Abstract
Background: This study aimed to evaluate serum lipoprotein(a) concentrations in Vietnamese patients with acute myocardial infarction and to investigate the relationship between high serum concentrations of lipoprotein(a) and major adverse cardiovascular events after acute myocardial infarction.
Methods: We conducted a prospective cohort study that included data from 199 patients with acute myocardial infarction admitted to the Cardiology Department, Cho Ray Hospital, Viet Nam. Data on demographics, and hematologic, and biochemical blood test results, including serum lipoprotein(a) concentrations and coronary angiography results, were collected. All major cardiovascular adverse events (MACE) were defined as cardiovascular mortality, non-fatal myocardial infarction, and non-fatal ischemic stroke in hospital 30 days after discharge.
Results: In patients with acute myocardial infarction, serum concentrations of lipoprotein(a) were not normally distributed, and skewed to the right, with a median of 17.8 mg/dL, interquartile range (IQR) 7.6-34.5 mg/dL. Overall, 29.1%, 17.1%, 12.6%, and 6.5% of patients had a serum lipoprotein(a) concentration of ≥ 30, ≥ 50, ≥ 70, and ≥ 90 mg/dL, respectively. Patients with a serum lipoprotein(a) concentration of ≥ 50 mg/dL had a higher BMI (p = 0.04), a higher rate of non-ST-elevation myocardial infarction (NSTEMI) (p = 0.035), a lower GRACE score (p = 0.038), higher levels of total cholesterol, high-density lipoprotein cholesterol (HDL-C) and unadjusted low-density lipoprotein cholesterol (LDL-C) concentrations (p = 0.002, 0.015, < 0.001, respectively), and a higher rate of three-vessel disease (p = 0.023) compared to patients with a serum lipoprotein(a) concentration < 50 mg/dL. The relative risk between lipoprotein(a) ≥ 50 mg/dL and MACE was 2.37.
Conclusions: Patients with acute myocardial infarction and serum lipoprotein(a) ≥ 50 mg/dL were more likely to have NSTEMI and a lower GRACE score. Lipoprotein(a) ≥ 50 mg/dL at the time of acute myocardial infarction was not associated with in-hospital MACE, 30-days-after-discharge MACE, nor with all-cause mortality within 6 months of study follow-up.
Introduction
Low-density lipoprotein cholesterol (LDL-C) has long been a focus of treatments aimed at reducing the risk of adverse cardiovascular events1. In addition, lipoprotein(a) is considered remarkable for its dual pathogenicity as a pro-atherosclerotic and prothrombotic agent2. Lipoprotein(a) is one of six types of human lipoprotein. The others are chylomicron, high-density lipoprotein (HDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL). Lipoprotein(a) is comprised of an LDL particle that contains a single molecule of apolipoprotein B100 and another protein, apolipoprotein(a)3, 4, 5, 6.
In the general population, meta-analyses of prospective population-based studies of adults show an increased risk of coronary heart disease and myocardial infarction at lipoprotein(a) concentrations greater than 30 mg/dL, and an increased risk of ischemic stroke at concentrations greater than 50 mg/dL2, 4, 7. However, in high-risk populations, such as patients with established atherothrombotic disease, there is ongoing controversy regarding the influence of elevated lipoprotein(a) on cardiovascular risk8. In a meta-analysis by Willeit et al., based on seven randomized, placebo-controlled, statin-outcomes trials, the association between both baseline (no statin) and statin treatment, the association of lipoprotein(a) with cardiovascular disease risk was approximately linear, with an increased risk at lipoprotein(a) values of ≥ 30 mg/dL for the baseline group, and ≥ 50 mg/dL for the statin group9. These findings suggest that patients with serum lipoprotein(a) of ≥ 50 mg/dL, who currently use statins, may benefit from lowering their serum lipoprotein(a) levels. Therefore, we used 50 mg/dL as the cut-off value in our study, instead of 30 mg/dL, which is the manufacturer’s cut-off value, as well as a common cut-off value in studies based on the general population.
No approved pharmacologic therapies to lower lipoprotein(a) concentrations are currently available10. Physical activity, diet, and statin use have not been shown to affect serum lipoprotein(a) concentrations9, 11. Various therapies have been suggested to treat elevated concentrations of lipoprotein(a), including niacin, PCSK9 inhibitors, and CETP inhibitors; however, no randomized controlled trial has demonstrated that a reduction in lipoprotein(a) leads to a lower risk of cardiovascular disease11, 12, 13, 14.
Lipoprotein(a) is currently emerging as a possible adjustable risk factor for cardiovascular disease. However, published data on lipoprotein(a) in Vietnamese patients is still insufficient, especially in patients with acute myocardial infarction. Therefore, in this trial, our objective was to estimate the proportion of patients with acute myocardial infarction who have serum lipoprotein(a) ≥ 50 mg/dL, and to describe the correlation between high concentrations of serum lipoprotein(a) and major adverse cardiovascular events and all-cause mortality after acute myocardial infarction.
Methods
This study was carried out in the Cardiology Department of Cho Ray Hospital, Vietnam, from January 2020 to May 2020. All patients were followed until June 2020.
Study design and participants
In this prospective cohort study, randomly selected patients diagnosed with acute myocardial infarction upon admission had blood samples collected for serum lipoprotein(a) analysis no later than 108 hours from the time of symptom onset, and no later than 36 hours from hospital admission. The serum fraction was separated from the blood sample and stored. Measurements of serum lipoprotein(a) concentration were performed weekly in the Biochemical Department of Cho Ray Hospital using an immunoturbidimetric assay with an Abbott Quantia Lp(a) kit by an Abbott ARCHITECT c16000 clinical chemistry analyzer. Participants were classified into two groups, namely the high lipoprotein(a) [Lp(a)] group (participants had serum lipoprotein(a) ≥ 50 mg/dL) and the low Lp(a) group (participants had serum lipoprotein(a) < 50 mg/dL). Data on demographics, hematologic and biochemical blood test results, and coronary angiography results (if available) were collected. Lipoprotein(a)-adjusted LDL-C values were calculated based on the Dahlen formula (LDL-C minus 30% lipoprotein(a)’s weight)15. Enrollment in the study did not affect treatment decisions; lipoprotein(a) results were not disclosed, and only provided to participants at discharge. All patients were followed for major adverse cardiovascular events (defined as cardiovascular-related death, nonfatal myocardial infarction, and nonfatal ischemic stroke) while in hospital and within 30 days after discharge. After 30 days of discharge, all-cause mortality was followed up in patients until June 30, 2020.
Patients 18 to 80 years of age, who were diagnosed with acute myocardial infarction using the Fourth Universal Definition of Myocardial Infarction16 and admitted within 72 hours from the onset of the symptoms, were eligible for enrollment. Exclusion criteria included septic shock, end-stage cancer, cardiac arrest with clear evidence of noncardiac origin, history of any hereditary cholesterol disorder, and patients with a history of using niacin, estrogen replacement therapy, PCSK9 inhibitors, CETP inhibitors, or lipoprotein apheresis. The trial participants provided their informed consent in writing prior to enrollment.
Sample size
To estimate the proportion of acute myocardial infarction patients with serum lipoprotein(a) ≥ 50 mg/dL, we calculated the sample size to be 163 for a 0.05 margin of error. To detect differences in the rates of significant adverse cardiovascular events between the two groups of high and low lipoprotein(a), the t-test with equal variance showed that the sample size should be 108 for 80% power.
Detailed definitions of the variables collected are presented in the Supplementary Appendix.
Statistical analysis
We used the Shapiro-Wilk test to examine the normality of our data. The results were presented as the mean ± standard deviation for variables with normality, and as the median and interquartile range (IQR) for variables without normality. The t-test equal variance was used to compare the two groups. We performed Fisher’s exact test for categorical variables if the expected value was less than six, and Pearson's chi-square test for other categorical variables.
Logarithmic rank testing was performed to assess the difference in survival between the two groups. Cox regression analysis was used to assess the impacts of the survival variable on independent factors. All hypotheses were tested as two tails, and a p-value < 0.05 was considered statistically significant. Statistical analyzes were performed using IBM SPSS Statistics 26.0 software and RStudio version 3.6.2.
Ethical considerations
Patients’ participation in the study was voluntary, and they were informed of the benefits and risks of participating in our study prior to enrollment. Patient information was kept confidential and used only for research purposes. Participants were informed that they could withdraw from the study at any time.
The Ethics Council approved the study on biomedical research at the University of Medicine and Pharmacy at Ho Chi Minh City on 11 October 2019, approval number 483/DHYD-HDDD, study number 19460-DHYD.
| Plasma lipoprotein (a) | Percentage (%) N = 199 | 95% CI |
|---|---|---|
| ≥ 30 mg/dL | 29.1 | 22.8-35.5 |
| ≥ 40 mg/dL | 20.1 | 14.5-25.7 |
| ≥ 50 mg/dL | 17.1 | 11.9-22.3 |
| ≥ 60 mg/dL | 14.6 | 9.7-19.5 |
| ≥ 70 mg/dL | 12.6 | 8.0-17.2 |
| ≥ 80 mg/dL | 10.1 | 5.9-14.2 |
| ≥ 90 mg/dL | 6.5 | 3.1-10.0 |
| ≥ 180 mg/dL | 1.5 | - |
| Variables | All (N = 199) | High Lp(a) group (n = 34) | Low Lp(a) group (n = 165) | P-value |
|---|---|---|---|---|
| Age, years (IQR) | 63.0 (54.0-70.0) | 57.0 (52.5-69.3) | 64.0 (55.0-71.0) | 0.074 |
| Female, n (%) | 71 (35.7) | 12 (35.3) | 59 (35.8) | 0.959 |
| BMI, kg/m2 (IQR) | 22.9 (20.8-24.6) | 23.9 (22.1-25.5) | 22.5 (20.8-24.4) | 0.040 |
| Risk factors, cardiovascular history and comorbidities | ||||
| Current smoking, n (%) | 98 (49.2) | 15 (44.1) | 83 (50.3) | 0.511 |
| Arterial hypertension, n (%) | 103 (51.8) | 16 (47.1) | 87 (52.7) | 0.547 |
| Diabetes mellitus, n (%) | 58 (29.1) | 7 (20.6) | 51 (30.9) | 0.228 |
| Family member with premature coronary heart disease, n (%) | 11 (5.5) | 1 (2.9) | 10 (6.1) | 0.694 |
| Myocardial infarction, n (%) | 14 (7.0) | 2 (5.9) | 12 (7.3) | 1.000 |
| Percutaneous coronary intervention, n (%) | 4 (2.0) | 1 (2.9) | 3 (1.8) | 0.530 |
| Coronary artery bypass graft procedure, n (%) | 1 (0.5) | 0% | 1(0.6) | 1.000 |
| Stroke, n (%) | 20 (10.1) | 2 (5.9) | 18 (10.9) | 0.537 |
| Heart failure, n (%) | 12 (6.0) | 0 (0) | 12 (7.3) | - |
| Chronic kidney disease grade ≥3 (KDIGO) , n (%) | 5 (2.5) | 0 (0) | 5 (3.0) | - |
| Presentation at admission | ||||
| Duration from symptom onset to hospital admission, hours (IQR) | 12.3 (6.3-22.7) | 13.2 (7.4-31.5) | 11.9 (6.0-22.4) | 0.321 |
| NSTEMI, n (%) | 96 (48.2) | 22 (64.7) | 74 (44.8) | 0.035 |
| Blood pressure, mmHg (IQR) | 120 (100-130) | 120 (100-133) | 120 (100-130) | 0.106 |
| Heart rate, bpm (IQR) | 79 (65-93) | 73 (65-89) | 79 (65-94) | 0.606 |
| Killip I, n (%) | 121 (60.8) | 21 (61.8) | 100 (60.6) | 0.900 |
| Killip ≥ II, n (%) | 78 (39.2) | 13 (38.2) | 65 (39.4) | |
| GRACE score (IQR) | 159 (136-190) | 147 (124-172) | 164 (137-193) | 0.038 |
| Left ventricular ejection fraction, % (IQR) | 44.0 (34.0-54.0) (n = 196) | 43 (34-55) (n = 33) | 44 (34-54) (n = 163) | 0.840 |
| Variables | All (N = 199) | High Lp(a) group (n = 34) | Low Lp(a) group (n = 165) | P- value |
| Laboratory tests | ||||
| Duration from symptom onset to first troponin I sample received, hour (IQR) | 13.5 (7.6-25.5) | 16.0 (9.0-33.8) | 13.0 (7.2-24.7) | 0.105 |
| First sample Troponin I, ng/mL (IQR) | 33.4 (8.4-50.0) | 28.7 (11.9-50.0) | 37.3 (8.4-50.0) | 0.563 |
| Second sample Troponin I, ng/mL (IQR) | 36.1 (11.8-168.1) | 34.4 (15.0-152.9) | 37.3 (10.2-168.5) | 0.683 |
| Troponin I level change, ng/mL (IQR) | 29.0 (6.8-194.8) | 23.2 (8.9-178.3) | 34.3 (6.6-199.9) | 0.975 |
| Duration between two troponin I samples, hours (IQR) | 11.3 (5.6-19.4) | 10.5 (5.7-19.8) | 11.3 (5.6-19.2) | 0.498 |
| Haemoglobin, g/L | 130.0 ± 17.7 | 132.5 ± 13.6 | 129.5 ± 18.4 | 0.366 |
| White blood cell count, G/L (IQR) | 12.4 (10.1-15.8) | 11.6 (9.9-14.0) | 12.6 (10.1-16.0) | 0.191 |
| Platelet count, G/L (IQR) | 261 (220-301) | 259 (217-305) | 261 (223-301) | 0.802 |
| Plasma creatinine, mg/dL (IQR) | 0.94 (0.75-1.17) | 0.81 (0.70-1.12) | 0.94 (0.76-1.19) | 0.099 |
| Plasma sodium, mmol/L (IQR) | 138 (136-140) | 138 (136-140) | 138 (136-140) | 0.525 |
| Plasma potassium, mmol/L (IQR) | 3.7 (3.4-4.0) | 3.7 (3.5-4.1) | 3.7 (3.4-4.0) | 0.602 |
| AST, U/L (IQR) | 86.0 (47.0-213.5) | 87.0 (48.5-149.5) | 85.5 (46.3-236.5) | 0.648 |
| ALT, U/L (IQR) | 44.0 (27.5-73.5) | 38.0 (26.5-50.5) | 47.5 (28.0-76.0) | 0.131 |
| Total cholesterol, mg/dL (IQR) | 179.0 (147.3-209.8) (n = 192) | 207.5 (162.3-228.3) (n = 34) | 174.0 (144.0-200.0) (n = 158) | 0.002 |
| LDL-C, mg/dL (IQR) | 120.5 (92.0-145.8) (n = 192) | 149.5 (130.0-177.5) (n = 34) | 112.5 (88.0-140.0) (n = 158) | < 0.001 |
| HDL-C, mg/dL(IQR) | 36.0 (30.0-42.0) (n = 192) | 38.5 (34.8-44.5) (n = 34) | 35.0 (29.0-41.3) (n = 158) | 0.015 |
| Triglyceride, mg/dL (IQR) | 156.0 (102.5-233.0) (n = 192) | 48.4 (27.5-59.1) (n = 34) | 41.5 (34.0-53.2) (n = 158) | 0.809 |
| Duration from symptom onset to lipoprotein(a) sample received, hours (IQR) | 42.7 (34.0-54.0) | 48.4 (27.5-59.1) | 41.5 (34.0-53.2) | 0.326 |
| Lipoprotein (a), mg/dL (IQR) | 17.8 (7.8-34.6) | 85.4 (68.2-105.2) | 12.9 (7.0-25.0) | - |
| Lipoprotein(a)-adjusted LDL-C, mg/dL (IQR) | 111.2 (87.1-136.1) (n = 192) | 118.2 (90.4-153.0) (n = 34) | 107.3 (86.6-134.6) (n = 158) | 0.090 |
| Medication during hospital stay | ||||
| Aspirin, (%) | 100% | 100% | 100% | - |
| Clopidogrel, (%) | 67.3% | 52.9% | 70.3% | 0.049 |
| Ticagrelor, (%) | 34.2% | 50.0% | 30.9% | 0.033 |
| ACEi/ARB, (%) | 91.5% | 94.1% | 90.9% | 0.742 |
| Beta blockers, (%) | 47.7% | 55.9% | 46.1% | 0.296 |
| Spironolactone, (%) | 36.2% | 32.4% | 37.0% | 0.610 |
| Loop diuretics, (%) | 26.1% | 29.4% | 25.5% | 0.632 |
| LMWH, (%) | 100% | 100% | 100% | - |
| NOAC, (%) | 1.0% | 0% | 1.2% | 1.000 |
| Anti-vitamin K, (%) | 0% | 0% | 0% | - |
| Statin, (%) | 100% | 100% | 100% | - |
| Coronary angiography | ||||
| Coronary angiography with/without PCI, n (%) | 153 (76.9) | 27 (79.4) | 126 (76.4) | 0.701 |
| Duration from symptom onset to coronary angiography, hours (IQR) | 62.1 (12.9-107.7) (n = 153) | 77.8 (17.8-110.3) (n = 27) | 56.4 (10.8-107.4) (n = 126) | 0.338 |
| Two-or-more-vessel disease, n (%) | 62.1% (n = 153) | 70.4% (n = 27) | 60.3% (n = 126) | 0.329 |
| Three-vessel disease, n (%) | 26.8% (n = 153) | 44.4% (n = 27) | 23.0% (n = 126) | 0.023 |
| Left main and/or three-vessel disease, n (%) | 29.4% (n = 153) | 44.4% (n = 27) | 26.2% (n = 126) | 0.059 |
| Other reperfusion therapies | ||||
| Coronary artery ypass grafting procedure, (%) | 1.5% | 2.9% | 1.2% | 0.432 |
| Fibrinolytic, (%) | 0% | 0% | 0% | - |
| Hospital stay, day (IQR) | 6 (5-8) | 6 (5-8) | 6 (4-9) | 0.558 |
| Variables | Sample size | Number (%) | P value | ||
| High Lp(a) group | Low Lp(a) group | High Lp(a) group | Low Lp(a) group | ||
| In-hospital MACEs, n (%) | 34 | 165 | 0 (0%) | 17 (10.3%) | 0.084 |
| MACEs since hospitalization up to 30 days of follow-up after discharge, n (%) | 30 | 149 | 2 (6.7%) | 23 (15.4%) | 0.206 |
| Rehospitalizationswithin 30 days after discharged, n (%) | 30 | 132 | 4 (13.3%) | 14 (10.6%) | 0.747 |
| Unplanned follow-up visitwithin 30 days after discharged, n (%) | 30 | 132 | 2 (6.7%) | 1 (0.8%) | 0.088 |
Results
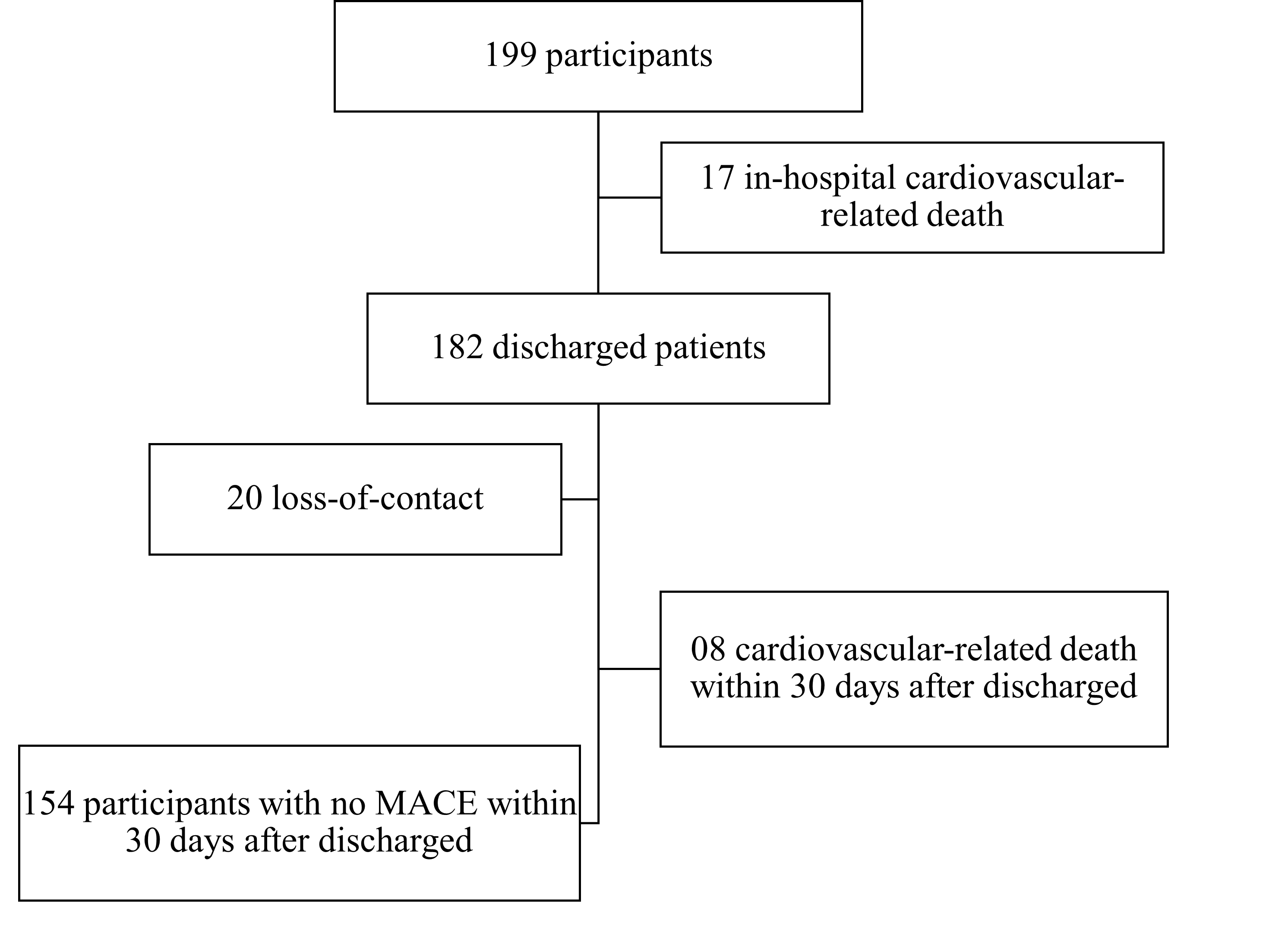
From January 16, 2020, to May 30, 2020, 200 patients were enrolled, and 199 patients were included in the final analysis. One patient was excluded from the analysis due to the loss of blood samples and the inability to contact the patient. Overall, 20 patients were lost to follow up after discharge (Figure 1). The median follow-up time was 88 days (IQR 54.0 – 125.0) (Figure 1).
Among the 199 patients who participated in the study, 128 patients (64.3%) were male. Most of the patients belonged to the Kinh ethnic group (195, 98.0%), and had a median age of 63.0 years (IQR 54.0 – 70.0) and a median BMI of 22.9 (IQR 20.8 – 24.6) kg/m2. The patients were born primarily in the provinces of the Mekong Delta.
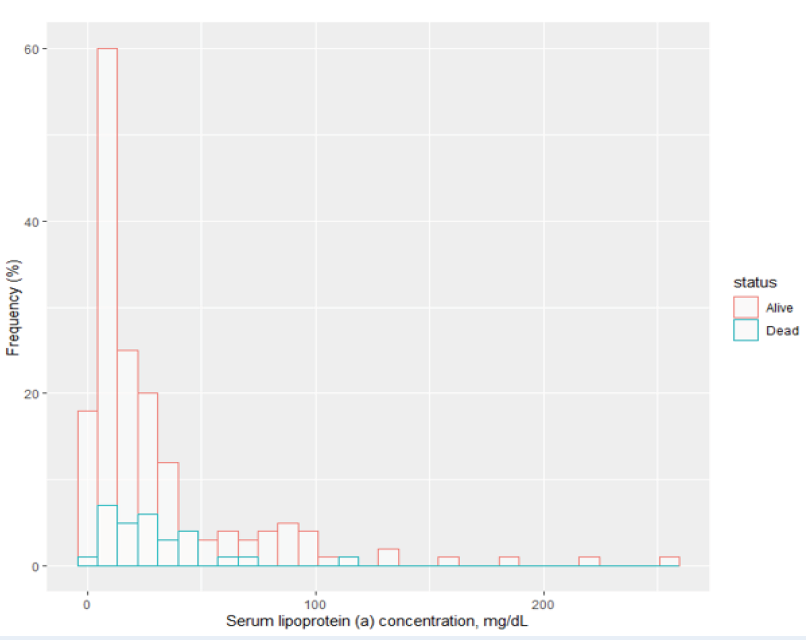
Blood samples for lipoprotein(a) measurements were received a median of 42.7 hours after the onset of symptoms of myocardial infarction (IQR 34.0 - 54.0). However, serum lipoprotein(a) concentrations in patients with acute myocardial infarction did not show a normal distribution (Shapiro-Wilk p < 0.001), with the histogram skewed to the right (Figure 2).
Table 1 shows the proportions of patients with acute myocardial infarction and various concentrations of serum lipoprotein(a). Among the study population, 34 patients (17.1%) had lipoprotein(a) ≥ 50 mg/dL, and 3 patients (1.5%) had very high lipoprotein(a) concentrations of ≥180 mg/dL. Additionally, 17.19% of male patients had lipoprotein(a) ≥ 50 mg/dL compared to 16.90% of female patients. Demographic and clinical characteristics are shown in Table 2. In our sample, 103 patients (51.8%) had been diagnosed with hypertension and 58 patients (29.1%) had been diagnosed with diabetes. Moreover, 11 patients (5.5%) had a family member(s) with premature coronary heart disease, and 14 patients (7.0%) had experienced a myocardial infarction. Furthermore, 4 patients (2.0%) had undergone a coronary intervention, 1 patient (0.5%) had undergone coronary artery bypass grafting surgery, and 20 patients (10.1%) had experienced at least a stroke. Consequently, 12 patients (6.0%) had been diagnosed with heart failure and 5 patients (2.5%) had been diagnosed with stage 3 or greater significant chronic kidney disease. Results from other hematologic and biochemical tests are presented in Table 3. Some patients may not have had all their test results collected. These cases are shown individually with a sample size n in the statistical results box.
Compared to patients with serum lipoprotein(a) < 50 mg/dL [low Lp(a) group], patients with serum lipoprotein(a) ≥ 50 mg/dL [high Lp(a) group] had significantly higher BMI (median 23.9 vs. 22.5, p = 0.0400), a higher rate of non-ST-elevation myocardial infarction (NSTEMI) (64.7% vs. 44.8%, p = 0.035), and a lower GRACE score (median 147 vs. 164, p = 0.038). No differences in patients’ medical history were found between the two groups. Total serum cholesterol, LDL-C, and HDL-C concentrations were significantly higher in the high Lp(a) group (mean total cholesterol, LDL-C, HDL-C: 207.5, 149.5, 38.5 mg/dL vs. 174.0, 112.5, 35.0 mg/dL; p = 0.002, < 0.001, 0.015, respectively). After adjusting for lipoprotein(a) cholesterol using a conservative number of 30% of its weight, lipoprotein(a)-adjusted LDL-C was found to be not statistically different between the two groups (118.2 mg/dL in the high Lp(a) group vs. 107.3 mg/dL, p = 0.090). The rate of three-vessel disease was significantly higher in the high Lp(a) group (44.4% vs. 23.0%, p = 0.0230) (Table 3). Patients in the high Lp(a) group were indicated for the use of ticagrelor at a higher rate compared to the low Lp(a) group (50.0% vs. 30.9%, p = 0.033) and for the use clopidogrel at a lower rate (52.9% vs. 70.3%, p = 0.049). Differences in the rate of aspirin use, ACEi or ARB, beta-blockers, spironolactone, loop diuretics, heparin, and statins between the two groups were not found. The rate of patients receiving coronary angiography, from the onset of symptoms to performance of the procedure, was not statistically different between the two groups. In addition, length of hospital stay was not statistically different between the two groups (both groups: median 6 days, p = 0.558).
In terms of major cardiovascular events among the 199 study patients, 17 patients (8.5%) reported in-hospital major cardiovascular events. All of these patients’ deaths were cardiovascular-related and occurred a median of 3 days (IQR 2.0-13.5) after admission. Among these, male patients (7.81%) were recorded with MACE during the study compared to 9.86% in female patients. We did not observe a statistically significant difference between the two groups of patients in the incidence of in-hospital MACE (0% in the high Lp(a) group compared to 10.3% in the low Lp(a) group, p = 0.084) or MACE after hospitalization up to 30 days of follow-up after discharge (6.7% in the high Lp(a) group vs. 15.4% in the low Lp(a) group, p = 0.206). At the same time, we also found no statistically significant differences in the rate of re-hospitalization (13.3% in the Lp(a) high group vs. 10.6% in the low Lp(a) group, p = 0.747) or unplanned follow-up visit (6.7% in the Lp(a) high group vs. 0.8% in the low Lp(a) group, p = 0.088) between the two groups within 30 days of follow-up after discharge (Table 4).
The relative risk associated with lipoprotein(a) ≥ 50 mg/dL and MACE following hospitalization up to 30 days after discharge was 2.37 (95% CI 0.59 – 9.58, p = 0.20) and not statistically significant. The adjusted hazard ratio for GRACE score 1.026 (95% CI 1.017 – 1.034, p < 0.001), was statistically significant. The Kaplan-Meier survival plots of the high Lp(a) group and the low Lp(a) group were not clearly separated (Figure 3). The survival difference between the two groups was not statistically significant, 𝛘2 (1) = 1.31, p = 0.31. The hazard ratio for lipoprotein(a) ≥ 50 mg/dL and all-cause mortality was 0.50 (95% CI 0.20 - 1.80) and was not statistically significant. Although we did not find statistically significant odds ratios between gender and MACE (odds ratio = 0.98, p = 0.9590), the hazard ratio for females with MACE compared to males was 4.88 (p < 0.0001).
Discussion
In this study, which was conducted in the context of the COVID-19 pandemic, we believe that our study population had some selection bias, in that the proportion of patients not referred to Cho Ray hospital may have increased, especially for NSTEMI patients who did not wish to have coronary angiography. Patients with mild symptoms could be admitted later and the proportion of patients with severe symptoms may have increased.
In patients with acute myocardial infarction, lipoprotein(a) concentrations had a right-skewed histogram, in line with other studies globally. Although direct comparison is difficult due to the lack of consistent results between different methods of measurement2, the median serum lipoprotein(a) concentration was higher than that of the general populations of Chinese and Japanese individuals (12.9 and 13.1 mg/dL, respectively17).
In our study population the proportion of patients with acute myocardial infarction and serum lipoprotein(a) ≥ 30 mg/dL was 29.1%, and the proportion with serum lipoprotein(a) ≥ 50 mg/dL was 17.1%, which is quite remarkable. This result was similar to that report in a study by Buuren et al. on a population with coronary artery disease, in which 26.6% of patients had lipoprotein (a) concentrations > 30 mg/dL and 18.4% of patients had lipoprotein(a) > 50 mg/dL18.
When comparing patients with acute myocardial infarction and serum lipoprotein(a) ≥ 50 mg/dL with patients whose serum lipoprotein(a) is < 50 mg/dL, our study found that patients in the high Lp(a) group were more likely to have NSTEMI and less likely to have STEMI compared to patients in the low Lp(a) group. If we had used 30 mg/dL as the cutoff value, the result would not have been statistically significant (the NSTEMI rates in the high and low Lp(a) groups were 58.6% and 44%, respectively, p = 0.060). Our study also found that the GRACE score was lower in the high Lp(a) group, but not statistically significant.At the time of writing, we could not find similar findings in other publications, in part due to the difference in cutoff values.
Coronary angiography resulted in a higher rate of three-vessel disease in the high Lp(a) group, suggesting that exposure to high concentrations of lipoprotein(a) may have caused a significant plaque burden in all coronary vessels.
Our study did not find correlations between increased lipoprotein(a) concentrations and major adverse cardiovascular events, both in-hospital and up to 30 days of follow-up after hospital discharge. We also found no association between increased lipoprotein(a) concentrations and the risk of death from all causes during the 6-month study follow-up (HR = 0.5, 95% CI 0.2‑1.8). Christian Roth et al. and Baris Gencer et al. reported similar findings, although different cutoff values were used (15, 30, 60 mg/dL in the study by Christian Roth et al. and 30 mg/dL in the study by Baris Gencer et al.)19, 20.
In summary, although an association between elevated serum lipoprotein(a) concentrations and cardiovascular diseases in patients who have had acute coronary syndrome or acute myocardial infarction has been shown, this association is not robust. Our study and the studies by Christian Roth et al. and Baris Gencer et al. have found that this association is not statistically significant19, 20. We hypothesize that the lack of statistical significance may be due to the short follow-up time, a less-selective target population, or an inappropriate cutoff value for lipoprotein(a). Interpretation of the results may also be a factor: currently, elevated lipoprotein(a) is considered a cardiovascular risk factor similar to other non-modifiable risk factors. A more robust conclusion that lipoprotein(a)-lowering treatment reduces the risk of cardiovascular events, may need confirmation from further trials, notably the lipoprotein(a) HORIZON study.
Our study has some limitations. First, this is a single-center study with a limited sample size. However, this is the first study to evaluate the association of lipoprotein(a) in Vietnamese patients with acute myocardial infarction. Second, the follow-up time was relatively short, and may not have been sufficient for enough events to occur and to increase the statistical validity of the analysis. Fewer reports found an association between lipoprotein(a) and MACE at a longer post-discharge follow-up at 1 year and 3 years21, 22. Therefore, further studies with larger sample sizes, multi-centers, and longer follow-up times are needed. Additionally, our endpoint at follow-up was all-cause mortality, and we did not report other data such as cardiovascular death, stroke, urgent coronary revascularization.
Conclusions
Patients with acute myocardial infarction with concentrations of serum lipoprotein(a) ≥ 50 mg/dL were more likely to have NSTEMI and lower GRACE scores. Lipoprotein(a) ≥ 50 mg/dL at the time of acute myocardial infarction was not associated with in-hospital MACE or 30-day-after-discharge MACE, nor with all-cause mortality within 6 months of study follow-up. A multi-center study with a larger sample size, and long-term follow-up is needed to evaluate with greater confidence the association between lipoprotein(a) and MACE.
Abbreviations
95% CI: 95% confident interval, HDL: high-density lipoprotein, HDL-C: high-density lipoprotein cholesterol, IDL: intermediate-density lipoprotein, IQR: interquartile range, LDL: low-density lipoprotein, LDL-C: low-density lipoprotein cholesterol, MACE: major cardiovascular adverse events, NSTEMI: non-ST-elevation myocardial infarction, VLDL: very low-density lipoprotein
Acknowledgments
This study was conducted at the Cardiology Department, Cho Ray Hospital. We want to thank the medical staff who helped with the data collection.
Author’s contributions
Study design: Hoang Van Sy, Hai Phuong Nguyen Tran; Collect data: Tra Thanh Ngo, Huu Nhat Minh Le; Analysis: Khoa Le Anh Huynh, Nguyen Minh Kha, Hoang Van Sy, Hai Phuong Nguyen Tran, Quang Duy Dang Pham; Manuscript: Khoa Le Anh Huynh, Nguyen Minh Kha, Hoang Van Sy, Hai Phuong Nguyen Tran, Quang Duy Dang Pham, Hoang Van Sy, Hai Phuong Nguyen Tran, Huu Nhat Minh Le, Tra Thanh Ngo; Supervisor: Hoang Van Sy, Hai Phuong Nguyen Tran. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The Ethics Council approved the study on biomedical research at the University of Medicine and Pharmacy at Ho Chi Minh City on 11 October 2019, approval number 483/DHYD-HDDD, study number 19460-DHYD.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Mach
F.,
Baigent
C.,
Catapano
A.L.,
Koskinas
K.C.,
Casula
M.,
Badimon
L.,
Scientific Document Group
ESC,
2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal.
2020;
41
(1)
:
111-88
.
View Article PubMed Google Scholar -
Wilson
D.P.,
Jacobson
T.A.,
Jones
P.H.,
Koschinsky
M.L.,
McNeal
C.J.,
Nordestgaard
B.G.,
Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. Journal of Clinical Lipidology.
2019;
13
(3)
:
374-92
.
View Article PubMed Google Scholar -
Berg
K.,
A new serum type system in man-The Lp system. Acta Pathologica et Microbiologica Scandinavica.
1963;
59
(3)
:
369-82
.
View Article PubMed Google Scholar -
Nordestgaard
B.G.,
Chapman
M.J.,
Ray
K.,
Borén
J.,
Andreotti
F.,
Watts
G.F.,
European Atherosclerosis Society Consensus Panel
Lipoprotein(a) as a cardiovascular risk factor: current status. European Heart Journal.
2010;
31
(23)
:
2844-53
.
View Article PubMed Google Scholar -
Tsimikas
S.,
A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. Journal of the American College of Cardiology.
2017;
69
(6)
:
692-711
.
View Article PubMed Google Scholar -
Tsimikas
S.,
Fazio
S.,
Ferdinand
K.C.,
Ginsberg
H.N.,
Koschinsky
M.L.,
Marcovina
S.M.,
NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. Journal of the American College of Cardiology.
2018;
71
(2)
:
177-92
.
View Article PubMed Google Scholar -
Nordestgaard
B.G.,
Langsted
A.,
Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. Journal of Lipid Research.
2016;
57
(11)
:
1953-75
.
View Article PubMed Google Scholar -
Boffa
M.B.,
Stranges
S.,
Klar
N.,
Moriarty
P.M.,
Watts
G.F.,
Koschinsky
M.L.,
Lipoprotein(a) and secondary prevention of atherothrombotic events: A critical appraisal. Journal of Clinical Lipidology.
2018;
12
(6)
:
1358-66
.
View Article PubMed Google Scholar -
Willeit
P.,
Ridker
P.M.,
Nestel
P.J.,
Simes
J.,
Tonkin
A.M.,
Pedersen
T.R.,
Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet.
2018;
392
(10155)
:
1311-20
.
View Article PubMed Google Scholar -
Tsimikas
S.,
Karwatowska-Prokopczuk
E.,
Gouni-Berthold
I.,
Tardif
J.C.,
Baum
S.J.,
Steinhagen-Thiessen
E.,
AKCEA-APO(a)-LRx Study Investigators
Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. The New England Journal of Medicine.
2020;
382
(3)
:
244-55
.
View Article PubMed Google Scholar -
Langsted
A.,
Nordestgaard
B.G.,
Antisense Oligonucleotides Targeting Lipoprotein(a). Current Atherosclerosis Reports.
2019;
21
(8)
:
30
.
View Article PubMed Google Scholar -
Boffa
M.B.,
Koschinsky
M.L.,
Therapeutic Lowering of Lipoprotein(a): A Role for Pharmacogenetics?. Circulation. Genomic and Precision Medicine.
2018;
11
(2)
:
e002052
.
View Article PubMed Google Scholar -
Sabatine
M.S.,
Giugliano
R.P.,
Keech
A.C.,
Honarpour
N.,
Wiviott
S.D.,
Murphy
S.A.,
Steering Committee
FOURIER,
Investigators
Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. The New England Journal of Medicine.
2017;
376
(18)
:
1713-22
.
View Article PubMed Google Scholar -
Schwartz
G.G.,
Steg
P.G.,
Szarek
M.,
Bhatt
D.L.,
Bittner
V.A.,
Diaz
R.,
Committees
ODYSSEY OUTCOMES,
Investigators
Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. The New England Journal of Medicine.
2018;
379
(22)
:
2097-107
.
View Article PubMed Google Scholar -
McNeal
C.J.,
Lipoprotein(a): its relevance to the pediatric population. Journal of Clinical Lipidology.
2015;
9
(5)
:
57-66
.
View Article PubMed Google Scholar -
Thygesen
K.,
Alpert
J.S.,
Jaffe
A.S.,
Chaitman
B.R.,
Bax
J.J.,
Morrow
D.A.,
Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction
Fourth Universal Definition of Myocardial Infarction (2018). Circulation.
2018;
138
(20)
:
e618-51
.
View Article PubMed Google Scholar -
Guan
W.,
Cao
J.,
Steffen
B.T.,
Post
W.S.,
Stein
J.H.,
Tattersall
M.C.,
Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology.
2015;
35
(4)
:
996-1001
.
View Article PubMed Google Scholar -
van Buuren
F.,
Horstkotte
D.,
Knabbe
C.,
Hinse
D.,
Mellwig
K.P.,
Incidence of elevated lipoprotein (a) levels in a large cohort of patients with cardiovascular disease. Clinical Research in Cardiology Supplements.
2017;
12
(1)
:
55-9
.
View Article PubMed Google Scholar -
Roth
C.,
Krychtiuk
K.A.,
Gangl
C.,
Schrutka
L.,
Distelmaier
K.,
Wojta
J.,
Lipoprotein(a) plasma levels are not associated with survival after acute coronary syndromes: an observational cohort study. PLoS One.
2020;
15
(1)
:
e0227054
.
View Article PubMed Google Scholar -
Gencer
B.,
Rigamonti
F.,
Nanchen
D.,
Vuilleumier
N.,
Kern
I.,
Aghlmandi
S.,
Prognostic value of elevated lipoprotein(a) in patients with acute coronary syndromes. European Journal of Clinical Investigation.
2019;
49
(7)
:
e13117
.
View Article PubMed Google Scholar -
O'Donoghue
M.L.,
Fazio
S.,
Giugliano
R.P.,
Lipoprotein(a), PCSK9 inhibition and cardiovascular risk: insights from the FOURIER trial. Circulation.
2018;
275
:
e9-10
.
-
Gencer
B.,
Rigamonti
F.,
Nanchen
D.,
Vuilleumier
N.,
Kern
I.,
Aghlmandi
S.,
Prognostic value of elevated lipoprotein(a) in patients with acute coronary syndromes. European Journal of Clinical Investigation.
2019;
49
(7)
:
e13117
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 1 (2022)
Page No.: 4873-4883
Published on: 2022-01-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6774 times
- PDF downloaded - 1757 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress