Abstract
Chimeric antigen receptor (CAR) T-cell therapy is a type of immunotherapy that uses the patient's immune system. It creates cancer-killing T cells through genetic modification that targets tumor antigens. CAR consists of three fundamental units, the extracellular, transmembrane, and intracellular domains. CARs are rapidly evolving with progress in the field of immunotherapy starting from first-generation CARs to next-generation CARs. Different cancer types, including B-cell malignancies, are being treated by CAR-T therapy. The FDA has approved two CAR-T therapies, namely, tisagenlecleucel and axicabtagene ciloleucel. The recently approved CAR-T products are Lisocabtagene maraleucel and Idecabtagene vicleucel. Despite the success of CAR-T therapy, several limitations, including cytokine release syndrome and neurotoxicity, need to be overcome. In the present review, we have provided an overview of CAR generations, their applications, potential limitations, and possible solutions for improving CAR-T therapy for a variety of tumor types.
Introduction
Cancer is one of the major causes of death worldwide. T cells, a type of white blood cell (WBC), are known to kill cancer cells. Chimeric antigen receptors (CARs) are WBCs specialized in targeting cancer cells by binding to their surface proteins. CAR-T-cell therapy harnesses the power of these T cells and CARs to kill cancer cells effectively. CAR-T cells are called the first living drugs, as once they are activated and infused, they remain in the body and show long-term results1. CAR-T-cell therapy mostly uses autologous T cells in which the patient’s own T cells are drawn out followed by genetic modifications and insertion into the same patient.
The FDA has approved a number of CAR-T therapies for patients with different types of cancers. Mainly two trademarks are registered: kymriah™ (tisagenlecleucel) and yescarta™ (axicabtagene ciloleucel). Recently, approved CAR-T therapy agents include lisocabtagene maraleucel (breyanzi™) and idecabtagene vicleucel (abecma™). Various antigens have been explored as potential targets for T-cell therapies in hematological and solid tumors. For instance, CD19 is used for B-cell acute lymphoblastic leukemia (B-ALL)2 and chronic lymphocytic leukemia (CLL). The majority of patients with relapsed or refractory B-ALL (R/R B–ALL) and CD19-targeted CAR-T cells have achieved complete disease remission3. Several toxicities are associated with the application of CAR-T-cell therapy, such as cytokine release syndrome (CRS), neurological toxicities, B-cell aplasia and hypogammaglobinemia. The two effective ways to cope with CRS and neurologic toxicity are to use tocilizumab and corticosteroids effectively.
This mini review highlights the recent developments in CAR-T therapy, its effective application in combating various types of cancers, updated information about the latest clinical trials and highlights its possible limitations.
Cancer Immunotherapy and Adoptive Cell Therapy
Immunotherapy is a treatment that makes use of the body's natural immune system, boosts its activity, and efficiently combats cancer and other disorders4. Immunotherapy helps in the direct control of killing cancerous cells by making the cells visible to the cancer fighting cells of the body5. Immunotherapy has successfully evolved in recent decades to address therapeutic barriers, bringing innovation to cancer treatment.
Active and passive immunotherapies are the two practiced immunotherapies. The former includes tumor vaccines and later includes monoclonal antibodies, oncolytic virotherapy and adoptive T-cell therapy (ACT). ACT is a type of passive immunotherapy that utilizes the body’s own T cells to treat cancer by manipulation of T cells outside the body, followed by their expansion and finally modified T-cell infusion back into the body6. The work was initiated in the 1980s when a new method was introduced to produce a large number of autologous lymphoid cells to fight cancer cells by Rosenberg’s team7.
Chimeric Antigen Receptor (CAR) and CAR-T-Cell Therapy
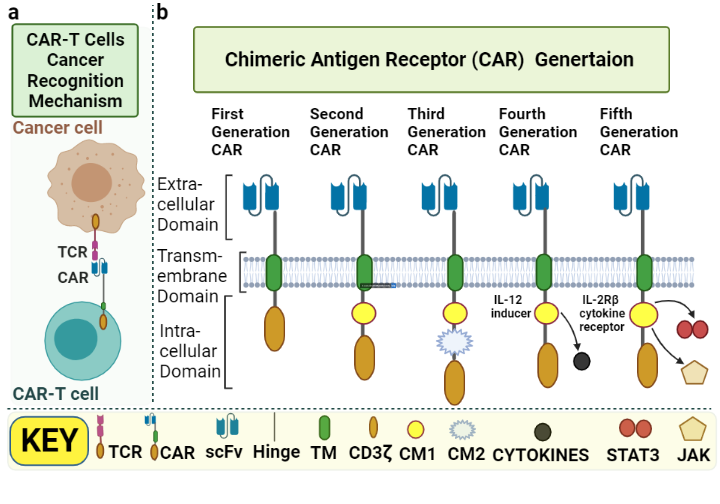
CARs are fusion proteins specifically manipulated to target antigens expressed on the surface of cells. In 1989, at the Weizmann Institute of Science, Eshhar’s group developed the first chimeric receptor8. In a very brief time span after their development and application, CAR-T cells have undergone an evolution of generations. CAR is a modified receptor consisting of three essential domains, including extracellular, transmembrane and intracellular domains9, 10. The intracellular domain helps in signaling T cells that allows the killing of cancerous cells in a human leukocyte antigen (HLA)-independent manner11, 12.
CAR-T therapy has revolutionized the field of treating various cancers. It is a way of providing personalized treatment according to the disease type and body requirements. It includes genetically modifying a person’s own T cells that will later own bind to the tumor antigen. This process is followed by proliferation and infusion back to the body to target and kill cancerous cells13.
Generalized Structural Design of Chimeric Antigen Receptors
Fundamentally, CAR consists of three main domains: the extracellular domain, transmembrane domain and intracellular domain1, 14. The extracellular domain is composed of a single-chain variable fragment (scFv) that is a fusion protein of the variable region of the light and heavy chains of the antibody. It is joined with the help of a spacer to the transmembrane domain that finally joins with the intracellular signaling domain, resulting in cytolysis of cancer cells1, 15, 16. The extracellular scFv aids CAR-T cells in binding to the targeted cells, whereas the intracellular domain helps activate T cells17, 18.
Generation of Chimeric Antigen Receptors
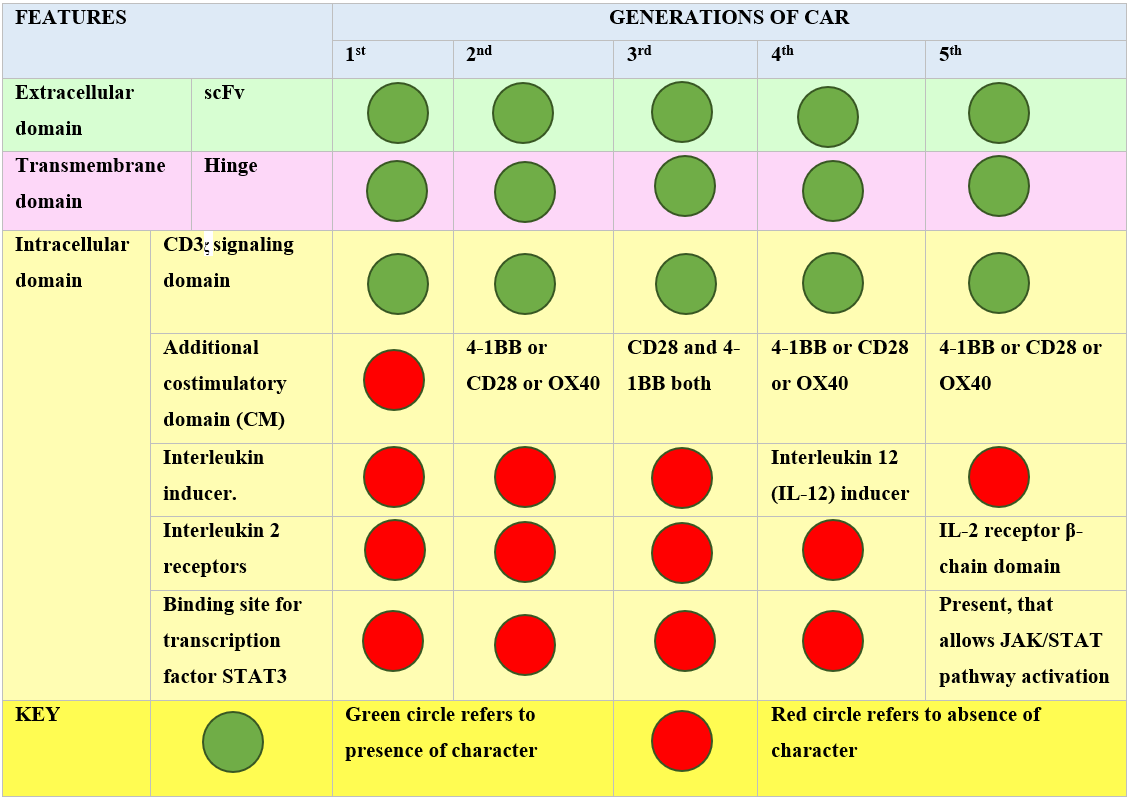
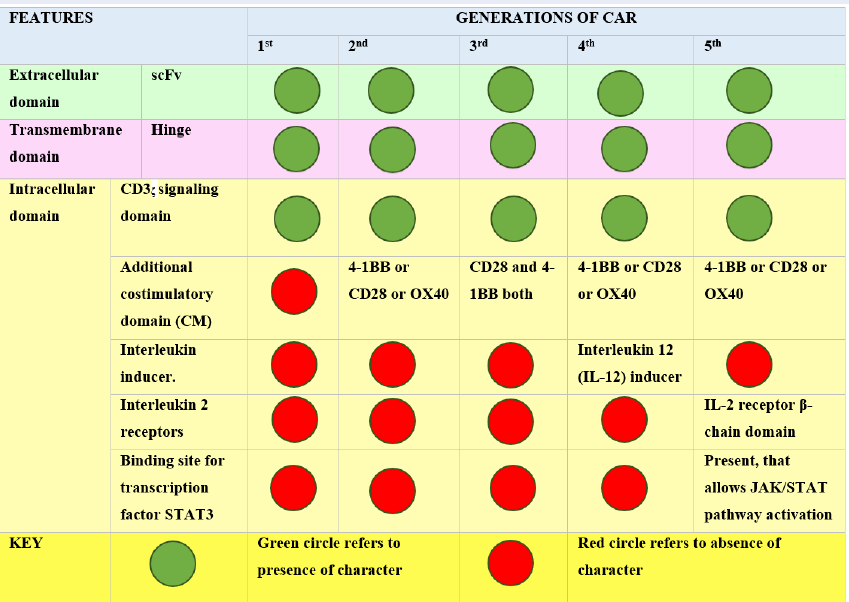
To date, there have been five main generations of CAR19. The difference in each generation CAR mainly lies in the structure and functionality of the intracellular domain20. Figure 1 summarizes the major differences and similarities among various CAR generations.
First-generation CARs are capable of activation only. Second-generation CARs perform dual signaling, and third-generation CARs are capable of multiple signaling1. Significant limitations have been noted for first-generation CARs, with less T-cell proliferation and inadequate release of cytokines21. The second and third generation have an additional structural modification, the intracellular costimulatory domains (CM)17. The CM in second-generation CARs may be 4-1BB22 or CD2823, 24. These modifications enhance the persistence and expansion of T cells. To further increase T-cell expansion and persistence, third-generation CARs were added to both CD28 and 4-1BB with no change in extracellular and transmembrane domain structures25, 26. TRUCK, the fourth-generation CAR, stands for Tcells redirected for universal cytokine-mediated killing20. This generation has resulted in a huge modification in the intracellular domain of the 2nd generation, i.e., the addition of cytokines such as interleukin 12 (IL-12), which activates natural killer (NK) cells in addition to other Tcells at the tumor site. IL-12 has the ability to cytolyse cancer cells that are not detected by CAR-T cells directly hence, IL-12 results in killing these cells by activating the innate immune response27. The fifth generation of CAR has anadditional modification of the binding site in the intracellular domain for the signal transducer and activator of transcription-3 (STAT3) transcription factor and another binding site for the interleukin-2 (IL-2) receptor28. Both these modifications provide better T-cell activation, persistence and proliferation by cytokine-inducing Janus kinase (JAK–STAT3/5) signaling29. The basic difference in each generation is shown in Figure 2.
CAR-T Therapy Procedure
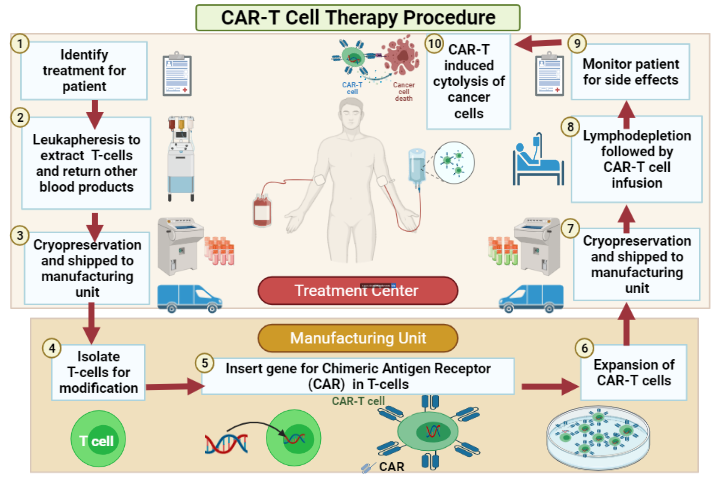
Leukapheresis is the first step to initiate CAR-T therapy. In this procedure, the patient goes to an apheresis machine that is used in autologous T-cell collection6. The patient’s own blood is drawn out, white blood cells are separated from the peripheral blood into a bag, and then they are cryopreserved so they can be sent for further manufacturing. If enough T cells were not collected, the process of leukapheresis was repeated the next day (Figure 3).
Upon successful collection of WBCs, the cells are selected based on the genetic code for the chimeric antigen receptor employing viral transduction. For example, T cells are genetically transduced ex vivo with a lentiviral vector encoding the anti-CD19 CAR30. The cells that express their chimeric antigen on their surface are then expanded to form many copies followed by cryopreservation and sent back to the treatment site. Simultaneously. Before introducing the CAR-T cells, the patient was given chemotherapy in the form of lymphodepleting treatment for three days, such as fludarabine or cyclophosphamide, to clear the immune system to make space for newly manufactured CAR-T cells31.
Finally, the CAR-T cells are infused into the patients’ blood. Inside the body, CAR-T cells target their cancer cells, expand rapidly and kill those cancer cells along with other B cells. The cytokines released by the destroyed cancer cells further lead to CAR-T-cell cell expansion32.
An effective response rate in various R/R B-cell malignancies is shown in patients who undergo leukopheresis followed by preparative lymphodepletion and finally treated with autologous T-cell infusion engineered for the expression of CD19-targeted CAR-T cells33, 34, 35, 36. The figure below shows the overall process of CAR-T therapy.
CAR-T Therapy Clinical Application
In recent years, various experiments and trials in clinical setup have been performed to check the efficiency of various CAR-T therapy agents for targeting different antigen receptors. Targeting CD19 has shown outstanding results in treating patients with CLL, B-ALL, Hodgkin lymphoma (HL) and B-NHL37, 38. The first efficient result was shown for CLL, but later, it showed a lower response rate, whereas in the case of R/R ALL, it showed a ninety percent remission rate 39. With patients suffering from B-ALL, using CAR-T cells that target CD19 resulted in complete remission rates of seventy to ninety-four percent37. After gaining efficient results, CAR-T therapy targeting CD19 is also used for patients suffering from FL and other kinds of B-cell-associated malignancies40. Other than targeting CD19, some studies have also shown efficient treatment by targeting CD2041 and CD3042. Recent advancements in CAR-T therapy have led to its effective use in treating solid tumors in addition to B-cell malignancies. It has been used for pediatric brain tumors that have caused fatalities in both adults and children. However, there are still some limitations to its effective use19.
CAR-T Therapy Approved Agents
Currently, various CAR-T therapy agents are in use and approved by different drug regulatory agencies, such as the United States Food and Drug Administration (US FDA) and European Medicines Agency32. The four main approved agents are given below.
Tisagenlecleucel by Kymriah™ is the first FDA approved CAR-T therapy43. It is used in patients suffering from R/R-ALL in both children and adults of approximately 3 years to 25 years of age44. On May 1, 2018, it was approved for use in adult patients suffering from B-cell lymphomas. Currently, tisagenlecleucel is also reviewed by the FDA for its use in CLL, R/R B-cell-ALL, R/R diffuse large B-cell lymphoma (DLBCL) and second-line DLBCL45. Despite its efficacy, this therapy comes with a high cost of nearly 475,000 dollars43.
Axicabtagene ciloleucel by Yescarta™ is another T-cell therapy agent that was approved by the FDA on October 18, 2017. It is prescribed therapy for patients suffering from DLBCL and R/R aggressive B-cell non-Hodgkin lymphoma (B-NHL)6. Currently, this therapy is also being applied and tested for mantle cell lymphoma (MCL) and follicular lymphoma (FL)45. It’s also an expensive therapy like tisagenlecleucel but its cost is little less, i.e., approximately 373,000 dollars43.
Lisocabtagene maraleucel by Breyanzi™ is the third CAR agent approved on February 5, 2021 by the FDA. It is approved for treating adult patients suffering from R/R B-ALL lymphomas. Quite recently, in March 2021, another FDA approved CAR-T therapy; Idecabtagene vicleucel by Abecma™ was used for treating patients suffering from R/R MM46, 47. Even though they are approved, therapies are still a topic of research and experiments for studying their effectiveness and any associated resulting toxicity45.
CAR-T Therapy-Associated Toxicity
With the outstanding results of CAR-T therapy, one may come across some toxic side effects induced by CAR-T-cell infusion. From mild to severe, some toxicities may be life-threatening or even fatal in some situations. The most common postprocedure toxicities for CAR-T-cell therapy include cytokine release syndrome (CRS) and neurological toxicities. The major CRS manifestations include fever, hypoxia, hypotension, cytopenia and fatigue48. CAR-T cells targeting CD19 may also damage B cells in addition to cancerous cells because CD19 is also found on the surface of normal B cells along with cancerous cells. In such cases, the patient may develop B-cell aplasia and hypogammaglobulinemia. There are multiple factors that affect the efficacy of CAR-T-cell therapy and induce posttreatment toxicity, including a high CAR-T-cell count in blood, peak cytokine levels and patient vulnerability to the immune system due to preparative chemotherapy48. The more common CRS-associated toxicity is usually treated with tocilizumab, which works on high interleukin-6 (IL-6) levels49. If tocilizumab shows ineffective treatment for CRs, corticosteroids may be given to the patient. Normally, corticosteroids are thought to be more effective against neurologic toxicities48.
Pre-Clinical Success of CAR-T Therapy
Chimeric antigen receptors have been studied widely in clinical and preclinical settings. The toxicities associated with CAR-T-cell application are different in human and animal models. Siegler and Wang (2018) summarized approximately all present preclinical studies performed on mice. The study provided information not only about xenograft, syngeneic, and transgenic models but also about humanized mouse models50. A xenotransplant murine model of B-cell malignancy targeting CD19 has also been studied51. For some past years, most preclinical studies were performed only on mice. However, there was a need to fill the gap, as there was no such reported neurotoxicity or cytokine release syndrome in murine models. To overcome this, primate models were then used as their immune system matches more closely with humans. However, primate models lack the ability to study antitumor efficacy. [50]. Nevertheless, the use of primate models such as rhesus macaques showed resulting neurotoxicity due to CAR-T application52.
Clinical Success of CAR-T Therapy
In 2003, the US led the first CAR-T clinical trial. It was focused on treating epithelial ovarian cancer53. This proves the significance of immunotherapy in enhancing the patient survival rate and reducing disease progression54. In 2010, Germany and the UK studied CD19-targeted CAR-T therapy in children suffering from ALL. In 2012, the 1st trial using CAR-T cells was performed by the PLAGH group, which focused on studying the effectiveness of CAR-T therapy in refractory lymphomas that are resistant to chemotherapies54. In 2013, there was a worldwide increase in the number of conducted trials on CAR-T, and they are still increasing to date53.
| No. | Study Title | Clinical Trial |
|---|---|---|
| 1 | Clinical Study of T Cell Infusion Targeting BCMA Chimeric Antigen Receptor. | NCT04650724 |
| 2 | 4th Generation Chimeric Antigen Receptor T Cells Targeting Glypican-3 | NCT03980288 |
| 3 | Cell Therapy for CD7 Positive T-cell Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma Using CD7-Specific CAR-T Cells | NCT04572308 |
| 4 | Pembrolizumab in Patients Failing to Respond to or Relapsing After CAR T Cell Therapy for Relapsed or Refractory Lymphomas | NCT02650999 |
| 5 | CAR-T Hepatic Artery Infusions and Sir-Spheres for Liver Metastases | NCT02416466 |
| 6 | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients With Malignant Gliomas Expressing EGFRvIII | NCT01454596 |
| 7 | CD19 /22 CAR T Cells (AUTO3) for the Treatment of B Cell Acute Lymphoblastic Leukemia (ALL) | NCT03289455 |
| 8 | Anti-CD19 Chimeric Antigen Receptor (CAR)-Transduced T Cell Therapy for Patients With B Cell Malignancies | NCT03076437 |
| 9 | Chimeric Antigen Receptor T Cells Targeting Glypican-3 | NCT03884751 |
| 10 | CD19-CAR-T in B-cell Malignancies Patients | NCT03952923 |
| 11 | CD19 CAR T Cells in Patients With Resistant or Refractory CD19+ Acute Lymphoblastic Leukemia | NCT02975687 |
| 12 | CD19-targeting, 3rd Generation CAR T Cells for Refractory B Cells Malignancy | NCT03068416 |
| 13 | A PhaseⅠb Study Evaluating Safety and Efficacy of C-CAR011 Treatment in B- NHL Subjects | NCT03483688 |
| 14 | CD19 CAR T Cells in Patients With Relapsed or Refractory CD19 Positive B-cell Lymphoma | NCT03029338 |
| 15 | Laboratory Treated T Cells in Treating Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia, Non-Hodgkin Lymphoma, or Acute Lymphoblastic Leukemia | NCT01865617 |
| 16 | Safety Study of Chimeric Antigen Receptor Modified T-cells Targeting NKG2D-Ligands | NCT02850536 |
| 17 | CAR-T Hepatic Artery Infusions or Pancreatic Venous Infusions for CEA-Expressing Liver Metastases or Pancreas Cancer | NCT02203825 |
| 18 | CD19 CAR T Cells for B Cell Malignancies After Allogeneic Transplant | NCT01475058 |
| 19 | Treatment of Relapsed and/or Chemotherapy Refractory B-cell Malignancy by Tandem CAR T Cells Targeting CD19 and CD20 | NCT03811457 |
| 20 | A Phase I Trial of T Cells Expressing an Anti-GD2 Chimeric Antigen Receptor in Children and Young Adults With GD2+ Solid Tumors | NCT02107963 |
| 21 | Immunotherapy With CD19 CAR T-cells in Patients With Relapsed or Refractory CD19+ Leukemia and Lymphoma | NCT03097770 |
| 22 | CAR T Cell Receptor Immunotherapy for Patients With B-cell Lymphoma | NCT03173417 |
| 23 | Safety and Efficacy Evaluation of IM19 CAR-T Cells (IM19CAR-T) | NCT00924326 |
| 24 | A Phase 1 Study Evaluating Safety and Efficacy of C-CAR011 Treatment in DLBCL Subjects | NCT02976857 |
| 25 | CD19-targeting 3rd Generation CAR T Cells for Refractory B Cell Malignancy - a Phase I/IIa Trial. | NCT02132624 |
| 26 | T Cells Expressing a Fully-Human Anti-CD30 Chimeric Antigen Receptor for Treating CD30-Expressing Lymphomas | NCT03049449 |
| 27 | A Phase I Trial of Anti-GD2 T-cells (1RG-CART) | NCT02761915 |
| 28 | CAR-GPC3 T Cells in Patients With Refractory Hepatocellular Carcinoma | NCT03146234 |
| 29 | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients With GBM | NCT01109095 |
| 30 | Dose Optimization Trial of CD19 Redirected Autologous T Cells | NCT01747486 |
| 31 | T-cells Expressing an Anti-SLAMF7 CAR for Treating Multiple Myeloma | NCT03958656 |
| 32 | Anti-CD19 White Blood Cells for Children and Young Adults With B Cell Leukemia or Lymphoma | NCT01593696 |
| Recruitment Status | Description | Clinical Trial No. |
|---|---|---|
| Not yet recruiting | The study has not started recruiting participants. | 71 |
| Recruiting | The study is currently recruiting participants. | 374 |
| Enrolling by invitation | The study is selecting its participants from a population, or group of people, decided on by the researchers in advance. These studies are not open to everyone who meets the eligibility criteria but only to people in that particular population, who are specifically invited to participate. | 5 |
| Active, not recruiting | The study is ongoing, and participants are receiving an intervention or being examined, but potential participants are not currently being recruited or enrolled. | 64 |
| Suspended | The study has stopped early but may start again. | 6 |
| Terminated | The study has stopped early and will not start again. Participants are no longer being examined or treated. | 18 |
| Completed | The study has ended normally, and participants are no longer being examined or treated (that is, the last participant's last visit has occurred). | 32 |
| Withdrawn | The study stopped early, before enrolling its first participant. | 12 |
| Unknown status | A study on ClinicalTrials.gov whose last known status was recruiting; not yet recruiting; or active, not recruiting but that has passed its completion date, and the status has not been last verified within the past 2 years. | 127 |
Numerous trials have successfully been completed using CAR-T cells to treat various diseases. To analyze the list of total completed trials, we searched ClinicalTrials.gov using the keywords “CAR-T,” “CAR-T therapy,” and “chimeric antigen receptor” with a filter for “completed” trials. Out of 32 reported trials from clinicaltrials.gov, fourteen were held in different institutions in China and fourteen separately in different regions of the United States. Two were in the United Kingdom, and one was held in Sweden (Table 1). The trials were performed to study the effect of CAR-T cells in curing various conditions, such as ALL, acute myeloid leukemia, relapsed or refractory CLL, B-ALL, brain cancer, FL, glioblastoma multiforme, gliosarcoma, lymphoid cancer, B-NHL, MCL, DLBCL, myelodysplastic syndrome, hepatocellular carcinoma, myeloma, neuroblastoma and osteosarcoma.
Present and Future work in CAR-T Therapy
To analyze the list of total trials, the same method was used as employed earlier. We searched overall trials either completed or ongoing by using the keywords “CAR-T,” “CAR-T therapy,” and “chimeric antigen receptor” on ClinicalTrials.gov. A list of a total of 706 results was obtained for trials that made use of CAR-T therapy in one way or another. The data were further filtered based on recruitment status to obtain more categorized data, as shown in Table 2.
Limitations of CAR-T
One of the limitations of CAR-T therapy is its inapplicability in solid tumors such as pediatric brain tumors, which hinders T-cell transport and proliferation19, although it has high enough efficacy for blood cancers28. Other limitations include antigenic escape, low persistence, an immunosuppressive microenvironment and other life-threatening conditions following CAR-T-cell infusion55. The high cost of the therapy is considered to be another limitation, ranging from 373,000 to 475,000 dollars for CAR-T therapy approved agents by the FDA43. Advancement is needed to effectively treat solid tumors and other diseases with minimum toxic effects.
Conclusion and Perspective
For successful application of CAR-T-cell therapy, better toxicity grading and management techniques are required to overcome the toxic side effects and other challenges that hinder effective CAR-T-cell therapy application. The two common and life-threatening CAR-T therapy-associated toxicities are CRS and neurotoxicity. Getting more knowledge about the underlying causes and mechanism of such toxicities will lead to better management of such toxicity and improved CAR-T efficacy. Additionally, clinical trials and studies to date are focusing more on liquid cancers such as leukemia and lymphomas, and it is urgently needed to expand CAR-T therapy advantages to solid tumors. In short, there is a dire need to perform clinical trials to dig more information so that targeted treatment can be performed with minimum normal cell cytolysis and less toxicity.
Abbreviations
ACT: Adoptive T-cell therapy, B-ALL: B-cell acute lymphoblastic leukemia, B-NHL: B-cell non-Hodgkin lymphoma, CAR: Chimeric antigen receptor, CLL: Chronic lymphocytic leukemia, CM: Costimulatory domains, CRS: Cytokine release syndrome, FL: Follicular lymphoma, HL: Hodgkin lymphoma, HLA: Human leukocyte antigen, IL-6: Interlelukin-6, IL-12: Interleukin 12, JAK: Janus kinases, MCL: Mantle cell lymphoma, NK: Natural killer, PLAGH: People's Liberation Army General Hospital, DLBCL: R/R diffuse large B-cell lymphoma, R/R B–ALL: Relapsed or refractory B-ALL, STAT3: Signal transducer and activator of transcription-3, scFv: Single-chain variable fragment, TCR: T-cell receptor, TRUCK: T-cells redirected for universal cytokine mediated killing, WBCs: White blood cells
Acknowledgments
The authors are thankful to the Vice-chancellor of the University of the Punjab, Lahore, Pakistan, and the Vice-chancellor of the University of Okara, Punjab, Pakistan, for providing support for the accomplishment of this study. All the figures were drawn using Biorender.com.
Author’s contributions
MHA, AR, and MBK collected the data, drew the figure, and wrote the manuscript. AF and AM revised the figure and the manuscript. MHA and NS proposed the idea, approved the final draft, and supervised the whole project. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sadelain
M.,
Brentjens
R.,
Rivière
I.,
The basic principles of chimeric antigen receptor design. Cancer Discovery.
2013;
3
(4)
:
388-98
.
View Article PubMed Google Scholar -
Greinix
H.T.,
Role of CAR-T cell therapy in B-cell acute lymphoblastic leukemia. memo-. Magazine of European Medical Oncology.
2020;
13
(1)
:
36-42
.
View Article Google Scholar -
Davila
M.L.,
Brentjens
R.J.,
CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clinical advances in hematology & oncology: H&O.
2016;
14
(10)
:
802
.
-
Haji-Fatahaliha
M.,
Hosseini
M.,
Akbarian
A.,
Sadreddini
S.,
Jadidi-Niaragh
F.,
Yousefi
M.,
CAR-modified T-cell therapy for cancer: an updated review. Artificial Cells, Nanomedicine, and Biotechnology.
2016;
44
(6)
:
1339-49
.
View Article PubMed Google Scholar -
Eggermont
L.J.,
Paulis
L.E.,
Tel
J.,
Figdor
C.G.,
Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends in Biotechnology.
2014;
32
(9)
:
456-65
.
View Article PubMed Google Scholar -
Miliotou
A.N.,
Papadopoulou
L.C.,
CAR T-cell therapy: a new era in cancer immunotherapy. Current Pharmaceutical Biotechnology.
2018;
19
(1)
:
5-18
.
View Article PubMed Google Scholar -
Rosenberg
S.A.,
Lotze
M.T.,
Muul
L.M.,
Leitman
S.,
Chang
A.E.,
Ettinghausen
S.E.,
Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. The New England Journal of Medicine.
1985;
313
(23)
:
1485-92
.
View Article PubMed Google Scholar -
Gross
G.,
Waks
T.,
Eshhar
Z.,
Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America.
1989;
86
(24)
:
10024-8
.
View Article PubMed Google Scholar -
Sadelain
M.,
Brentjens
R.,
Rivière
I.,
The basic principles of chimeric antigen receptor design. Cancer Discovery.
2013;
3
(4)
:
388-98
.
View Article PubMed Google Scholar -
Srivastava
S.,
Riddell
S.R.,
Engineering CAR-T cells: design concepts. Trends in Immunology.
2015;
36
(8)
:
494-502
.
View Article PubMed Google Scholar -
Park
J.H.,
Brentjens
R.J.,
Adoptive immunotherapy for B-cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discovery Medicine.
2010;
9
(47)
:
277-88
.
PubMed Google Scholar -
Park
J.H.,
Geyer
M.B.,
Brentjens
R.J.,
CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood.
2016;
127
(26)
:
3312-20
.
View Article PubMed Google Scholar -
Feins
S.,
Kong
W.,
Williams
E.F.,
Milone
M.C.,
Fraietta
J.A.,
An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. American Journal of Hematology.
2019;
94
:
3-9
.
View Article PubMed Google Scholar -
Srivastava
S.,
Riddell
S.R.,
Engineering CAR-T cells: design concepts. Trends in Immunology.
2015;
36
(8)
:
494-502
.
View Article PubMed Google Scholar -
Park
J.H.,
Brentjens
R.J.,
Adoptive immunotherapy for B-cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discovery Medicine.
2010;
9
(47)
:
277-88
.
PubMed Google Scholar -
Park
J.H.,
Rivière
I.,
Gonen
M.,
Wang
X.,
Sénéchal
B.,
Curran
K.J.,
Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. The New England Journal of Medicine.
2018;
378
(5)
:
449-59
.
View Article PubMed Google Scholar -
Abate-Daga
D.,
Davila
M.L.,
CAR models: next-generation CAR modifications for enhanced T-cell function. Molecular Therapy Oncolytics.
2016;
3
:
16014
.
View Article PubMed Google Scholar -
Sadelain
M.,
Rivière
I.,
Brentjens
R.,
Targeting tumours with genetically enhanced T lymphocytes. Nature Reviews. Cancer.
2003;
3
(1)
:
35-45
.
View Article PubMed Google Scholar -
Wu
W.T.,
Lin
W.Y.,
Chen
Y.W.,
Lin
C.F.,
Wang
H.H.,
Wu
S.H.,
New Era of Immunotherapy in Pediatric Brain Tumors: Chimeric Antigen Receptor T-Cell Therapy. International Journal of Molecular Sciences.
2021;
22
(5)
:
2404
.
View Article PubMed Google Scholar -
Zhang
C.,
Liu
J.,
Zhong
J.F.,
Zhang
X.,
Engineering CAR-T cells. Biomarker Research.
2017;
5
(1)
:
22
.
View Article PubMed Google Scholar -
Firor
A.E.,
Jares
A.,
Ma
Y.,
From humble beginnings to success in the clinic: chimeric antigen receptor-modified T-cells and implications for immunotherapy. Experimental Biology and Medicine (Maywood, N.J.).
2015;
240
(8)
:
1087-98
.
View Article PubMed Google Scholar -
Imai
C.,
Mihara
K.,
Andreansky
M.,
Nicholson
I.C.,
Pui
C.H.,
Geiger
T.L.,
Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia.
2004;
18
(4)
:
676-84
.
View Article PubMed Google Scholar -
Brentjens
R.J.,
Santos
E.,
Nikhamin
Y.,
Yeh
R.,
Matsushita
M.,
La Perle
K.,
Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clinical Cancer Research.
2007;
13
(18 Pt 1)
:
5426-35
.
View Article PubMed Google Scholar -
Finney
H.M.,
Lawson
A.D.,
Bebbington
C.R.,
Weir
A.N.,
Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. Journal of Immunology (Baltimore, Md.: 1950).
1998;
161
(6)
:
2791-7
.
PubMed Google Scholar -
Heczey
A.,
Louis
C.U.,
Savoldo
B.,
Dakhova
O.,
Durett
A.,
Grilley
B.,
CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Molecular Therapy.
2017;
25
(9)
:
2214-24
.
View Article PubMed Google Scholar -
Morgan
R.A.,
Yang
J.C.,
Kitano
M.,
Dudley
M.E.,
Laurencot
C.M.,
Rosenberg
S.A.,
Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy.
2010;
18
(4)
:
843-51
.
View Article PubMed Google Scholar -
Chmielewski
M.,
Abken
H.,
TRUCKs: the fourth generation of CARs. Expert Opinion on Biological Therapy.
2015;
15
(8)
:
1145-54
.
View Article PubMed Google Scholar -
Tokarew
N.,
Ogonek
J.,
Endres
S.,
von Bergwelt-Baildon
M.,
Kobold
S.,
Teaching an old dog new tricks: next-generation CAR T cells. British Journal of Cancer.
2019;
120
(1)
:
26-37
.
View Article PubMed Google Scholar -
Kagoya
Y.,
Tanaka
S.,
Guo
T.,
Anczurowski
M.,
Wang
C.H.,
Saso
K.,
A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nature Medicine.
2018;
24
(3)
:
352-9
.
View Article PubMed Google Scholar -
Cordes
N.,
Kolbe
C.,
Lock
D.,
Holzer
T.,
Althoff
D.,
Schäfer
D.,
Anti-CD19 CARs displayed at the surface of lentiviral vector particles promote transduction of target-expressing cells. Molecular Therapy. Methods & Clinical Development.
2021;
21
:
42-53
.
View Article PubMed Google Scholar -
Wall
D.A.,
Krueger
J.,
Chimeric antigen receptor T cell therapy comes to clinical practice. Current Oncology (Toronto, Ont.).
2020;
27
(12)
:
115-23
.
View Article PubMed Google Scholar -
Priceman
S.J.,
Forman
S.J.,
Brown
C.E.,
Smart CARs engineered for cancer immunotherapy. Current Opinion in Oncology.
2015;
27
(6)
:
466-74
.
View Article PubMed Google Scholar -
Maude
S.L.,
Laetsch
T.W.,
Buechner
J.,
Rives
S.,
Boyer
M.,
Bittencourt
H.,
Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. The New England Journal of Medicine.
2018;
378
(5)
:
439-48
.
View Article PubMed Google Scholar -
Neelapu
S.S.,
Locke
F.L.,
Bartlett
N.L.,
Lekakis
L.J.,
Miklos
D.B.,
Jacobson
C.A.,
Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. The New England Journal of Medicine.
2017;
377
(26)
:
2531-44
.
View Article PubMed Google Scholar -
Turtle
C.J.,
Hanafi
L.A.,
Berger
C.,
Gooley
T.A.,
Cherian
S.,
Hudecek
M.,
CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. The Journal of Clinical Investigation.
2016;
126
(6)
:
2123-38
.
View Article PubMed Google Scholar -
Turtle
C.J.,
Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor–modified T cells. Science translational medicine.
2016;
8
(355)
:
355ra116-355ra116
.
View Article Google Scholar -
Grupp
S.A.,
American Society of Hematology Washington: DC; 2015.
View Article Google Scholar -
Kochenderfer
J.N.,
Dudley
M.E.,
Kassim
S.H.,
Somerville
R.P.,
Carpenter
R.O.,
Stetler-Stevenson
M.,
Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of Clinical Oncology.
2015;
33
(6)
:
540-9
.
View Article PubMed Google Scholar -
Singh
N.,
Frey
N.V.,
Grupp
S.A.,
Maude
S.L.,
CAR T cell therapy in acute lymphoblastic leukemia and potential for chronic lymphocytic leukemia. Current Treatment Options in Oncology.
2016;
17
(6)
:
28
.
View Article PubMed Google Scholar -
Johnson
L.A.,
June
C.H.,
Driving gene-engineered T cell immunotherapy of cancer. Cell Research.
2017;
27
(1)
:
38-58
.
View Article PubMed Google Scholar -
Zhang
W.Y.,
Wang
Y.,
Guo
Y.L.,
Dai
H.R.,
Yang
Q.M.,
Zhang
Y.J.,
Treatment of CD20-directed Chimeric Antigen Receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduction and Targeted Therapy.
2016;
1
(1)
:
16002
.
View Article PubMed Google Scholar -
Wang
C.M.,
Wu
Z.Q.,
Wang
Y.,
Guo
Y.L.,
Dai
H.R.,
Wang
X.H.,
Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clinical Cancer Research.
2017;
23
(5)
:
1156-66
.
View Article PubMed Google Scholar -
Prasad
V.,
Immunotherapy: Tisagenlecleucel - the first approved CAR-T-cell therapy: implications for payers and policy makers. Nature Reviews. Clinical Oncology.
2018;
15
(1)
:
11-2
.
View Article PubMed Google Scholar -
Ahmad
A.,
Uddin
S.,
Steinhoff
M.,
Car-t cell therapies: an overview of clinical studies supporting their approved use against acute lymphoblastic leukemia and large b-cell lymphomas. International Journal of Molecular Sciences.
2020;
21
(11)
:
3906
.
View Article PubMed Google Scholar -
Yip
A.,
Webster
R.M.,
The market for chimeric antigen receptor T cell therapies. Nature Reviews. Drug Discovery.
2018;
17
(3)
:
161-2
.
View Article PubMed Google Scholar -
Mullard
A.,
FDA approves first BCMA-targeted CAR-T cell therapy. Nature reviews. Drug Discovery, 2021..
.
-
Munshi
N.C.,
Anderson
L.D.,
Shah
N.,
Madduri
D.,
Berdeja
J.,
Lonial
S.,
Idecabtagene vicleucel in relapsed and refractory multiple myeloma. The New England Journal of Medicine.
2021;
384
(8)
:
705-16
.
View Article PubMed Google Scholar -
Brudno
J.N.,
Kochenderfer
J.N.,
Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Reviews.
2019;
34
:
45-55
.
View Article PubMed Google Scholar -
Si
S.,
Teachey
D.T.,
Spotlight on Tocilizumab in the Treatment of CAR-T-Cell-Induced Cytokine Release Syndrome: Clinical Evidence to Date. Therapeutics and Clinical Risk Management.
2020;
16
:
705-14
.
PubMed Google Scholar -
Siegler
E.L.,
Wang
P.,
Preclinical Models in Chimeric Antigen Receptor-Engineered T-Cell Therapy. Human Gene Therapy.
2018;
29
(5)
:
534-46
.
View Article PubMed Google Scholar -
Lee
J.C.,
Hayman
E.,
Pegram
H.J.,
Santos
E.,
Heller
G.,
Sadelain
M.,
In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Research.
2011;
71
(8)
:
2871-81
.
View Article PubMed Google Scholar -
Taraseviciute
A.,
Tkachev
V.,
Ponce
R.,
Turtle
C.J.,
Snyder
J.M.,
Liggitt
H.D.,
Chimeric Antigen Receptor T Cell-Mediated Neurotoxicity in Nonhuman Primates. Cancer Discovery.
2018;
8
(6)
:
750-63
.
View Article PubMed Google Scholar -
Wei
J.,
Guo
Y.,
Wang
Y.,
Wu
Z.,
Bo
J.,
Zhang
B.,
Clinical development of CAR T cell therapy in China: 2020 update. Cellular & Molecular Immunology.
2021;
18
(4)
:
792-804
.
View Article PubMed Google Scholar -
Zhang
L.,
Conejo-Garcia
J.R.,
Katsaros
D.,
Gimotty
P.A.,
Massobrio
M.,
Regnani
G.,
Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England Journal of Medicine.
2003;
348
(3)
:
203-13
.
View Article PubMed Google Scholar -
Sterner
R.C.,
Sterner
R.M.,
CAR-T cell therapy: current limitations and potential strategies. Blood Cancer Journal.
2021;
11
(4)
:
69
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 2 (2022)
Page No.: 4920-4929
Published on: 2022-02-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 12503 times
- PDF downloaded - 2742 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress