Abstract
In most people, COVID-19 presents as a mild disease. However, in many people, especially those with comorbidities, profound inflammation manifesting as a cytokine storm may lead to acute respiratory distress syndrome and multi-organ failure. This novel study reports two severe COVID-19 patients from Koja Regional Hospital: a male aged 52 and a female aged 65. Both patients had poor prognoses based on prognostic biomarkers, disseminated intravascular coagulation, nosocomial infections, and were reintubated more than once. Both patients were treated with adjunct autologous activated platelet-rich plasma — a safe and promising therapy — and were ultimately discharged. Thus, this study reports the potential of autologous activated platelet-rich plasma as supportive therapy for severe COVID-19 patients.
Introduction
In the majority of the population, COVID-19 presents as a mild disease with symptoms such as cough, fever, fatigue, and anosmia1. However, for those with comorbidities, COVID-19 may cause acute respiratory distress syndrome (ARDS) and multi-organ failure. This is the devastating consequence of a “cytokine storm”, a condition characterized by profound inflammation2.
Autologous activated platelet-rich plasma (aaPRP) is a blood product rich in various growth factors and anti-inflammatory proteins, such as the cytokine interleukin-1 receptor antagonist (IL-1RA)3. Based on previous studies, aaPRP is safe and capable of ameliorating inflammation as reflected by the lowering of the pro-inflammatory biomarker4. Additionally, it also has an anti-fibrotic effect, which is beneficial for COVID-19 patients at risk of pulmonary fibrosis5.
With surges in COVID-19 cases and hospital collapses caused by the delta variant of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Indonesia and other countries, aaPRP may reduce the length of stay, improve clinical outcome, and hasten hospital bed availability, making precious room for other patients. In this uncontrolled case series, we report two severe COVID-19 patients treated successfully with adjunct intravenous aaPRP therapy. To our knowledge, this report is the first to utilize three or more doses of aaPRP to treat severely ill COVID-19 patient.
Case presentation
Case 1
The first case was a 65-year-old female who was presented to the emergency department on 19 February 2021. Four days prior, the patient began experiencing a worsening nonproductive cough and breathlessness, which prompted medical attention. The patient also experienced a high refractory fever during the same period. A reverse transcription-polymerase chain reaction test confirmed a COVID-19 diagnosis. The patient achieved an oxygen saturation (SpO2) of only 65% after administration of 15 liters per minute (lpm) oxygen through a non-rebreather mask (NRM). Consequently, the patient was transferred to another hospital with an intensive care unit (ICU) facility the next day.
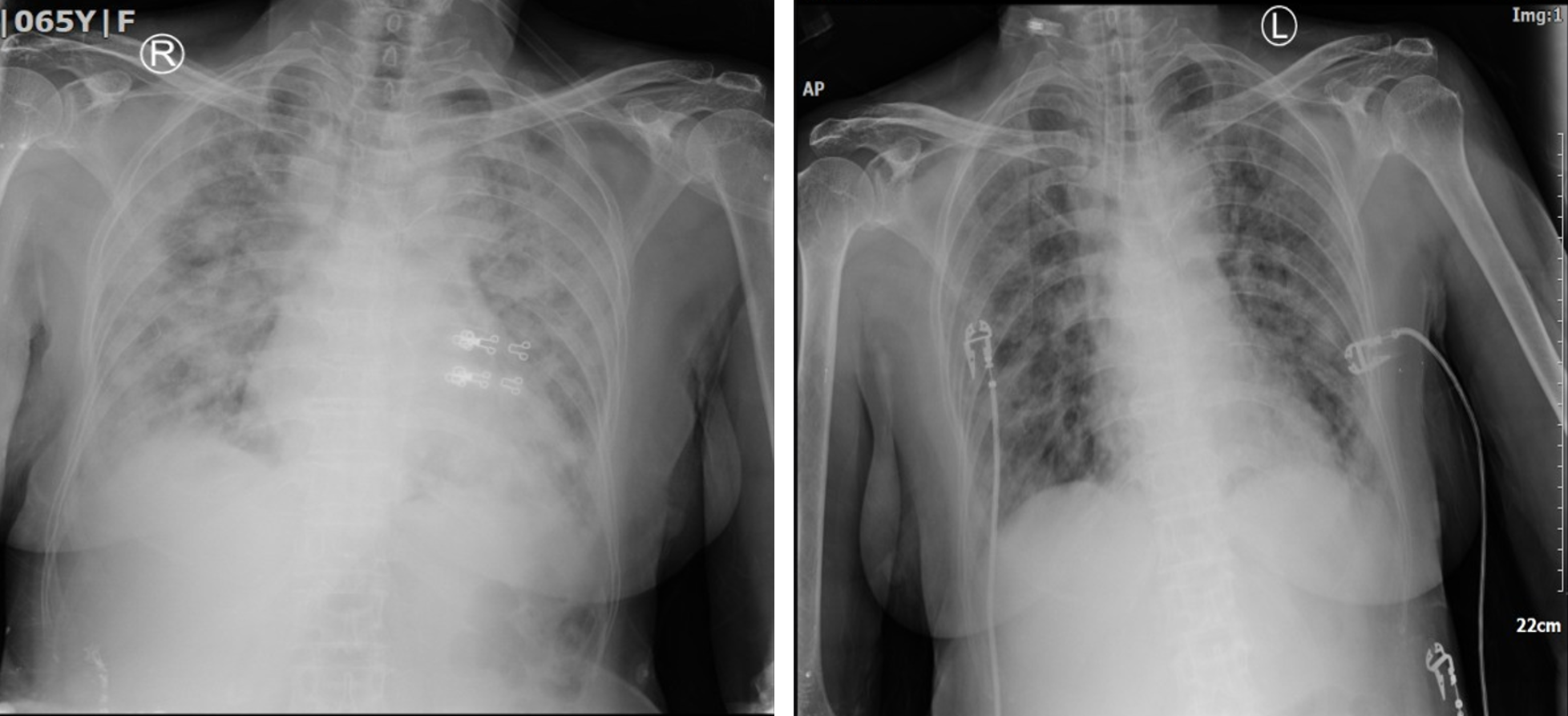
At the time of arrival, the patient was severely dyspneic. A high flow nasal cannula (HFNC) was started and a SpO2 of about 85% was attained after two hours. The patient’s PaO2/FiO2 ratio was 90.7, qualifying for severe ARDS. The routine hematological test revealed an absolute lymphocyte count (ALC) of 497/μL and a neutrophil-lymphocyte ratio (NLR) of 26.11. Electrolyte and chemistry test values were unremarkable, except for a moderately elevated aspartate transaminase. Subsequent chest radiographs also revealed extensive bilateral lung infiltration (Figure 1).
| Day | Neutrophil-lymphocyte ratio | Absolute lymphocyte count (/μL) | Platelet (×10 3 /μL) | d-Dimer (ng/mL) | Prothrombin Time (s) | Activated Partial Thromboplastin Time (s) |
|---|---|---|---|---|---|---|
| 2 | 26.11 | 497 | 395 | 1,126 | 10.4 | 85 |
| First dose of aaPRP | ||||||
| 3 | 43.41 | 444 | 390 | 1,711 | 10.9 | 67.9 |
| 4 | Second dose of aaPRP | |||||
| 5 | 22.85 | 367 | 406 | 10,000 | 12.4 | 40.4 |
| 6 | Third dose of aaPRP | |||||
| 7 | 43.23 | 421 | 196 | 10,000 | 12.4 | 34 |
| 9 | - | - | - | - | 13.4 | >180 |
| 12 | 27.94 | 514 | - | - | - | - |
| 13 | - | - | - | - | 11.8 | 39.9 |
| 14 | - | - | - | 5,341 | 12.7 | 40.3 |
Consent was obtained for intubation, mechanical ventilation, and aaPRP therapy, all of which were performed thereafter. At the same time, informed consent for study enrollment and clinical data publication was also obtained. Before aaPRP administration, the patient’s c-reactive protein (CRP) was 14.09 mg/dL. The immunology test showed elevated procalcitonin (25.9 ng/mL). Later, blood cultures turned out positive for Staphlycoccus haemolyticus and were treated appropriately.
To prepare aaPRP, 24 mL of venous blood was collected into ten vacutainer tubes (each containing 0.5 mL of anticoagulant) which were then centrifuged at 1,000 rotations per minute. Afterward, the plasma was equally distributed into two 15 mL screw cap centrifuge tubes and recentrifuged at 3,000 rotations per minute. The resulting platelet-poor portion at the top of the plasma was discarded until 2.5 mL of PRP remained in each tube. Afterward, 0.15 mL of calcium activator was added to the two tubes6. Subsequent clots were then removed, followed by the combination of aaPRP into one tube (resulting in 5 mL of aaPRP) and the addition of 10 mL of normal saline. The tube was then given a gentle shake. Finally, the 15mL of aaPRP was suspended in 100 mL of normal saline and administered intravenously.
The following day after beginning the intubation and administration of remdesivir 200 mg once daily (followed by 100 mg once daily up to day 7), dexamethasone, heparin, and antibiotics, the patient’s fever subsided, and the PaO2/FiO2 ratio increased slightly. The laboratory test showed further CRP elevation (30.6 mg/dL). On day 4, the second dose of aaPRP was administered, and laboratory tests the next day showed a CRP level of 24.06 mg/dL and d-Dimer of 10,000 ng/mL. On day 6, the patient received a third dose of aaPRP; tests did the next day showed a great reduction in CRP (3.74 mg/dL), although d-Dimer values remained high.
From day 12 onwards, the patient was successfully weaned from mechanical ventilation. Before this, a weaning attempt had failed, necessitating reintubation. On day 14, the patient’s CRP and d-Dimer were 1.44 mg/dL and 5,341 ng/mL, respectively. The next day (5 March 2021), the patient was discharged from the ICU (Table 1).
Case 2
The second case was an obese 52-year-old male who arrived at the hospital on 15 May 2021 with complaints of fever, sore throat, and a productive cough for the past five days. In the last two days, he complained of persistent dyspnea and cold sweat. Upon arrival, the patient’s room-air SpO2 was only 50%. 60 lpm of oxygen was then administered via HFNC and a SpO2 of 90% was achieved. A rapid SARS-CoV2 antigen test on the patient showed positive results. A chest radiograph showed extensive bilateral infiltration (Figure 2), and the patient had a PaO2/FiO2 ratio of 72.5, qualifying for severe ARDS. Chemistry test values were within reference ranges, but the electrolyte test revealed low serum sodium (114 mmol/L), which was later corrected. Remdesivir, dexamethasone, heparin, and antibiotics were also administered.
On day 3—still, on HFNC—the patient’s SpO2 dropped to 71%; he was consequently transferred to the ICU. Lab tests showed an ALC of 576/μL, NLR of 16.98, and CRP of 14.9 mg/dL. Informed consent was then obtained for intubation, aaPRP therapy, and study enrollment; the next day, a CRP reading of 7,54 mg/dL was taken. A second dose of aaPRP was given on day 5, and the next day the patient was weaned to HFNC with a CRP of 14.9 mg/dL. On day 7, the third dose of aaPRP was given and tests the next day showed a decrease in CRP (3.29 mg/dL) but a high d-Dimer. After a fourth dose of aaPRP on day 9, the patient’s SpO2 dropped to 81% on the following day with a decrease in d-Dimer (2,579 ng/mL) but an increase in CRP (15.64 mg/dL).
On day 11, the patient was reintubated, and the fifth dose of aaPRP was given. CRP level the next day was 13.66 mg/dL, and procalcitonin was 1.5 ng/mL. Later, blood cultures showed Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae. As the patient showed clinical improvement, the patient was gradually weaned from day 21 onward until discharge on day 25 (8 June 2021) (Table 2).
| Day | Neutrophil-lymphocyte ratio | Absolute lymphocyte count (/μL) | Platelet (×10 3 /μL) | d-Dimer (ng/mL) | Prothrombin Time (s) | Activated Partial Thromboplastin Time (s) |
|---|---|---|---|---|---|---|
| 3 | 16.98 | 576 | 240 | - | 10.8 | 36 |
| First dose of aaPRP | ||||||
| 5 | Second dose of aaPRP | |||||
| 6 | 8.42 | 1199 | - | - | - | - |
| 7 | Third dose of aaPRP | |||||
| 8 | 28.42 | 603 | 251 | 10,000 | 11.6 | 35.2 |
| 9 | Fourth dose of aaPRP | |||||
| 10 | 20.71 | 963 | 220 | 2,579 | 12.3 | 84.8 |
| 11 | Fifth dose of aaPRP | |||||
| 11 | 23.85 | 914 | - | - | - | - |
| 12 | - | - | 181 | 6,542 | 11.9 | 41.1 |
| 13 | 26.08 | 700 | - | - | - | - |
| 16 | 15.81 | 781 | 237 | - | 10.8 | 19.7 |
| 19 | 10.37 | 1084 | 350 | 6,475 | 10.9 | 32.8 |
Discussions
As previously described, aaPRP is an autologous blood product containing bioactive molecules such as various cytokines and growth factors3, 4, 5. Furthermore, after thorough activation and processing, intravenous administration is deemed safe as it contains no leukocytes or platelets6.
Despite administration of remdesivir, corticosteroid, anticoagulant, and antibiotics, both patients still experienced severe ARDS to the point of requiring intubation along with progressive bilateral infiltration. Furthermore, leukocyte disorder, disseminated intravascular coagulation (DIC), and profound inflammation was also observed. The patients were given multiple doses of aaPRP before clinical improvements and discharge.
Along with the remdesivir, dexamethasone is recommended for severe and critical COVID-19 in Indonesia7. Such immunosuppressive therapy along with invasive device usage, such as an endotracheal tube, contributes to nosocomial infection risk in ICU patients8.
Carbapenem-resistant Klebsiella pneumoniae infection is associated with a high mortality and morbidity rate; currently, it is one of the most commonly encountered carbapenem-resistant Enterobacteriaceae in hospitals9, while nosocomial Staphylococcus haemolyticus infection is associated with insertion of medical devices10. Pseudomonas aeruginosa is among the most common pathogen for nosocomial infection; infection by a multi-drug resistant strain similar to our case carries a high mortality rate11. Both patients underwent more than one intubation and experienced nosocomial infection. The use of aaPRP, in this case, is rationalized owing to its antimicrobial quality. Molecules present in aaPRP such as connective tissue-activating peptide-III, chemokine ligand-5, thymosin β-4, and fibrinopeptide can impede bacterial protein synthesis and enzyme activity12.
The ALC and NLR observed are typical findings in COVID-19. The two parameters are prognostic, with marked lymphopenia and neutrophilia associated with poorer outcomes in COVID-19. Restoration of the lymphocyte level has been suggested as crucial to COVID-19 recovery13, 14. Lymphopenia in COVID-19 may be caused by the SARS-CoV-2 infection of lymphocytes, among others15. Not only compromising adaptive antiviral responses, a mouse model of SARS also demonstrated hyper inflammation associated with lymphopenia.
Meanwhile, neutrophilia is associated with bacterial infection and/or corticosteroids14. In this sense, aaPRP may alleviate inflammation and infection through its anti-inflammatory activity, allowing the return of normal neutrophil and lymphocyte levels4. The reduction in inflammation also attenuates the cytokine storm responsible for ARDS2.
Another hematological disorder found in COVID-19 is coagulopathy. A high d-Dimer and prothrombin time is significantly associated with a higher mortality risk in COVID-1913, 16. Although fibrinogen levels were not assessed, both patients qualified for DIC as per the International Society Thrombosis and Haemostasis criteria17. The pathogenesis of COVID-19-induced coagulopathy is yet fully elucidated. Still, excess inflammatory cytokines, vascular endothelial damage, and an overload of the damage-associated molecular pattern are among the major causes of coagulopathy in any severe infection18. In the context of coagulopathy, besides being anti-inflammatory, aaPRP also contains growth factors such as the vascular endothelial growth factor, which may be beneficial for treating DIC in COVID-1919.
Elevated CRP levels in COVID-19 patients can be attributed to the inflammation brought about by a cytokine storm. Ramifications of this profound inflammation include vascular and epithelial damage, suppressed T-cell activity, and ARDS2. In the first patient, a remarkable decrease in CRP level was observed at the end of the study. Meanwhile, patient two also had a noteworthy decrease in CRP level. Although the level rose again on day 10, CRP levels decreased slightly after another dose of aaPRP. A previous study with aaPRP in COVID-19 patients has shown a significant reduction in CRP levels4. Anti-inflammatory cytokines such as IL-1RA and innate immunomodulatory properties within aaPRP are thought to be credited for these particular effects3, 20.
Conclusion
Cytokine storm-induced ARDS, nosocomial infections, and coagulopathy are some of the challenges met when treating severe COVID-19 cases in the ICU. Therefore, the current situation warrants a more cautious usage of antibiotics to prevent an exacerbation of resistance. Furthermore, prognostic factors such as CRP and d-Dimer should be closely monitored. aaPRP is a safe and promising adjunct treatment for dealing with these challenges and improving clinical outcomes. Nevertheless, limitations, including the lack of control and the small sample size, warrant further studies to be conducted.
Abbreviations
ALC: Absolute lymphocyte count
ARDS: Acute respiratory distress syndrome
aaPRP: Autologous activated platelet-rich plasma
COVID-19: Coronavirus disease 2019
CRP: C-reactive protein
DIC: isseminated intravascular coagulation
HFNC: High flow nasal cannula
ICU: Intensive care unit
Lpm: Liters per minute
NLR: Neutrophil-lymphocyte ratio
NRM: Non-rebreather mask
SpO2: Oxygen saturation
SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2
Acknowledgments
The authors would like to thank Dr. Ida Bagus Nyoman Banjar, MKM as director of Koja Regional Hospital for their support in conducting during the study. The authors would also like thank the participants who took part in the trial.
Author’s contributions
KK, LM, RN were the principal investigators. I Rosadi, I Rosliana, SR, SS, NF, NP, YL, IA, DE, TW, SAD, AZ, NA isolated the plasma, did laboratory procedures, and processed the data. HA, I Rosadi, I Rosiana, SR analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Boban
M.,
Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments. Int J Clin Pract.
2021;
75
(4)
:
e13868
.
View Article PubMed Google Scholar -
de la Rica
R.,
Borges
M.,
Gonzalez-Freire
M.,
COVID-19: In the Eye of the Cytokine Storm. Front Immunol.
2020;
11
:
558898
.
View Article PubMed Google Scholar -
Ziegler
C.G.,
Van Sloun
R.,
Gonzalez
S.,
Whitney
K.E.,
DePhillipo
N.N.,
Kennedy
M.I.,
Characterization of Growth Factors, Cytokines, and Chemokines in Bone Marrow Concentrate and Platelet-Rich Plasma: A Prospective Analysis. Am J Sports Med.
2019;
47
(9)
:
2174-87
.
View Article PubMed Google Scholar -
Karina
K.,
Rosliana
I.,
Rosadi
I.,
Sobariah
S.,
Christoffel
L.M.,
Novariani
R.,
Phase I/II Clinical Trial of Autologous Activated Platelet Rich Plasma (aaPRP) in the Treatment of Severe Coronavirus Disease 2019 (COVID-19) Patients. Int J Inflam.
2021;
2021
:
5531873
.
View Article PubMed Google Scholar -
Karina
K.,
Christoffel
L.M.,
Novariani
R.,
Rosadi
I.,
Rosliana
I.,
Rosidah
S.,
The Effect of Intravenous Autologous Activated Platelet-Rich Plasma Therapy on “Profibrotic Cytokine” IL-1β Levels in Severe and Critical COVID-19 Patients: A Preliminary Study. Scientifica.
2021;
2021
:
1-7
.
View Article Google Scholar -
Karina
K.,
Ekaputri
K.,
Biben
J.A.,
Purwoko
R.H.,
Sibuea
T.P.,
Astuti
S.L.,
Evaluating the Safety of Intravenous Delivery of Autologous Activated Platelet-rich Plasma. Journal of Health Sciences.
2021;
11
(2)
:
61-65
.
View Article Google Scholar -
Burhan
E.,
Susanto
A.D.,
Nasution
S.A.,
Ginanjar
E.,
Pitoyo
C.W.,
Susilo
A.,
Pedoman tatalaksana COVID-19 2020.
Google Scholar -
He
Y.,
Li
W.,
Wang
Z.,
Chen
H.,
Tian
L.,
Liu
D.,
Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol.
2020;
41
(8)
:
982-3
.
View Article PubMed Google Scholar -
Dumitru
I.M.,
Dumitrascu
M.,
Vlad
N.D.,
Cernat
R.C.,
Ilie-Serban
C.,
Hangan
A.,
Carbapenem-resistant Klebsiella pneumoniae associated with COVID-19. Antibiotics (Basel).
2021;
10
(5)
:
561
.
View Article PubMed Google Scholar -
Daniel
B.,
Saleem
M.,
Naseer
G.,
Fid
A.,
Significance of Staphylococcus hemolyticus in nosocomial associated Infections. J Pioneer Med Sci.
2014;
4
(3)
:
119-25
.
-
Matos
E.C. de,
Andriolo
R.B.,
Rodrigues
Y.C.,
Lima
P.D. de,
Mortality in patients with multidrug-resistant Pseudomonas aeruginosa infections: A meta-analysis. Rev Soc Bras Med Trop.
2018;
51
(4)
:
415-420
.
View Article Google Scholar -
Zhang
W.,
Guo
Y.,
Kuss
M.,
Shi
W.,
Aldrich
A.L.,
Untrauer
J.,
Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng Part B Rev.
2019;
25
(3)
:
225-36
.
View Article PubMed Google Scholar -
Agbuduwe
C.,
Basu
S.,
Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur J Haematol.
2020;
105
(5)
:
540-6
.
View Article PubMed Google Scholar -
Henry
B.,
Cheruiyot
I.,
Vikse
J.,
Mutua
V.,
Kipkorir
V.,
Benoit
J.,
Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed.
2020;
91
(3)
:
e2020008
.
PubMed Google Scholar -
Tavakolpour
S.,
Rakhshandehroo
T.,
Wei
E.X.,
Rashidian
M.,
Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett.
2020;
225
:
31-2
.
View Article PubMed Google Scholar -
Tang
N.,
Li
D.,
Wang
X.,
Sun
Z.,
Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost.
2020;
18
(4)
:
844-7
.
View Article PubMed Google Scholar -
Toh
C.H.,
Hoots
W.K.,
on Disseminated Intravascular Coagulation of the ISTH
SSC,
The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost.
2007;
5
(3)
:
604-6
.
View Article PubMed Google Scholar -
Iba
T.,
Levy
J.H.,
Levi
M.,
Thachil
J.,
Coagulopathy in COVID-19. J Thromb Haemost.
2020;
18
(9)
:
2103-9
.
View Article PubMed Google Scholar -
Yamakawa
S.,
Hayashida
K.,
Advances in surgical applications of growth factors for wound healing. Burns Trauma.
2019;
7
(7)
:
10
.
View Article PubMed Google Scholar -
Andia
I.,
Rubio-Azpeitia
E.,
Maffulli
N.,
Platelet-rich plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clinical Orthopaedics and Related Research.
2015;
473
(5)
:
1624-1634
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 10 (2021)
Page No.: 4614-4619
Published on: 2021-10-17
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5866 times
- PDF downloaded - 1689 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress




