Polymorphisms in TSHR and IL1RN genes and the risk and prognosis of Graves’ disease in Tunisian population
Abstract
Graves’ disease (GD) is a complex genetic autoimmune thyroid diseases (AITD). TSHR is considered as candidate gene in GD. IL1RN gene shown to be related to the pathogenesis of a number of autoimmune diseases. These finding prompted us to investigate the association of TSHR and IL1RN genes polymorphism with the risk and the prognosis of GD in Tunisia. A total of 249 healthy controls and 68 GD were genotyped for TSHR D727E and IL1RNVNTR polymorphism. No significant difference was found for D727E polymorphism between GD patients and healthy controls. For IL1RNVNTR, we found an association between GD and IL1RN A1A2 genotype. TSHR polymorphism was associated with GD susceptibility in patients older than 40 years. We found for the first time an association of IL1RNVNTR polymorphism with the production of anti-thyroglobulin and anti-thyroid peroxidase antibody at the onset of disease. These preliminary results suggest that TSHR polymorphism may be a risk factor for late onset of GD, and that IL1RNVNTR polymorphism may be associated with GD susceptibility and may represent prognostic factor for predicting the severity of GD.
Introduction
Graves’ diseases (GD) is an autoimmune thyroid diseases (AITD) characterized by lymphocytic infiltrates reactive against thyroid antigens including thyroglobulin (Tg), thyroid peroxidase (TPO) and thyroidstimulating hormone receptor (TSHR). The production of thyroid-specific auto-antibodies leads to hyperthyroidism in people with Graves’ disease (GD) or to thyroid gland damage and hypothyroidism in people with Hashimoto’s thyroiditis (HT).
Multiple genes, including both immune regulatory genes and thyroid-specific genes have been reported to contribute to AITD susceptibility Invernizzi et al., 2009Smith et al., 2012.
TSHR is a thyroid specific gene, located on chromosome 14q31, close to the main locus of susceptibility to Graves’ disease Tomer et al., 1998Tomer et al., 1997. TSHR gene is considered a candidate gene in GD since the TSHR protein is a major auto-antigen in this disease Menconi, 2008.
Several polymorphisms were detected in the TSHR gene. The D727E (rs1991517) dimorphism results in an amino acid substitution of aspartate (D) to glutamate (E) at codon 727 in the intracellular domain of TSHR molecule Tonacchera and Pinchera,2000. Functional analysis of this polymorphism reveled an association with a significantly higher cyclic adenosine 3',5' monophosphate (cAMP) response to thyroid-stimulating hormone stimulation in vitro than the wild-type receptor. cAMP molecules regulate the secretion of thyroid hormones and the thyrocytes growth Gabriel et al.,1999.
Intrathyroidal inflammatory cells and thyroid follicular cells produce a variety of cytokines including the interleukin-1 alpha (IL1α), IL1 beta (IL1β) and a specific receptor antagonist (IL1Ra) Ajjan et al., 1998. IL1Ra inhibits IL1 induced inflammation action by blocking the binding of IL1 to IL1 receptor (Dinarello, 1997)
The IL1RN gene, coding for IL1Ra protein, is localized on the chromosome 2. The polymorphism (rs2234663) in intron 2 of IL1RN gene is caused by 86 bp variable Number of Tandem Repeats (VNTR) Tarlow et al., 1993. This region contains binding sites for transcriptional factors that regulate IL1Ra production Vamvakopoulos et al.,2002a. Depending to the number of 86 bp repeats there are six alleles. The most common alleles have been termed allele 1 (A1, four repeats) and allele 2 (A2, two repeats) Steinkasserer et al., 1991Tarlow et al., 1993Vamvakopoulos et al., 2002b. Allele 2 has been shown to be associated with increased production of IL1Ra in vitro Danis et al., 1995 and in healthy individuals Hurme and Santtila, 1998 and it is shown to be related to the pathogenesis of a number of autoimmune diseases Blakemore et al., 1995Fang et al., 1999.
These findings prompted us to investigate the potential association of the functional polymorphisms of TSHR and IL1RN genes with the increased risk and the prognosis of GD in Tunisia.
Subjects and Methods
Patients and controls
This study was conducted on 249 healthy controls and 68 patients with Graves’ disease. Controls and patients were selected from the same population living in the middle coast of Tunisia.
Clinical data were determined from medical records of patients enrolled in the Department of Endocrinology, University Hospital FattoumaBourguibaMonastir. The average age of patients with Grave’s disease (56 women and 12 men) had an average age of 46 ± 12 years. Controls individuals (21 men and 228 women) having a mean age of 46 ± 12 years were healthy blood donors with no autoimmune disease.
Graves’ disease is diagnosed by standard criteria (clinical examination, thyroid function tests, thyroglobin and thyroid peroxidase antibody titres, scintigraphy…).
Written informed consent was obtained from all patients and control and the study protocol was approved by the ethics committee of university hospital FattoumaBourguiba, Monastir.
Genotyping of thyroid-stimulating hormone receptor D727E polymorphism
Genomic DNA was obtained from peripheral blood leukocytes by the salting out technique Olerup and Zetterquist, 1992. The D727E polymorphism was analyzed using specific primers 5’- AACGCCAGGCTCAGGCATAC-3' and 5'- AAGTTCCCCTACCATTGTGA-3' as previously described Muhlberg et al., 2000. PCR was performed according to the following program: an initial denaturation at 94°C for 5 minutes followed by 35 cycles each consisting of denaturation for 45 seconds at 94°C, a primer annealing at 58°C for 1 minute, an extension at 72 °C for 1 minute. After 35 cycles, the PCR is terminated by a final elongation for 5 minutes at 72°C. The PCR cocktail contained 50 ng of genomic DNA, 0.6 μmol of each primer, 10χ PCR buffer, 2 mM MgCl2, 200 mM of each dATP, dGTP, dTTP, dCTP, and 1 unit of AmpliTaq DNA polymerase. The verification of the amplification and the specificity of thereaction is carried out by electrophoresis on an agarose 2% gel.
The PCR product (10 μl) was incubated overnight at 37°C in the presence of 10μl of a reaction mixture containing 0.15ql of enzyme NlaIII (5 units/μl), 2 μl digestion buffer (10χ) and a sufficient amount of distilled water to 10μl. The revelation of the product of digestionis performed onan agarose 3% gel.
Genotyping of interleukin-1 receptor antagonist VNTR polymorphism
The IL1RN intron 2 polymorphism was analyzed using oligonucleotide primers 5’-CTCAGCA AC ACTCCTAT-3’ and 5’-TCCTGGTCTGCAGGTAA-3’ as previously described Settin et al., 2007. Amplification conditions consist of an initial denaturation at 96°C for 1 min, followed by 35 cycles of amplification, each cycle consisting of 1 min at 94°C, 1 min at 58°C and 1 min at 70°C, and final extension at 70°C for 5 min. The reaction mix (25 μl) contained 50 ng of DNA, 0.6 μmol of each primer, 10χ PCR buffer, 2 mM MgCl2, 200 mM of each dATP, dGTP, dTTP, dCTP, and 1 unit of AmpliTaq DNA polymerase. Resulting PCR products of 410 bp (A1, four repeats), 240 bp (A2, two repeats), 325 bp (A3, three repeats), 500 bp (A4, five repeats) and 595 bp (A5, six repeats) were run on 2% agarose gel using molecular ladder of 50 bp to estimate the size of the PCR fragments.
Thyroid function and autoantibodies
The sera were obtained from each GD patients at the onset of the disease. The measurements of serum thyroid stimulating hormone (TSH), Tetraiodothyroxine (T4), anti-thyroglobuline (anti-tg) and anti-thyroid peroxidase (anti-TPO) were performed by the clinical chemistry laboratory of university hospital Fattouma-Bourguiba, using commercial reagent kits following the manufacturer's instruction.
The normal range of serum TSH is 0.15-5 mIU/l and of T4 is 8.6-25 pmol/l. For anti-tg and anti-TPO a reciprocal titer of >1:100 was considered positive.
Statistical analysis
Allele and genotype distribution between groups were evaluated using Chi-square test or Fischer exact test. The difference in frequencies between the case and control groups was analysed for statistical significance at 95% confidence interval using χ2 test and Yates’ correction. The allele's frequency of TSHR and IL1RN gene were in Hardy-Weinberg equilibrium. Odds ratios (OR) at 95% confidence intervals were calculated. Each clinical feature is compared with TSHR or IL1RN genotype counts in the GD patients using the following segregations: age of patients (< 40 years old vs ≥ 40 years old), sex (women vs men), thyroglobulin antibody and thyroid peroxidase antibody (positive vs negative). Mean and standard deviation were calculated for T4 and TSH profile for GD patients. ANOVA test was performed to find out whether there was significant difference in hormone profile between the TSHR or IL1RN genotype in GD patients. All statistical analyses were performed using the SPSS 18 program. A p-value of ≤ 0.05 was considered statistically significant.
Results
The TSHR D727E polymorphism
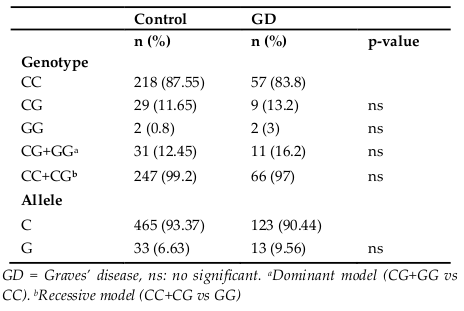
Genotype and allele frequencies for the TSHR (D727E) gene in healthy controls and GD patients are shown in Table 1 . There was no significant difference in codon 727 polymorphism frequencies between patients with GD and healthy control group either with additive or dominant models ( Table 1 ).
The IL1RNVNTRpolymorphism
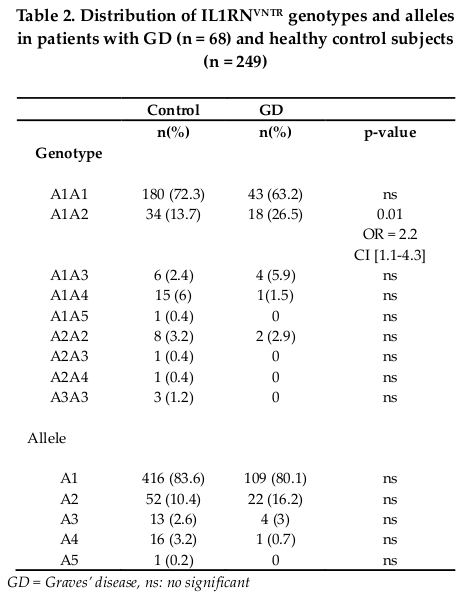
Five alleles were observed in GD and control subjects. Two alleles, 1 and 2 were the most frequent. The most widespread genotypes are A1A1 and A1A2 ( Table 2 ). The A1A2 genotype was more common in patients with GD than in controls (26.5% vs 13.7%) and showed significant association (OR = 2.2; CI = 1.1-4.3; p = 0.01). No significant difference was observed in allelic distribution between controls and GD patients ( Table 2 ).
Clinical characteristics and TSHR D727E polymorphism
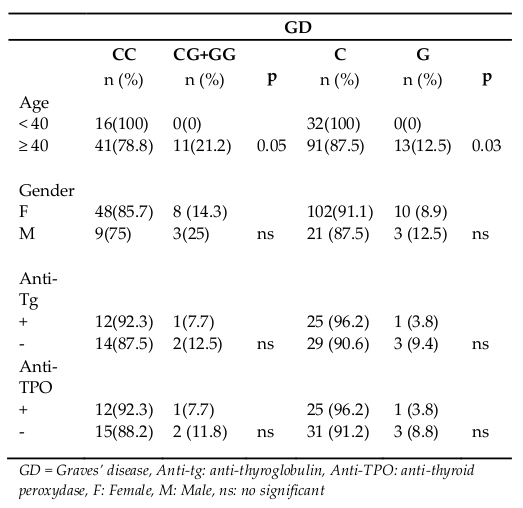
Genotypes and alleles frequency of D727E polymorphism according to clinical parameters of all patients are found in Table 3 . We found no association between D727E polymorphism and the gender of the patients with GD. No association was found with the presence or absence of thyroglobulin antibodyand thyroid peroxidase antibody. The statistically significant association was detected only with age at time of first diagnosis. The genotypes with the mutated G allele (CG+GGa) and the G allele are more frequent in GD patients older than 40 years with p = 0.05 and p = 0.03 respectively ( Table 3 ).
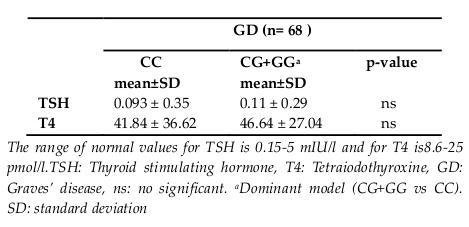
In Table 4 , serum TSH and T4 levels of GD patients were compared according to TSHR genotypes. We did not demonstrate a significant difference in TSH and T4 levels in GD patients between TSHR genotypes ( Table 4 ).
Clinical characteristics and IL-1RNVNTR polymorphism
Genotypes and alleles frequency of IL1RNVNTR polymorphism according to clinical parameters are found in Table 5 for GD patients. No association between IL1RNVNTRpolymorphism and gender or age of GD patients at the time of first diagnosis was found.
The proportion of A1A1 genotype and A1 allele decreased significantly in presence of anti-tg antibody (30.7% and 57.7%) than in absence of this antibody (81.25% and 90.6%). A1A1 genotype and A1 allele frequencies were different in the presence or absence of anti-tg antibody; p = 0.009; OR = 0.1; CI = 0.01-0.5 for A1A1 genotype and p = 0.005; OR = 0.14; CI = 0.03-0.5 for A1 allele ( Table 5 ).
In contrast, increased A1A2 genotype and A2 allele frequency were observed with the presence of anti-tg antibody (54% and 42.3%) compared with the absence of this antibody (6.25% and 3.15%). A1A2 genotype and A2 allele frequencies were different in the presence or absence of anti-tg antibody, with (p = 0.009; OR = 17.5; CI = 1.7-174.4) for the A1A2 genotype and (p < 0.001; OR = 22.7; CI = 2.6-192.8) for A2 allele ( Table 5 ).
Discussion
This study investigated the association of functional polymorphism in the TSHR and IL1RN genes with the development and the prognosis of Graves’ disease (GD).
No significant difference was found for TSHR D727E polymorphism between GD patients and healthy controls.
D727E Polymorphism of TSHR gene has not been associated with Graves’ disease. The mutated allele G (or 727E) does not seem to be related to the risk of developing Graves’ disease. This result is in agreement with several other studies with different populations such as Caucasian U.S. population Ban et al., 2002Gabriel et al., 1999, the German population Muhlberg et al., 2000 and the Asian population (Ho et al.,2003). However, one study, conducted with the Russian population has shown a very strong combination (p = 7.5 x 10-6) of this polymorphism with Graves’ disease Chistiakov, 2002.
For IL1RNVNTR polymorphism, we found an association between GD and IL1RN A1A2 genotype. People with heterozygous A1A2 genotype are 2.2 times more likely to have had GD than people carrying the other genotypes. Our results suggest that the IL1RN polymorphism can be a risk factor for GD if the number of repeat is lower than four (two and three repeats). We can hypothesize that the IL1RNVNTR effect depends on the number of repeat. Since Tarlow et al. have shown that the repeat region contains three potential proteinbinding sites and so the variable copy number may have functional significance Tarlow et al., 1993. Therefore, the functional ef fects of this polymorphism on IL1Ra production remain to be evaluated in GD Tunisian patients.
Our data does not show an association between GD and the A2 allele of the IL1 receptor antagonist gene. We don't support the A2 allele as a genetic susceptibility marker for GD. These finding are in agreement with previous studies conducted on the Tunisian population Kammoun-Krichen et al., 2007, Russian Chistiakov, 2000, United states Cuddihy and Bahn, 1996 and in the Belgium population Muhlberg et al.,1998. However, only data of Blakemore et al. have proved the predisposing role of allele 2 in the development of GD Blakemore et al., 1995.
The analysis of our results according to age and gender of patient showed that only TSHR polymorphism was associated with Graves’ disease susceptibility in patients older than 40 years. Indeed, the presence of the mutated G allele of D727E polymorphism could be a risk factor for late onset of GD in the Tunisian population. However, neither TSHR, nor IL1RN gene polymorphisms affect the etiology of GD between men and women. On the other hand, we investigated the association of the D727E and IL1RNVNTR polymorphism with the severity and the prognosis of GD. We have failed to show a relationship between the D727E polymorphism and the presence of circulation anti-tg and anti-TPO antibody in GD, suggesting that the humoral relations in the thyroid may be independent of TSHR polymorphism.
We did not observe a variation in TSH and T4 levels in GD patients which can be due to the small sample numbers of patients. Also, in agreement with Blakemore et al. we were unable to find any significant association between IL1RNVNTR polymorphism and change in the serum concentrations of TSH and T4 in AITD patients Blakemore et al.,1995.
The most striking findings in this study may be the association of IL1RNVNTRpolymorphism with the production of anti-tg and anti-TPO antibody at the onset of disease. The A1A2 genotype and the A2 allele seemed to be a risk factor for Tg and TPO autoantibody expression in GD patients. However, the A1A1 genotype and A1 allele seemed to be protected only from the production of Tg antibodies at the onset of GD. These findings suggest that the A2 allele of IL1RN gene may be associated with a severity of GD.
This finding is consistent with the majority of studies which supported the notion that the A2 allele may represent a marker of disease severity rather than one of disease susceptibility Bioque et al., 1996Blakemore et al., 1994Tarlow, 1997.
Conclusion
In conclusion, this study examined the prevalence of TSHR and IL1RN genotype polymorphism and their association with GD and with clinical parameters. These preliminary results suggest that TSHR polymorphism may be a risk factor for late onset of GD. Nevertheless, the IL1RNVNTR polymorphism may be associated with the development of GD. We found for the first time an association of IL1RNVNTRpolymorphism with the production of anti-thyroglobulin and anti-thyroid peroxidase antibody at the onset of disease. The A2 allele of IL1RNVNTR represents a prognostic variable for predicting the severity of GD.
Additional studies, particularly with a functional analysis of D727E and IL1RNVNTRpolymorphism are necessary to clarify the precise role of these polymorphisms on the GD in the Tunisian population.
References
-
R.
Ajjan,
C.
Findlay,
R.
Metcalfe,
P.
Watson,
M.
Crisp,
M.
Ludgate,
A.
Weetman.
The Modulation of the Human Sodium Iodide Symporter Activity by Graves’ Disease Sera 1. The Journal of Clinical Endocrinology & Metabolism.
1998;
83
:
1217-1221
.
-
Y.
Ban,
D.A.
Greenberg,
E.S.
Concepcion,
Y.
Tomer.
A germline single nucleotide polymorphism at the intracellular domain of the human thyrotropin receptor does not have a major effect on the development of Graves’ disease. Thyroid.
2002;
12
:
1079-1083
.
-
G.
Bioque,
G.
Bouma,
J.B.A.
Crusius,
P.J.
Kostense,
S.G.
Meuwissen,
A.S.
Pena.
Evidence for genetic heterogeneity in IBD: 1. The interleukin-1 receptor antagonist in the predisposition to suffer from ulcerative colitis.. European journal of gastroenterology & hepatology.
1996;
8
:
105-110
.
-
A.
Blakemore,
P.F.
Watson,
A.P.
Weetman,
G.W.
Duff.
Association of Graves’ disease with an allele of the interleukin-1 receptor antagonist gene. The Journal of Clinical Endocrinology & Metabolism.
1995;
80
:
111-115
.
-
A.I.
Blakemore,
J.K.
Tarlow,
M.
J Cork,
C.
Gordon,
P.
Emery,
G.W.
Duff.
Interleukin-1 receptor antagonist gene polymorphism as a disease severity factor in systemic lupus erythematosus. Arthritis & Rheumatism.
1994;
37
:
1380-1385
.
-
D.A.
Chistiakov,
K.V.
Savost’anov,
R.I.
Turakulov,
N.A.
Petunina,
M.I. Nosikov
Balabolkin.
Further studies of genetic susceptibility to Graves’ disease in Russian population. Med Sci Monit.
2002;
8
:
180-184
.
-
D.A.
Chistiakov,
K.V.
Savost’anov,
R.I.
Turakulov,
N.A.
Petunina,
L.V.
Trukhina,
A.V.
Kudinova,
M.I. Nosikov
Balabolkin.
Complex association analysis of Graves’ disease using a set of polymorphic markers molecular. Mol Genet Metab.
2000;
70
:
214-218
.
-
R.
Cuddihy,
R.
Bahn.
Lack of an association between alleles of interleukin-1 alpha and interleukin-1 receptor antagonist genes and Graves’ disease in a North American Caucasian population. The Journal of Clinical Endocrinology & Metabolism.
1996;
81
:
4476-4478
.
-
V.
Danis,
M.
Millington,
V.
Hyland,
D.
Grennan.
Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clinical and experimental immunology 99, 303. Dinarello, C.A. (1997). Interleukin-1.. Cytokine & growth factor reviews.
1995;
8
:
253-265
.
-
X.M.
Fang,
S.
Schroder,
A.
Hoeft,
F.
Stuber.
Comparison of two polymorphisms of the interleukin-1 gene family: interleukin-1 receptor antagonist polymorphism contributes to susceptibility to severe sepsis. Critical care medicine.
1999;
27
:
1330-1334
.
-
E.M.
Gabriel,
E.R.
Bergert,
C.S.
Grant,
J.A.
van Heerden,
G.B.
Thompson,
J.C.
Morris.
Germline Polymorphism of Codon 727 of Human Thyroid-Stimulating Hormone Receptor Is Associated with Toxic Multinodular Goiter 1. The Journal of Clinical Endocrinology & Metabolism.
1999;
84
:
3328-3335
.
-
S.-C.
Ho,
S.-S.
Goh,
D.H.
Khoo.
Association of Graves’ disease with intragenic polymorphism of the thyrotropin receptor gene in a cohort of Singapore patients of multi-ethnic origins. Thyroid.
2003;
13
:
523-528
.
-
M.
Hurme,
S.
Santtila.
IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1β genes. European journal of immunology.
1998;
28
:
2598-2602
.
-
P.
Invernizzi,
S.
Pasini,
C.
Selmi,
M.E.
Gershwin,
M.
Podda.
Female predominance and X chromosome defects in autoimmune diseases. Journal of autoimmunity.
2009;
33
:
12-16
.
-
M.
Kammoun-Krichen,
N.
Bougacha-Elleuch,
K.
Makni,
M.
Rebai,
S.
Peraldi-Roux,
A.
Rebai,
M.
Mnif,
M.
Abid,
J.
Jouida,
H.
Ayadi.
Association analysis of interleukin gene polymorphisms in autoimmune thyroid diseases in the Tunisian population. European cytokine network.
2007;
18
:
196-200
.
-
F.
Menconi,
Y.L.
Oppenheim,
Y.
Tomer.
Graves’ Disease. pp. 231-235. In:Shoenfeld, Y., Cervera, R., and Gershwin, M.E., (eds), Diagnostic Criteria in Autoimmune Diseases. Humana Press Totowa (2008). Graves’ Disease. pp. 231-235.. In:Shoenfeld, Y., Cervera, R., and Gershwin, M.E., (eds), Diagnostic Criteria in Autoimmune Diseases. Humana Press Totowa.
2008;
:
231-235
.
-
T.
Muhlberg,
K.
Herrmann,
W.
Joba,
M.
Kirchberger,
H.-J.
Heberling,
A.
Heufelder.
Lack of Association of Nonautoimmune Hyperfunctioning Thyroid Disorders and a Germline Polymorphism of Codon 727 of the Human Thyrotropin Receptor in a European Caucasian Population 1. The Journal of Clinical Endocrinology & Metabolism.
2000;
85
:
2640-2643
.
-
T.
Muhlberg,
M.
Kirchberger,
C.
Spitzweg,
F.
Herrmann,
H.
Heberling,
A.
Heufelder.
Lack of association of Graves’ disease with the A2 allele of the interleukin-1 receptor antagonist gene in a white European population. European journal of endocrinology.
1998;
138
:
686-690
.
-
O.
Olerup,
H.
Zetterquist.
HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue antigens.
1992;
39
:
225-235
.
-
A.
Settin,
H.
Abdel-Hady,
R.
El-Baz,
I.
Saber.
Gene Polymorphisms of TNF-α- 308, IL-10-1082, IL-6-174, and IL-1RaVNTR Related to Susceptibility and Severity of Rheumatic Heart Disease. Pediatric cardiology.
2007;
28
:
363-371
.
-
T.J.
Smith,
L.
Hegedüs,
R.S.
Douglas.
Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves’ orbitopathy. Best Practice & Research Clinical Endocrinology & Metabolism.
2012;
26
:
291-302
.
-
A.
Steinkasserer,
K.
Koelble,
R.
Sim.
Length variation within intron 2 of the human IL-1 receptor antagonist protein gene (IL1RN). Nucleic acids research.
1991;
19
:
5095-5095
.
-
J.K.
Tarlow,
A.I.
Blakemore,
A.
Lennard,
R.
Solari,
H.N.
Hughes,
A.
Steinkasserer,
G.W.
Duff.
Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Human genetics.
1993;
91
:
403-404
.
-
J.K.
Tarlow,
M.J.
Cork,
F.E.
Clay,
M.
Schmitt-Egenolf,
A.M.
Crane,
C.
Stierle,
W.H.
Boehncke,
T.H.
Eiermann,
A.I.
Blakemore,
S.S.
Bleehen,
W.
Sterry,
G.V
Duff.
Association between interleukin-1 receptor antagonist (IL-1ra) gene polymorphism and early and late-onset psoriasis. Br J Dermatol.
1997;
136
:
147-148
.
-
Y.
Tomer,
G.
Barbesino,
D.A.
Greenberg,
E.
Concepcion,
T.F.
Davies.
A new Graves disease-susceptibility locus maps to chromosome 20q11.. The American Journal of Human Genetics.
1998;
63
:
1749-1756
.
-
Y.
Tomer,
G.
Barbesino,
M.
Keddache,
D.A.
Greenberg,
T.F.
Davies.
Mapping of a major susceptibility locus for Graves’ disease (GD-1) to chromosome 14q31. The Journal of Clinical Endocrinology & Metabolism.
1997;
82
:
1645-1645
.
-
M.
Tonacchera,
A.
Pinchera.
Thyrotropin receptor polymorphisms and thyroid diseases. The Journal of clinical endocrinology and metabolism.
2000;
85
:
263-7
.
-
J.
Vamvakopoulos,
C.
Green,
S.
Metcalfe.
Genetic control of IL-1β bioactivity through differential regulation of the IL-1 receptor antagonist. European journal of immunology.
2002a;
32
:
2988-2996
.
-
J.
Vamvakopoulos,
C.
Taylor,
G.
Morris-Stiff,
C.
Green,
S.
Metcalfe.
The interleukin-1 receptor antagonist gene: a single-copy variant of the intron 2 variable number tandem repeat (VNTR) polymorphism. European journal of immunogenetics.
2002b;
29
:
337-340
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 12 (2015)
Page No.: 418-425
Published on: 2015-12-23
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4935 times
- Download PDF downloaded - 1691 times
- View Article downloaded - 4 times
 Biomedpress
Biomedpress