet Sci.
2018;
21
(3)
:
643-9
.
View Article PubMed Google Scholar -
Rappuoli
R.,
Pizza
M.,
Del Giudice
G.,
De Gregorio
E.,
Vaccines, new opportunities for a new society. Proc Natl Acad Sci USA.
2014;
111
(34)
:
12288-93
.
View Article PubMed Google Scholar -
Mascola
J.R.,
Fauci
A.S.,
Novel vaccine technologies for the 21st century. Nat Rev Immunol.
2020;
20
(2)
:
87-8
.
View Article PubMed Google Scholar -
Nabel
G.J.,
Designing tomorrow's vaccines. N Engl J Med.
2013;
368
(6)
:
551-60
.
View Article PubMed Google Scholar -
World Health Organization. Immunization. World Health Organization. 2019. Available from: https://www.who.int/news-room/facts-in-pictures/detail/immunization (accessed on September 3, 2021)..
.
-
World Health Organization. Vaccines and immunizations. World Health Organization. Available from: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on February 3, 2021)..
.
-
Li
Y.,
Huang
X.,
Zhang
Z.,
Li
S.,
Zhang
J.,
Xia
N.,
Prophylactic Hepatitis E Vaccines: Antigenic Analysis and Serological Evaluation. Viruses.
2020;
12
(1)
:
109
.
View Article PubMed Google Scholar -
Viswanathan
R.,
A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res.
1957;
45
:
145-55
.
PubMed Google Scholar -
Teshale
E.H.,
Hu
D.J.,
Hepatitis
E.,
Hepatitis E: epidemiology and prevention. World J Hepatol.
2011;
3
(12)
:
285-91
.
View Article PubMed Google Scholar -
Teshale
E.H.,
Grytdal
S.P.,
Howard
C.,
Barry
V.,
Kamili
S.,
Drobeniuc
J.,
Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis.
2010;
50
(7)
:
1006-10
.
View Article PubMed Google Scholar -
Arankalle
V.A.,
Chobe
L.P.,
Hepatitis E virus: can it be transmitted parenterally?. J Viral Hepat.
1999;
6
(2)
:
161-4
.
View Article PubMed Google Scholar -
Robson
S.C.,
Adams
S.,
Brink
N.,
Woodruff
B.,
Bradley
D.,
Hospital outbreak of hepatitis E. Lancet.
1992;
339
(8806)
:
1424-5
.
View Article PubMed Google Scholar -
Khuroo
M.S.,
Kamili
S.,
Khuroo
M.S.,
Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat.
2009;
16
(7)
:
519-23
.
View Article PubMed Google Scholar -
Li
P.,
Liu
J.,
Li
Y.,
Su
J.,
Ma
Z.,
Bramer
W.M.,
The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int.
2020;
40
(7)
:
1516-28
.
View Article PubMed Google Scholar -
Pallerla
S.R.,
Harms
D.,
Johne
R.,
Todt
D.,
Steinmann
E.,
Schemmerer
M.,
Hepatitis E Virus Infection: Circulation, Molecular Epidemiology, and Impact on Global Health. Pathogens.
2020;
9
(10)
:
856
.
View Article PubMed Google Scholar -
Purdy
M.A.,
Harrison
T.J.,
Jameel
S.,
Meng
X.J.,
Okamoto
H.,
Van der Poel
W.H.,
Ictv Report Consortium
ICTV Virus Taxonomy Profile: hepeviridae. J Gen Virol.
2017;
98
(11)
:
2645-6
.
View Article PubMed Google Scholar -
Sridhar
S.,
Teng
J.L.,
Chiu
T.H.,
Lau
S.K.,
Woo
P.C.,
Hepatitis
E.,
Hepatitis E Virus Genotypes and Evolution: Emergence of Camel Hepatitis E Variants. Int J Mol Sci.
2017;
18
(4)
:
869
.
View Article PubMed Google Scholar -
Carratalà
A.,
Joost
S.,
Population density and water balance influence the global occurrence of hepatitis E epidemics. Sci Rep.
2019;
9
(1)
:
10042
.
View Article PubMed Google Scholar -
Melgaço
J.G.,
Gardinali
N.R.,
de Mello
V.D.,
Leal
M.,
Lewis-Ximenez
L.L.,
Pinto
M.A.,
Hepatitis E: Update on Prevention and Control. BioMed Res Int.
2018;
2018
:
5769201
.
View Article PubMed Google Scholar -
Webb
G.W.,
Dalton
H.R.,
Hepatitis E: an underestimated emerging threat. Ther Adv Infect Dis.
2019;
6
:
2049936119837162
.
View Article PubMed Google Scholar -
Osundare
F.A.,
Klink
P.,
Majer
C.,
Akanbi
O.A.,
Wang
B.,
Faber
M.,
Hepatitis E Virus Seroprevalence and Associated Risk Factors in Apparently Healthy Individuals from Osun State, Nigeria. Pathogens.
2020;
9
(5)
:
392
.
View Article PubMed Google Scholar -
Aslan
A.T.,
Balaban
H.Y.,
Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol.
2020;
26
(37)
:
5543-60
.
View Article PubMed Google Scholar -
Raji
Y.E.,
Toung
O.P.,
Mohd Taib
N.,
Sekawi
Z.B.,
A systematic review of the epidemiology of Hepatitis E virus infection in South - Eastern Asia. Virulence.
2021;
12
(1)
:
114-29
.
View Article PubMed Google Scholar -
Emerson
S.U.,
Purcell
R.H.,
Recombinant vaccines for hepatitis E. Trends Mol Med.
2001;
7
(10)
:
462-6
.
View Article PubMed Google Scholar -
Cao
Y.,
Bing
Z.,
Guan
S.,
Zhang
Z.,
Wang
X.,
Development of new hepatitis E vaccines. Hum Vaccin Immunother.
2018;
14
(9)
:
2254-62
.
View Article PubMed Google Scholar -
Qian
C.,
Liu
X.,
Xu
Q.,
Wang
Z.,
Chen
J.,
Li
T.,
Recent Progress on the Versatility of Virus-Like Particles. Vaccines (Basel).
2020;
8
(1)
:
139
.
View Article PubMed Google Scholar -
Shouval
D.,
Hepatitis B vaccines. J Hepatol.
2003;
39
:
70-6
.
View Article PubMed Google Scholar -
Li
S.,
Tang
X.,
Seetharaman
J.,
Yang
C.,
Gu
Y.,
Zhang
J.,
Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog.
2009;
5
(8)
:
e1000537
.
View Article PubMed Google Scholar -
Zhao
Q.,
Li
S.,
Yu
H.,
Xia
N.,
Modis
Y.,
Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol.
2013;
31
(11)
:
654-63
.
View Article PubMed Google Scholar -
Hepatitis E vaccine: WHO position paper, May 2015. Wkly Epidemiol Rec. 2015;90(18):185-200. English, French.
.
-
Nan
Y.,
Wu
C.,
Zhao
Q.,
Sun
Y.,
Zhang
Y.J.,
Zhou
E.M.,
Vaccine Development against Zoonotic Hepatitis E Virus: Open Questions and Remaining Challenges. Front Microbiol.
2018;
9
:
266
.
View Article PubMed Google Scholar -
Shukla
P.,
Nguyen
H.T.,
Torian
U.,
Engle
R.E.,
Faulk
K.,
Dalton
H.R.,
Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc Natl Acad Sci USA.
2011;
108
(6)
:
2438-43
.
View Article PubMed Google Scholar -
Wang
D.,
Zhang
Y.,
Ma
C.,
Ma
D.,
Zhao
Q.,
Wang
F.,
Live recombinant Lactococcuslactis expressing avian hepatitis virus ORF2 protein: immunoprotection against homologous virus challenge in chickens. Vaccine.
2018;
36
(8)
:
1108-15
.
View Article PubMed Google Scholar -
Takahashi
M.,
Tanaka
T.,
Takahashi
H.,
Hoshino
Y.,
Nagashima
S.,
Jirintai
Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol.
2010;
48
(4)
:
1112-25
.
View Article PubMed Google Scholar -
Meng
J.,
Dai
X.,
Chang
J.C.,
Lopareva
E.,
Pillot
J.,
Fields
H.A.,
Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology.
2001;
288
(2)
:
203-11
.
View Article PubMed Google Scholar -
Wen
J.,
Behloul
N.,
Dai
X.,
Dong
C.,
Liang
J.,
Zhang
M.,
Shi
C.,
Meng
J.,
Immunogenicity difference between two hepatitis E vaccines derived from genotype 1 and 4. Antiviral Res.
2016;
128
:
36-42
.
View Article PubMed Google Scholar -
Jiménez de Oya
N.,
Escribano-Romero
E.,
Blázquez
A.B.,
Lorenzo
M.,
Martín-Acebes
M.A.,
Blasco
R.,
Characterization of hepatitis E virus recombinant ORF2 proteins expressed by vaccinia viruses. J Virol.
2012;
86
(15)
:
7880-6
.
View Article PubMed Google Scholar -
Farshadpour
F.,
Makvandi
M.,
Taherkhani
R.,
Design, Construction and Cloning of Truncated ORF2 and tPAsp-PADRE-Truncated ORF2 Gene Cassette From Hepatitis E Virus in the pVAX1 Expression Vector. Jundishapur J Microbiol.
2015;
8
(12)
:
e26035
.
View Article PubMed Google Scholar -
Dong
C.,
Dai
X.,
Meng
J.H.,
The first experimental study on a candidate combined vaccine against hepatitis A and hepatitis E. Vaccine.
2007;
25
(9)
:
1662-8
.
View Article PubMed Google Scholar -
Xiang
K.,
Kusov
Y.,
Ying
G.,
Yan
W.,
Shan
Y.,
Jinyuan
W.,
A Recombinant HAV Expressing a Neutralization Epitope of HEV Induces Immune Response against HAV and HEV in Mice. Viruses.
2017;
9
(9)
:
260
.
View Article PubMed Google Scholar -
Gao
Y.,
Su
Q.,
Yi
Y.,
Jia
Z.,
Wang
H.,
Lu
X.,
Enhanced mucosal immune responses induced by a combined candidate mucosal vaccine based on Hepatitis A virus and Hepatitis E virus structural proteins linked to tuftsin. PLoS One.
2015;
10
(4)
:
e0123400
.
View Article PubMed Google Scholar -
Li
H.Z.,
Gang
H.Y.,
Sun
Q.M.,
Liu
X.,
Ma
Y.B.,
Sun
M.S.,
Production in Pichia pastoris and characterization of genetic engineered chimeric HBV/HEV virus-like particles. Chin Med Sci J.
2004;
19
(2)
:
78-83
.
PubMed Google Scholar -
Wang
L.,
Cao
D.,
Wei
C.,
Meng
X.J.,
Jiang
X.,
Tan
M.,
A dual vaccine candidate against norovirus and hepatitis E virus. Vaccine.
2014;
32
(4)
:
445-52
.
View Article PubMed Google Scholar -
Makvandi
M.,
Teimoori
A.,
Neisi
N.,
Samarbafzadeh
A.,
Designing, Construction and Expression of a Recombinant Fusion Protein Comprising the Hepatitis E Virus ORF2 and Rotavirus NSP4 in the Baculovirus Expression System. Jundishapur J Microbiol.
2016;
9
(11)
:
e40303
.
View Article PubMed Google Scholar -
Li
T.,
Takeda
N.,
Miyamura
T.,
Oral administration of hepatitis E virus-like particles induces a systemic and mucosal immune response in mice. Vaccine.
2001;
19
:
3476-3484
.
View Article PubMed Google Scholar -
Li
T.C.,
Suzaki
Y.,
Ami
Y.,
Dhole
T.N.,
Miyamura
T.,
Takeda
N.,
Protection of cynomolgus monkeys against HEV infection by oral administration of recombinant hepatitis E virus-like particles. Vaccine.
2004;
22
(3-4)
:
370-7
.
View Article PubMed Google Scholar -
Dong
C.,
Meng
J.H.,
Expression, purification and immunogenicity of a novel hepatitis E virus-like particle. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (Chinese).
2006;
22
(3)
:
339-342
.
PubMed Google Scholar -
Purdy
M.A.,
McCaustland
K.A.,
Krawczynski
K.,
Spelbring
J.,
Reyes
G.R.,
Bradley
D.W.,
Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV). J Med Virol.
1993;
41
(1)
:
90-4
.
View Article PubMed Google Scholar -
Qi
Y.,
Fan
J.,
Huang
W.,
Zhao
C.,
Wang
Y.,
Kong
F.T.,
Expression and characterization of hepatitis E virus-like particles and non-virus-like particles from insect cells. Biotechnol Appl Biochem.
2016;
63
(3)
:
362-70
.
View Article PubMed Google Scholar -
Sehgal
D.,
Malik
P.S.,
Jameel
S.,
Purification and diagnostic utilityof a recombinant hepatitis E virus capsid protein expressed in insect larvae. Protein Expr Purif.
2003;
27
:
27-34
.
View Article PubMed Google Scholar -
Robinson
R.A.,
Burgess
W.H.,
Emerson
S.U.,
Leibowitz
R.S.,
Sosnovtseva
S.A.,
Tsarev
S.,
Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif.
1998;
12
(1)
:
75-84
.
View Article PubMed Google Scholar -
Zhang
M.,
Emerson
S.U.,
Nguyen
H.,
Engle
R.,
Govindarajan
S.,
Blackwelder
W.C.,
Recombinant vaccine against hepatitis E: duration of protective immunity in rhesus macaques. Vaccine.
2002;
20
(27-28)
:
3285-91
.
View Article PubMed Google Scholar -
Shrestha
M.P.,
Scott
R.M.,
Joshi
D.M.,
Mammen
M.P.,
Thapa
G.B.,
Thapa
N.,
Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med.
2007;
356
(9)
:
895-903
.
View Article PubMed Google Scholar -
Su
C.X.,
Gu
M.R.,
Zhang
P.,
Jin
Z.J.,
Meng
F.H.,
Chen
E.J.,
Yang
Z.,
Liu
Y.,
Wang
Y.C.,
Expression of ORF2 protein of HEV genotype IV in Hansenula polymorpha. Sheng Wu Gong Cheng Xue Bao (Chinese).
2007;
23
(1)
:
73-78
.
PubMed Google Scholar -
Tong
Y.,
Zhan
M.,
Lu
J.,
Bai
Y.,
Bi
S.,
Immunogenicity of recombinant HEV ORF2 protein expressed in pichia pastoris. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi (Chinese).
2002;
16
(1)
:
23-26
.
PubMed Google Scholar -
Trabelsi
K.,
Kamen
A.,
Kallel
H.,
Development of a vectored vaccine against hepatitis E virus. Vaccine.
2014;
32
:
2808-2811
.
View Article PubMed Google Scholar -
Cao
Y.F.,
Tao
H.,
Hu
Y.M.,
Shi
C.B.,
Wu
X.,
Liang
Q.,
A phase 1 randomized open-label clinical study to evaluate the safety and tolerability of a novel recombinant hepatitis E vaccine. Vaccine.
2017;
35
(37)
:
5073-80
.
View Article PubMed Google Scholar -
Zhu
F.C.,
Zhang
J.,
Zhang
X.F.,
Zhou
C.,
Wang
Z.Z.,
Huang
S.J.,
Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet.
2010;
376
(9744)
:
895-902
.
View Article PubMed Google Scholar -
Xia
M.,
Wei
C.,
Wang
L.,
Cao
D.,
Meng
X.J.,
Jiang
X.,
A trivalent vaccine candidate against hepatitis E virus, norovirus, and astrovirus. Vaccine.
2016;
34
(7)
:
905-13
.
View Article PubMed Google Scholar -
Rani
D.,
Saxena
R.,
Nayak
B.,
Srivastava
S.,
Cloning and expression of truncated ORF2 as a vaccine candidate against hepatitis E virus. 3 Biotech.
2018;
8
(10)
:
414
.
View Article PubMed Google Scholar -
Kmush
B.L.,
Yu
H.,
Huang
S.,
Zhang
X.,
Wu
T.,
Nelson
K.E.,
Labrique
A.B.,
Erratum: Long-term antibody persistence after hepatitis E virus infection and vaccination in Dongtai, China. Open Forum Infect Dis.
2019;
6
(8)
:
ofz224
.
View Article PubMed Google Scholar -
Yu
X.Y.,
Chen
Z.P.,
Wang
S.Y.,
Pan
H.R.,
Wang
Z.F.,
Zhang
Q.F.,
Safety and immunogenicity of hepatitis E vaccine in elderly people older than 65% UNKNOWN UNICODE CHARACTER 0202F (NARROW NO-BREAK SPACE) years. Vaccine.
2019;
37
(32)
:
4581-6
.
View Article PubMed Google Scholar -
Zaman
K.,
Dudman
S.,
Stene-Johansen
K.,
Qadri
F.,
Yunus
M.,
Sandbu
S.,
HEV study protocol : design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open.
2020;
10
(1)
:
e033702
.
View Article PubMed Google Scholar -
Khuroo
M.S.,
Khuroo
M.S.,
Hepatitis E: an emerging global disease - from discovery towards control and cure. J Viral Hepat.
2016;
23
(2)
:
68-79
.
View Article PubMed Google Scholar -
Cao
Y.,
Bing
Z.,
Guan
S.,
Zhang
Z.,
Wang
X.,
Development of new hepatitis E vaccines. Hum Vaccin Immunother.
2018;
14
(9)
:
2254-62
.
View Article PubMed Google Scholar -
Walker
C.M.,
Adaptive Immune Responses in Hepatitis A Virus and Hepatitis E Virus Infections. Cold Spring Harb Perspect Med.
2019;
9
(9)
:
a033472
.
View Article PubMed Google Scholar -
Wu
X.,
Chen
P.,
Lin
H.,
Hao
X.,
Liang
Z.,
Hepatitis E virus: current epidemiology and vaccine. Hum Vaccin Immunother.
2016;
12
(10)
:
2603-10
.
View Article PubMed Google Scholar -
Smith
J.,
Lipsitch
M.,
Almond
J.W.,
Vaccine production, distribution, access, and uptake. Lancet.
2011;
378
(9789)
:
428-38
.
View Article PubMed Google Scholar -
Centers for Disease Control and Prevention (CDC). Possible side-effects from vaccines. http://www.cdc.gov/vaccines/vac-gen/side-effects.html. Accessed March 10, 2021. .
.
-
Beirne
P.V.,
Hennessy
S.,
Cadogan
S.L.,
Shiely
F.,
Fitzgerald
T.,
MacLeod
F.,
Needle size for vaccination procedures in children and adolescents. Cochrane Database Syst Rev.
2018;
8
(8)
.
View Article PubMed Google Scholar -
Spencer
J.P.,
Trondsen Pawlowski
R.H.,
Thomas
S.,
Vaccine Adverse Events: Separating Myth from Reality. Am Fam Physician.
2017;
95
(12)
:
786-94
.
PubMed Google Scholar -
Health Resources and Services Administration. National vaccine injury compensation program. http://www.hrsa.gov/vaccinecompensation/vaccinetable.html. Accessed March 10, 2021. .
.
-
Miller
L.,
Reynolds
J.,
Autism and vaccination-the current evidence. J Spec Pediatr Nurs.
2009;
14
(3)
:
166-72
.
View Article PubMed Google Scholar -
Stratton
K.R.,
Howe
C.J.,
Johnston
R.B.,
Adverse Events Associated with Childhood Vaccines: Evidence Bearing on CausalityNational Academy Press: Washington (DC); 1994.
Google Scholar -
Babl
F.E.,
Lewena
S.,
Brown
L.,
Vaccination-related adverse events. Pediatr Emerg Care.
2006;
22
(7)
:
514-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 9 (2021)
Page No.: 4514-4524
Published on: 2021-09-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8029 times
- PDF downloaded - 1913 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress





