Abstract
Background: This study aims to evaluate serum concentrations of anti-collagen type II (anti-CII) antibodies in Iraqi patients with a severe rheumatoid arthritis (RA) phenotype and investigate the relationship between higher concentrations of anti-CII antibodies and higher levels of inflammation.
Methods: This study was conducted with 100 patients with RA who were admitted to the Arthritis Consulting Clinic, Baghdad Teaching Hospital, Iraq. The patients selected were patients diagnosed with RA about 2 to 3 years ago. Data on the patients were collected, including the results of tests for anti-CII concentrations, and were compared with data from 30 healthy subjects. Healthcare workers aspirated 5 ml of blood from each control and patient subject, divided into two parts. The workers transferred the first one (3 ml) into a plain tube, allowed 30 minutes for it to clot, and then isolated the serum by centrifugation at 2500 rpm for 10 minutes for measurement of the anti-collagen type II antibodies by enzyme-linked immunosorbent assay (ELISA). The workers transferred the second part (2 ml) to the tube containing EDTA for hematological measurement of the erythrocyte sedimentation rate (ESR).
Results: This study found that the levels of anti-collagen type II antibodies were significantly higher in patients with RA than in healthy controls (p-value > 0.05). The study found a statistically significant positive moderate correlation with ESR (r = 0.56, p-value = 0.000) and a statistically significant positive strong correlation between disease activity score-28 (DAS28) and anti-collagen type II antibodies (r = 0.65, p-value = 0.000) in RA patients.
Conclusions: Anti-CII antibodies can be considered an important parameter for the early detection of RA and can be used to determine the activity of RA.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that affects multiple joints on both sides of the body. It is a long-term condition that causes inflammation of the joints and surrounding tissues and is characterized by tendinitis, which leads to the erosion of cartilage and bone1. Worldwide, RA is the most common type of systemic arthritis. Women, smokers, and those with a family history of the disease are the most vulnerable groups2. Toxic elements and trace elements are among the many contributing factors proposed to participate in the pathogenesis of this disease3. Synovitis is represented at the onset of clinical symptoms and systemic comorbidities that affect blood vessels, metabolism, and bones4. Histological features of RA synovitis have been broadly characterized. The synovial appearance does not change significantly in the pre-rheumatoid stage5.
Collagen type II is the dominant hyaline cartilage collagen. Native human collagen type II (anti-CII) patients form a distinct phenotype of RA, which is associated with acute inflammatory disease6. The early emergence of anti-CII antibodies characterizes the early inflammation and destruction phenotype in adult patients with RA7. Studies have shown that anti-CII stimulates pro‑inflammatory cytokines; these findings are consistent with previous reports and research that have shown that elevated levels of anti-CII are associated with higher levels of ESR, CRP, TNFα, and IL-6. Anti-CII-positive RA patients also have a lower delay in diagnosis, possibly due to higher inflammatory activity7. Disease activity score-28 (DAS28) is an instrument used to monitor and measure disease activity in patients with RA. DAS28 is among the rheumatic joint disease activity measures recommended by the American College of Rheumatology (ACR)8. It is a system commonly used in daily clinical practice to score and assess the activity of the disease. The score is derived from a set of four variables: number of painful joints, number of swollen joints, ESR, and the patient’s overall health assessment9.
In clinical cases, clinical examinations and laboratory tests assess the activity of the disease by regularly measuring acute phase reagents such as ESR and CRP, which are elevated in most RA patients10. This is important for changing patients’ drugs from synthetic to biological or from one biological agent to another11.
Methods
This study was conducted on 100 patients with RA admitted to the Arthritis Consulting Clinic, Baghdad Teaching Hospital, Iraq. The patients selected were patients diagnosed with RA about 3 years ago. Data on the patient group, including results of tests for anti-CII concentrations, were collected and compared with data from 30 healthy subjects.
Healthcare workers aspirated 5 ml of blood from each control and patient subject and divided it into two parts. The workers transferred the first one (3 ml) into a plain tube and waited for 30 minutes for it to clot. The serum was then isolated by centrifugation at 2500 rpm for 10 minutes to measure the anti-collagen type II antibodies by enzyme-linked immunosorbent assay (ELISA). The workers transferred the second part (2 ml) to the tube containing EDTA for hematological measurement of the erythrocyte sedimentation rate (ESR).
| Patients | Control | ||||
|---|---|---|---|---|---|
| Group | % | % | |||
| Gender | Female | 74 | 74.0% | 22 | 73.3% |
| Male | 26 | 26.0% | 8 | 26.7% | |
| Age group (years) | 21-30 | 8 | 8.0% | 2 | 6.7% |
| 31-40 | 20 | 20.0% | 7 | 23.3% | |
| 41-50 | 38 | 38.0% | 10 | 33.3% | |
| 51-60 | 23 | 23.0% | 8 | 26.7% | |
| 61-70 | 9 | 9.0% | 3 | 10.0% | |
| equal to or above 71 | 2 | 2.0% | 2 | 6.7% | |
| Total | 100 | 100.0% | 30 | 100% | |
| Variables | Groups | Mean ± SE | P-Value |
|---|---|---|---|
| Anti-collagen type II antibodies (anti-CII) (ng/dl) | Control | 0.63 ± 0.03 | 0.00* |
| Patients | 29.69 ± 1.10 |
| Mean ± SE | Between Groups | Within groups | p-value | ||
|---|---|---|---|---|---|
| (anti-CII)(ng/dl) | Remission | 11.51 ± 0.94 | 5200.42 | 6942.53 | 0.000* |
| Low disease activity | 17.06 ± 4.30 | ||||
| Moderate disease activity | 21.32 ± 2.08 | ||||
| High disease activity | 34.22 ± 1.00 | ||||
| Total | 29.69 ± 1.10 |
Results
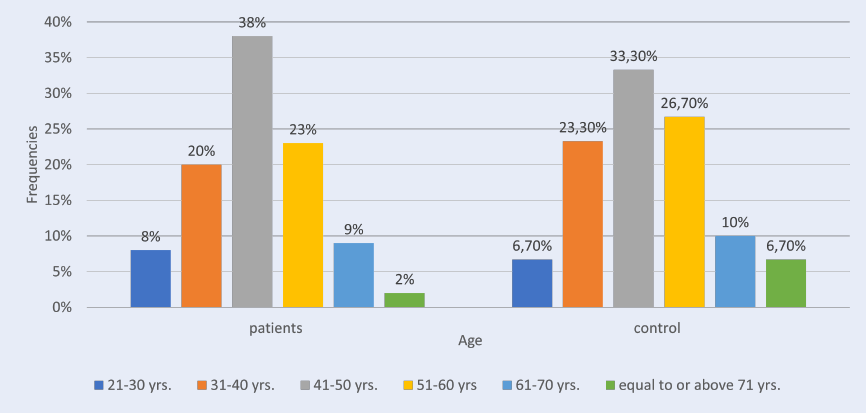
This study included 96 females (74%) and 34 males (26%). In the patient group, the greatest plurality of participants (38%) was aged between 41 and 50 yrs., followed by 51 – 60 yrs. (23%), 31 – 40 yrs. (20 %), 61 – 70 yrs. (9%), 21 – 30 yrs. (8%) and ≥ 71 yrs. (2%).
In the control group of 30, 22 (73.3%) were female and 8 (26.7%) were male. Regarding age, 33.3% were 41 – 50 yrs. old, 26.7% were 51 – 60 yrs., 23.3% were 31 – 40 yrs., 10.0% were 61 – 70 yrs., 6.7% were 21 – 30 yrs. old, and 6.7% were ≥ 71 yrs., as shown in (Table 1, Figure 1). The mean age in the patient group was 46.3 ± 1.13 and in the control group was 46.43 ± 2.13, with no statistically significant differences between the two groups (p-value = 0.95). The current study showed that RA patients had a higher level of anti-CII than those in the control group. The patient group had a statistically significant higher mean (29.69 ± 1.10) than the control group (0.63 ± 0.03), with a p-value of 0.00 (Table 2, Figure 2).
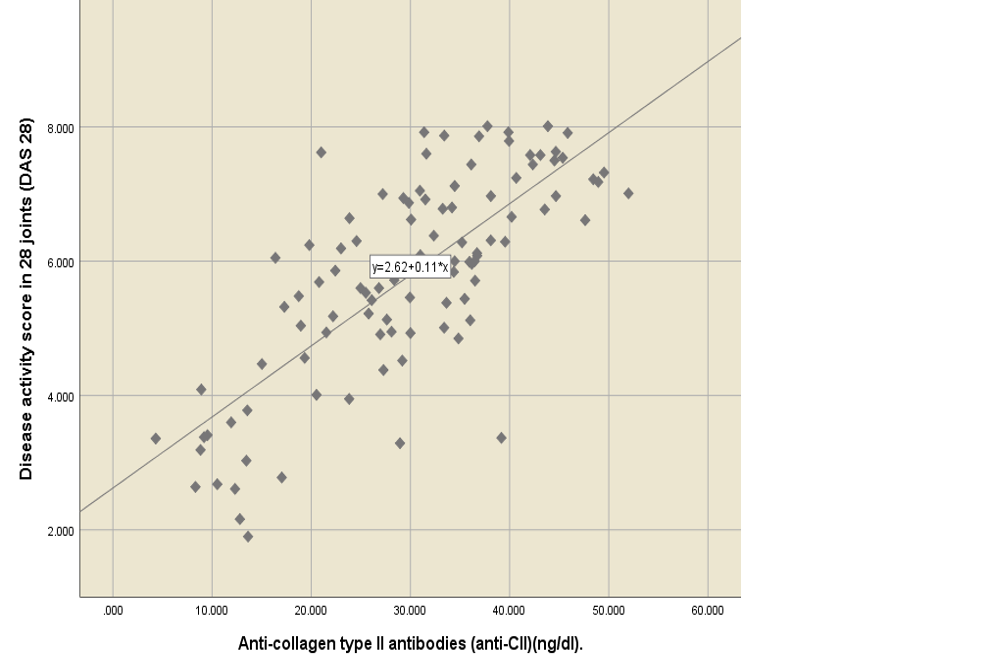
RA patients have higher levels of anti-CII compared to healthy people[12]. When comparing the mean level of anti-CII, according to the DAS28 result, the results revealed that the mean of anti-CII was 11.51 ± 0.94 for remission state, 17.06 ± 4.30 for low disease activity, 21.32 ± 2.08 for moderate disease activity, and 34.22 ± 1.00 for high disease activity. There was a statistically significant difference in the anti-CII mean, which increased with an increase in the DAS28 score, p-value of 0.000 (Table 3 ). Anti-CII had a statistically significant positive moderate correlation with ESR (r = 0.56, p-value = 0.000) and a statistically significant positive strong correlation with DAS28 (r = 0.65, p-value = 0.000) (Figure 3, Figure 4).
Discussion
In the patients with RA, concentrations of anti-collagen type II antibodies were much higher than in the healthy subjects, in line with other studies worldwide. Although direct comparison is difficult due to the lack of consistent results among different measurement methods, in one study the mean concentration of anti-CII in RA patients was higher than in the general Swedish population12.
This study aims to assess serum concentrations of anti-CII antibodies in Iraqi patients with a severe RA phenotype and to investigate the relationship between higher concentrations of anti-CII associated with RA and a low degree of inflammation. Therefore, patients with a high degree of inflammation and with severe symptoms of inflammation had a high proportion of anti-CII antibody concentrations. This study found a statistically significant positive strong correlation between anti-CII and DAS 28 (r = 0.65 p-value = 0.000).
There are a couple of caveats to this study: First, this is a single centralized study with a limited sample size. However, this is the first study to evaluate the association of anti-CII with RA in Iraqi patients. Second, the follow-up time was relatively short, and may not have been enough for sufficient events to occur and increase the statistical reliability of the analysis. Therefore, it is necessary to conduct additional studies and research with larger samples at bigger, multiple healthcare centers, and longer follow-up times in terms of years.
Conclusions
Anti-CII antibodies can be considered an important parameter for the early detection of RA and can be used to determine the activity of RA.
Abbreviations
anti-CII: Anti-collagen type II antibodies, ACR: American College of Rheumatology, DAS28: Disease Activity Score 28, ESR: erythrocyte sedimentation rate.
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Lin
Y.J.,
Anzaghe
M.,
Schülke
S.,
Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells.
2020;
9
(4)
:
880
.
View Article Google Scholar -
Wasserman
A.,
Rheumatoid arthritis: common questions about diagnosis and management. American Family Physician.
2018;
97
(7)
:
455-62
.
-
Afridi
H.I.,
Kazi
T.G.,
Brabazon
D.,
Naher
S.,
Association between essential trace and toxic elements in scalp hair samples of smokers rheumatoid arthritis subjects. The Science of the Total Environment.
2011;
412-413
:
93-100
.
View Article Google Scholar -
Firestein
G.S.,
McInnes
I.B.,
Immunopathogenesis of rheumatoid arthritis. Immunity.
2017;
46
(2)
:
183-96
.
View Article Google Scholar -
de Hair
M.J.,
van de Sande
M.G.,
Ramwadhdoebe
T.H.,
Hansson
M.,
Landewé
R.,
van der Leij
C.,
Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis {&}amp; Rheumatology (Hoboken, N.J.).
2014;
66
(3)
:
513-22
.
View Article Google Scholar -
Mullazehi
M.,
Mathsson
L.,
Lampa
J.,
Rönnelid
J.,
High anti-collagen type-II antibody levels and induction of proinflammatory cytokines by anti-collagen antibody-containing immune complexes in vitro characterise a distinct rheumatoid arthritis phenotype associated with acute inflammation at the time of disease onset. Annals of the Rheumatic Diseases.
2007;
66
(4)
:
537-41
.
View Article Google Scholar -
Berntson
L.,
Nordal
E.,
Fasth
A.,
Aalto
K.,
Herlin
T.,
Nielsen
S.,
Nordic Study Group of Pediatric Rheumatology (NoSPeR)
Anti-type II collagen antibodies, anti-CCP, IgA RF and IgM RF are associated with joint damage, assessed eight years after onset of juvenile idiopathic arthritis (JIA). Pediatric Rheumatology Online Journal.
2014;
12
(1)
:
22
.
View Article Google Scholar -
Nielung
L.,
Validity and agreement between the 28-joint disease activity score based on C-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. Arthritis.
2015;
2015
:
401690
.
View Article Google Scholar -
Hansen
I.M.,
Emamifar
A.,
Andreasen
R.A.,
Antonsen
S.,
No further gain can be achieved by calculating Disease Activity Score in 28 joints with high-sensitivity assay of C-reactive protein because of high intraindividual variability of C-reactive protein: A cross-sectional study and theoretical consideration. Medicine.
2017;
96
(1)
:
e5781
.
View Article Google Scholar -
Hurnakova
J.,
Hulejova
H.,
Zavada
J.,
Komarc
M.,
Cerezo
L.A.,
Mann
H.,
Serum calprotectin may reflect inflammatory activity in patients with active rheumatoid arthritis despite normal to low C-reactive protein. Clinical Rheumatology.
2018;
37
(8)
:
2055-62
.
View Article Google Scholar -
Sengul
I.,
Akcay-Yalbuzdag
S.,
Ince
B.,
Goksel-Karatepe
A.,
Kaya
T.,
Comparison of the DAS28-CRP and DAS28-ESR in patients with rheumatoid arthritis. International Journal of Rheumatic Diseases.
2015;
18
(6)
:
640-5
.
View Article Google Scholar -
Manivel
V.A.,
Mullazehi
M.,
Padyukov
L.,
Westerlind
H.,
Klareskog
L.,
Alfredsson
L.,
Anticollagen type II antibodies are associated with an acute onset rheumatoid arthritis phenotype and prognosticate lower degree of inflammation during 5 years follow-up. Annals of the Rheumatic Diseases.
2017;
76
(9)
:
1529-36
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 4 (2022)
Page No.: 5023-5028
Published on: 2022-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4254 times
- PDF downloaded - 1391 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress