Abstract
Background: Early secretory antigenic target protein 6-kDa (ESAT-6), culture filtrate 10-kDa (CFP- 10), and Mycobacterium tuberculosis (Mtb) 64 (MPT-64) antigens are secreted by actively growing Mtb in active tuberculosis (TB) patients. Increased levels of ESAT-6, CFP-10, and MPT-64 at the site of infection facilitate the entry of antigens into the systemic circulation. These low molecular weight proteins (< 67 kDa) can be excreted into the urine via the kidneys. Therefore, this study evaluated the urinary Mtb antigen cocktail (ESAT-6, CFP-10, and MPT-64) levels in active [pulmonary (P) and extrapulmonary (EP)] TB and latent tuberculosis infections (LTBI) individuals.
Methods: This study was conducted in Dr. Hasan Sadikin General Hospital Bandung from June 2016 to June 2017, using 474 participants. The specimens for TB diagnosis were taken and investigated for acid-fast bacilli (AFB) using the Ziehl Neelsen (ZN) technique. The remaining sputum was cultured on Lowenstein- Jensen (LJ) medium, and TB Ag MPT-64 rapid tests were performed to identify Mtb. In addition, LTBI individuals were screened in a TB outpatient clinic who had close contact with patients with active TB and underwent an interferon g release assay (IGRA). Urine samples were collected for urinary Mtb antigen cocktail (ESAT-6, CFP-10, and MPT-64) testing using the quantitative immunochromatographic method.
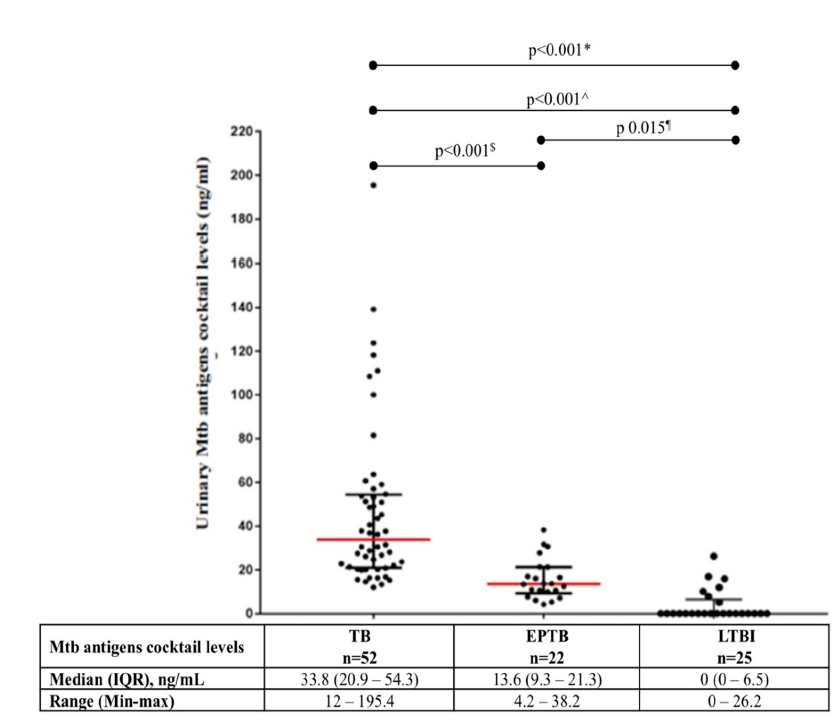
Results: The 99 patients were divided into three groups, PTB (n = 52), EPTB (n = 22) and LTBI (n = 25). There were significant differences in urinary Mtb antigen cocktail levels between PTB and EPTB (33.8 vs 13.6 ng/ml; p < 0.001), between PTB and LTBI (33.8 vs 0 ng/ml; p < 0.001), and between EPTB and LTBI (13.6 vs 0 ng/ml; p = 0.015).
Conclusion: Urinary Mtb antigen cocktail levels were increased in patients with active TB and undetected in those with LTBI. This result was obtained using the urinary Mtb antigen cocktail as a potential marker for differentiating active TB and LTBI.
Introduction
Tuberculosis (TB) is the second leading cause of death globally, caused by a single infectious agent, Mycobacterium tuberculosis (Mtb). The World Health Organization (WHO) has estimated that in 2019, there were 7.1 million new TB cases and 1.2 million TB-related deaths, approximately 44% occurring in Southeast Asia. In Indonesia, notifications of new and relapsed cases increased from 331,703 in 2015 to 562,049 in 2019, a rise of 69%1. The WHO has estimated that one-third of the global population has a latent Mtb infection (LTBI), of whom 5–10% develop active TB disease. The lungs are the primary site of infection. However, the incidence of extrapulmonary TB (EPTB) has increased to over 15% of all cases2, 3.
The detection of Mtb remains challenging in the modern innovative diagnostic era. A combination of clinical symptoms and laboratory tests, such as sputum microscopic examination, remain key to diagnosing active pulmonary TB. Microscopic examination of sputum is inexpensive and requires minimal biological safety standards, but its sensitivity is low1, 4. Mtb culturing is considered the gold standard but is time-consuming since growth depends on the number of viable bacteria.1 In addition, EPTB diagnosis becomes challenging in the clinical setting. Despite the absence of clinically manifested disease, the positivity of the EPTB test also varies, with a previous study reporting 5% EPTB cases while culture tests instead indicated approximately 10 — 30%5, 6, 7. A new molecular-based Mtb diagnostic assay called the nucleic acid amplification test (NAAT) has become the standard for Mtb detection. However, it requires advanced laboratory equipment and highly trained technicians.
Currently, the number of individuals with LTBI is increasing, and they are more challenging to diagnose. The tuberculin test was identified as the standard diagnostic assay for LTBI. However, it cannot differentiate between Mtb infection and sensitization with other environmental mycobacteria. Therefore, cross-reactivity can occur with patients given the Bacillus Calmette-Guerin (BCG) vaccine8. Previous studies proposed using the interferon γ release assay (IGRA) as a marker of T-cell reactivity against Mtb antigen for diagnosing LTBI. However, its ability to differentiate between active and latent TB appears problematic9, 10.
Previous studies identified Mtb antigens as potential markers that can aid TB diagnosis. These antigens are placed into envelope cell and culture filtrate protein (CFP) groups11, 12. The CFP group contains antigens actively secreted in Mtb cultures, including the early secretory antigenic target protein 6-kDa (ESAT-6), culture filtrate protein 10-kDa (CFP-10), and Mtb protein 64 (MPT-64) encoded by genes in regions of difference 1-3 (RD1, RD2, and RD3). Because RDs 1-3 are not found in Mycobacterium bovis used in BCG, they are considered Mtb-specific genomic regions13, 14, 15, 16, 17. The ESAT-6, CFP-10, and MPT-64 antigens play an important role in TB pathogenesis. Numerous studies have found that their levels in examination materials increase proportionally with bacterial replication activity18, 19, 20, 21, 22. Moreover, they are categorized as low molecular weight proteins and freely filtered through the glomerulus. Therefore, this study evaluates urinary Mtb antigens cocktail (ESAT6, CFP10, and MPT-64) levels in TB, EPTB, and LTBI patients.
Methods
Study Population
This study was performed between June 1, 2016, and June 30, 2017, at Dr. Hasan Sadikin General Hospital in West Java Province, Indonesia. Patients visiting the outpatient clinic or admitted to the TB ward were screened for eligibility. Eligible patients were included if they met the following criteria: (1) adult (≥ 18 years old); (2) suspected to have active pulmonary or extrapulmonary TB or LTBI; and (3) had clinical specimens obtained on their inclusion day. The exclusion criteria were: (1) urinary tract infections diagnosed with dipstick and culture testing; (2) abnormal kidney function tests or any history of renal disease; (3) HIV infection; and (4) severe infection.
Study participants were placed into three groups: (a) an active pulmonary TB group containing those with clinical TB manifestations according to the International Standards of Tuberculosis Care (ISTC) guidelines23 confirmed by laboratory (acid-fast bacilli [AFB] or culture) and chest imaging tests; (b) an active EPTB group containing those with clinical TB manifestations according to ISTC guidelines and confirmed by laboratory (acid-fast bacilli or culture) and imaging tests on associated TB infection sites; and (c) an LTBI group containing those who had close contact with active TB patients and confirmed by a positive IGRA test24.
Specimen Collection
Expectorate sputum was obtained from participants in the TB group. pecific clinical specimens, such as pus, cerebrospinal fluid, or pleural fluid, were obtained from participants in the EPTB group according to the hospital standard operating procedure for AFB direct smear and Mtb culture. On the same day, each included participant was also required to submit a random urine specimen with a clean-catch collection technique25.
Laboratory Testing for TB
Sputum, cerebrospinal fluid, pus, or pleural fluid specimens were assessed for AFB with the Ziehl Neelsen (ZN) technique (ST Reagensia Company, Jakarta, Indonesia) according to the standard protocol26. Remaining sputum specimens were decontaminated using a standard N-acetyl-L-cysteine-NaOH (NaLC-NaOH) method and then concentrated via centrifugation at 3000 g for 15 minutes26 before culturing on Lowenstein-Jensen (LJ) medium (bioMérieux SA, Marcy l’Etoile, France). Subsequently, Mtb culture growth was observed weekly for 3 to 8 weeks and was considered positive when mycobacterial growth was >1 colony-forming unit (CFU).
The urine specimen was collected in a sterile container and directly centrifuged at 1006 g for 15 minutes to separate the low molecular weight analytes and then kept at -80°C for further examination of the Mtb antigen cocktail (ESAT6, CFP10, and MPT64). The cocktail was detected using the semiquantitative immunochromatography test (ICT) method (Jei Daniel Biotech Corp., Taiwan), in which 100 µL of sample buffer was placed into the specimen collection box with 100 µL of urine sediment and mixed well by dropper for 30 – 60 seconds then left to stand for 30 minutes. Four ~60 µL drops were applied to the “S” region of the testing card, and the result was read after 30 minutes using an automatic reader (JD Reader) to determine the Mtb antigen cocktail concentration of the clinical specimen. Quality control and instrument assurance were performed according to the manufacturer’s recommendations.
Data Analysis
The urinary Mtb antigen cocktail concentration was assessed against the clinical TB, EPTB, and LTBI diagnoses. Population characteristics are presented descriptively: (1) as numbers and percentages for categorical data such as age, clinical symptoms, and gender; (2) as median and range (minimum-maximum) for numerical data. The significance of differences in median urinary Mtb antigen cocktail concentration among groups was evaluated using the Kruskal-Wallis test. The significance of differences in urinary Mtb antigen cocktail concentration between the following pairs of groups was independently assessed using the Mann-Whitney U test: (a) TB vs. EPTB; (b) TB vs. LTBI; (c) EPTB vs. LTBI. We considered tests with p < 0.05 to be significant. All data analysis was performed using SPSS software v.23 (IBM, Armonk, NY, USA).
| Variable | Tuberculosis Group | ||
| TB n = 52 | EPTB n = 22 | LTBI n = 25 | |
| Gender (n, %) | |||
| Male | 34 (65.4) | 15 (68.2) | 11 (44.0) |
| Female | 18 (34.6) | 7 (31.8) | 14 (56.0) |
| Age (year) | |||
| Median | 36 | 27 | 25 |
| Range (min-max) | 17 – 73 | 18 – 53 | 15 – 58 |
| BMI (kg/m 2 ) | |||
| Median | 19.0 | 18.0 | 22.1 |
| Range (min - max) | 14.4 – 26.2 | 14.2 – 26.7 | 17.7 – 33.6 |
| Symptoms (n, %) | |||
| Cough | 46 (88.5) | 7 (38.9) | NA |
| Fever | 27 (71.1) | 12 (37.5) | NA |
| Breathless | 16 (42.1) | 10 (31.3) | NA |
| Chest pain | 16 (42.1) | 6 (18.8) | NA |
Results
A total of 474 participants were enrolled in this study, and their positivity in direct smear (AFB) and Mtb cultures was 23% and 30% for TB and EPTB, respectively. These findings underscore the low positivity of AFB and culture tests in standard TB and EPTB diagnostics. However, the positivity of the standard IGRA diagnostic test for LTBI was 59% (Figure 1). Among participants in the EPTB group, four had spondylitis, nine had meningitis, eight had pleuritis, and one had abdominal.
The proportion of males among participants in the TB, EPTB, and LTBI groups was 65.4%, 68.2%, and 44%, respectively, with a median age of 36, 27, and 31 years, respectively. Median BMI across the three groups was 18-22.1 and categorized as normal. Clinical symptoms, such as cough and fever, were most prevalent in the TB and EPTB groups (Table 1).
The urinary Mtb antigen cocktail was successfully detected in the TB and EPTB groups. A higher median urinary Mtb cocktail antigen concentration was found in the TB group compared to the other groups (p < 0.001; Figure 2). Independent pairwise group comparisons found higher median urinary Mtb antigen cocktail concentrations in the EPTB (p < 0.001) and LTBI groups (p = 0.015) than in the TB group. In the LTBI group, 18 of the 25 participants had a urinary Mtb antigen cocktail concentration of 0 ng/mL, while the remaining 7 had urinary Mtb antigen cocktail concentrations between 5.2 and 26.2 ng/ml.
Discussion
TB infection remains a challenging global threat, particularly in laboratory diagnostics. This study explored the urinary Mtb antigen cocktail in three TB groups: TB, EPTB, and LTBI. Higher urinary Mtb antigen cocktail concentrations were found in the TB group than in the other two groups. This finding indicates that in active pulmonary TB, ESAT-6, CFP-10, and MPT-64 antigens are secreted by Mtb replication and metabolism. However, a lower urinary antigen concentration was found in the EPTB group. A previous study observed fewer Mtb cells with EPTB, called paucibacillary, compared to active-pulmonary TB4, 27, 28, reflecting the lower urinary Mtb antigen cocktail concentration in our EPTB group. Moreover, Song et al. showed higher TB antigen concentrations in cerebrospinal fluid29. Therefore, the positivity performance of urinary TB antigens provides improved diagnostic performance with active TB.
Urine is an ideal clinical specimen because it is excreted in large quantities, and the collection process does not require invasive methods. The additional value of urinary Mtb antigen cocktails to improve pulmonary TB diagnosis, particularly in peripheral health settings, has been shown30.
This study also evaluated the performance of urinary TB antigens in those with LTBI, finding negative antigen results. In those with LTBI, mycobacterial proliferation is inhibited by immune cell interactions and granuloma formation. Chronic stimulation of proinflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-12, IL-17, and IL-23 stimulates macrophages to differentiate into epithelioid and giant cells. Moreover, the chemokines CCL2, CCL3, CCL5, CXCL8, and CXCL10 induce T-cell aggregation to form granulomas. Cytokines limit primary TB infection to increase its stability. In more than 90% of latent infections, foci caseoses contain mycobacteria lined by granuloma walls. A good host cellular immune response will prevent the replication and spread of mycobacteria31, 32, 33, 34. In vitro studies have found that the ESAT-6, CFP-10, and MPT-64 antigens are not secreted when there is no replication or interaction between Mtb and host receptors22, 31, 32.
However, seven participants with LTBI had urinary Mtb antigen cocktail concentrations between 5.2 and 26.3 ng/ml, returning a median TB antigen level of 0. One possible explanation for urinary TB antigens being detected in LTBI urine is that IGRA positivity cannot differentiate between active and latent TB. Therefore, the possibility of these participants having active early-phase TB cannot be discounted31. Second, granuloma is a dynamic battleground between host and pathogen35. Macrophages egress from the lesion and spread the infection to other lung regions during latency. However, this finding is at odds with the very definition of a latent infection following the migration of infective macrophages through the airspaces. Instead, it may reflect the flaring-up of quiescent lesions during apparent “latency.” An emerging consensus is that active and latent TB has a spectrum of lesional activity.
Several challenges in diagnostic accuracy with urinary lipoarabinomannan (LAM) tests have been previously reported36 that may impact urinary TB antigens: (1) The non-sputum-based test represents an imperfect reference standard, particularly for EPTB and paucibacillary disease; (2) the probability of cross-reactivity with other pathogens and colonizing organisms; and (3) user subjectivity in test interpretation when visual reading is applicable. The standard TB diagnosis by AFB and culture was used as the reference in the active TB group. The number of growth colonies in TB and EPTB cultures that contribute to urinary antigens was not observed. Therefore, the concentration of urinary Mtb still corresponds to the positive culture.
This observation highlights the cross-reactivity due to Mtb antigen strains instead of other pathogenic and colonizing organisms. Choundhry et al. showed that a higher concentration of urinary Mtb antigens detected using the CFP polyclonal antigen Mtb H37Ra strain were present among TB patients37. Meanwhile, a cocktail of ESAT-6, CFP-10, MPT-64, and immunodominant Mtb antigens were also detected. Alexandra et al. previously reported that using polyclonal antibodies in cocktail form increases the sensitivity and specificity of TB examination38. The urinary TB antigens were measured with an automatic reader, eliminating possible user subjectivity in test interpretation, which can be considered a TB diagnostic test even with limited information for urinary Mtb antigens.
Conclusion
The urinary Mtb antigen cocktail levels were elevated in patients with active TB and undetected in those with LTBI. Therefore, this study used the antigen cocktail as a potential marker for differentiating active TB and LTBI.
Abbreviations
AFB: acid-fast bacilli, AUC: Area Under Curve, BCG: Bacillus Calmette-Guerin, CFP: culture filtrate protein, CFP-10: culture filtrate protein 10-kDa, CFU: colony-forming unit, EPTB: extra-pulmonary tuberculosis, ESAT-6: Early secretory antigenic target protein 6-kDa, ICT: immunochromatography test, IFN: Interferon, IGRA: Interferon γ Release Assay, IL: Interleukin, LCS: Liquor cerebrospinal, LTBI: latent tuberculosis infections, MPT-64: Mtb protein 64, Mtb: M. tuberculosis, NAAT: nucleic acid amplification test, NaLC: N-acetyl-L-cysteine, PTB: pulmonary tuberculosis, RD: Regions of Differences, TB: Tuberculosis, TNF: Tumor Necrosis Factor, WHO: World Health Organization, ZN: Ziehl Neelsen
Acknowledgments
The authors are grateful to Bachti Alisyahbana for providing the clinical samples and also to the patients who participated in the study.
Author’s contributions
Dewi Kartika Turbawaty, Ida Parwati, and Adhi Kristianto Sugianli had full access to all of the data in the study and took responsibility for the data’s integrity and accuracy data analysis. Ahmad Rizal Ganiem contributed substantially to the study design, data analysis, and interpretation. Dewi Kartika Turbawaty, Ida Parwati, Adhi Kristianto Sugianli was the primary author and Dewi Kartika Turbawaty was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Universitas Padjadjaran through grant-in-aid for HIU for Dewi Kartika Turbawaty and Academic Leadership Grant for Professor Ida Parwati.
Availability of data and materials
The corresponding author will provide the data used during the current analysis on reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the Faculty of Medicine, Universitas Padjadjaran, and Dr. Hasan Sadikin General Hospital (number 493/UN6.C1.3.2/KEPK/PN/2016 and L.B.02.01/C02/10856/VIII/2016). In addition, written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
Dewi Kartika Turbawaty is an academic author, a clinical pathologist, and the Head of Clinical Pathology, staff of Microbiology and Biomolecular Department, Faculty of Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital. Ida Parwati is an academic author, a professor of the clinical pathologist, and the Head of Microbiology and Biomolecular Department, Faculty of Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital. Adhi Kristianto Sugianli is an academic author, a clinical pathologist, and staff of the Microbiology and Biomolecular Department, Faculty of Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital. Ahmad Rizal Ganiem is a Neurologist and the Head of the Neurology Department, Faculty of Medicine Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital. The remaining authors declare no relevant financial disclosures or conflicts of interest.
References
-
World Health Organization. World Health Organization Global Tuberculosis Report 2020 .
Google Scholar -
Munk
M.E.,
Arend
S.M.,
Brock
I.,
Ottenhoff
T.H.,
Andersen
P.,
Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. The Journal of Infectious Diseases.
2001;
183
(1)
:
175-6
.
View Article PubMed Google Scholar -
Attallah
A.M.,
Osman
S.,
Saad
A.,
Omran
M.,
Ismail
H.,
Ibrahim
G.,
Application of a circulating antigen detection immunoassay for laboratory diagnosis of extra-pulmonary and pulmonary tuberculosis. Clinica Chimica Acta.
2005;
356
(1-2)
:
58-66
.
View Article PubMed Google Scholar -
Perkins
M.D.,
New diagnostic tools for tuberculosis. The International Journal of Tuberculosis and Lung Disease.
2000;
4
(12)
:
182-8
.
PubMed Google Scholar -
Farhana
A.,
Islam
M.,
Rehena
Z.,
Yasmin
F.,
Nurullah
A.,
Talukder
S.,
Comparative Study of Adenosine Deaminase and Other Conventional Diagnostic Parameters in Diagnosis of Tuberculous Pleural Effusion. Mymensingh Medical Journal.
2013;
6
(2)
:
105-12
.
PubMed Google Scholar -
Kashyap
R.S.,
Ramteke
S.S.,
Morey
S.H.,
Purohit
H.J.,
Taori
G.M.,
Daginawala
H.F.,
Diagnostic value of early secreted antigenic target-6 for the diagnosis of tuberculous meningitis patients. Infection.
2009;
37
(6)
:
508-13
.
View Article PubMed Google Scholar -
Ferrer
J.,
Pleural tuberculosis. The European Respiratory Journal.
1997;
10
(4)
:
942-7
.
PubMed Google Scholar -
Nahid
P.,
Pai
M.,
Hopewell
P.C.,
Advances in the diagnosis and treatment of tuberculosis. Proceedings of the American Thoracic Society.
2006;
3
(1)
:
103-10
.
View Article PubMed Google Scholar -
Bekmurzayeva
A.,
Sypabekova
M.,
Kanayeva
D.,
Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland).
2013;
93
(4)
:
381-8
.
View Article PubMed Google Scholar -
Diel
R.,
Loddenkemper
R.,
Meywald-Walter
K.,
Niemann
S.,
Nienhaus
A.,
Predictive value of a whole blood IFN-γ assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. American Journal of Respiratory and Critical Care Medicine.
2008;
177
(10)
:
1164-70
.
View Article PubMed Google Scholar -
Khomenko
A.G.,
Bayensky
A.V.,
Chernousova
L.N.,
Kulikovskaya
N.V.,
Demianenko
N.V.,
Litvinov
V.I.,
Serodiagnosis of tuberculosis: detection of mycobacterial antibodies and antigens. Tubercle and Lung Disease.
1996;
77
(6)
:
510-5
.
View Article PubMed Google Scholar -
Bassey
E.O.,
Catty
D.,
Kumararatne
D.S.,
Raykundalia
C.,
Candidate antigens for improved serodiagnosis of tuberculosis. Tubercle and Lung Disease.
1996;
77
(2)
:
136-45
.
View Article PubMed Google Scholar -
Shen
G.H.,
Chiou
C.S.,
Hu
S.T.,
Wu
K.M.,
Chen
J.H.,
Rapid identification of the Mycobacterium tuberculosis complex by combining the ESAT-6/CFP-10 immunochromatographic assay and smear morphology. Journal of Clinical Microbiology.
2011;
49
(3)
:
902-7
.
View Article PubMed Google Scholar -
Mahairas
G.G.,
Sabo
P.J.,
Hickey
M.J.,
Singh
D.C.,
Stover
C.K.,
Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. Journal of Bacteriology.
1996;
178
(5)
:
1274-82
.
View Article PubMed Google Scholar -
Brosch
R.,
Gordon
S.V.,
Billault
A.,
Garnier
T.,
Eiglmeier
K.,
Soravito
C.,
Use of a Mycobacterium tuberculosis H37Rv bacterial artificial chromosome library for genome mapping, sequencing, and comparative genomics. Infection and Immunity.
1998;
66
(5)
:
2221-9
.
View Article PubMed Google Scholar -
Gordon
S.V.,
Brosch
R.,
Billault
A.,
Garnier
T.,
Eiglmeier
K.,
Cole
S.T.,
Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Molecular Microbiology.
1999;
32
(3)
:
643-55
.
View Article PubMed Google Scholar -
Parkash
O.,
Singh
B.P.,
Pai
M.,
Regions of differences encoded antigens as targets for immunodiagnosis of tuberculosis in humans. Scandinavian Journal of Immunology.
2009;
70
(4)
:
345-57
.
View Article PubMed Google Scholar -
Pathak
S.K.,
Basu
S.,
Basu
K.K.,
Banerjee
A.,
Pathak
S.,
Bhattacharyya
A.,
Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nature Immunology.
2007;
8
(6)
:
610-8
.
View Article PubMed Google Scholar -
Krishnan
N.,
Robertson
B.D.,
Thwaites
G.,
The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland).
2010;
90
(6)
:
361-6
.
View Article PubMed Google Scholar -
Kumar
V.G.,
Urs
T.A.,
Ranganath
R.R.,
MPT 64 Antigen detection for Rapid confirmation of M.tuberculosis isolates. BMC Research Notes.
2011;
4
(1)
:
79
.
View Article PubMed Google Scholar -
Ernst
J.D.,
Trevejo-Nuñez
G.,
Banaiee
N.,
Genomics and the evolution, pathogenesis, and diagnosis of tuberculosis. The Journal of Clinical Investigation.
2007;
117
(7)
:
1738-45
.
View Article PubMed Google Scholar -
Raghavan
S.,
Manzanillo
P.,
Chan
K.,
Dovey
C.,
Cox
J.S.,
Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature.
2008;
454
(7205)
:
717-21
.
View Article PubMed Google Scholar -
TB CARE I. International Standards for Tuberculosis Care, Edition 3. TB CARE I, The Hague, 2014..
.
-
Castro
K.G.,
Goldberg
S.,
Jereb
J.A.,
LoBue
P.,
Mazurek
G.H.,
Vernon
A.,
Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep.
2010;
59
(RR-5)
:
1-25
.
PubMed Google Scholar -
Thomas
C.E.,
Sexton
W.,
Benson
K.,
Sutphen
R.,
Koomen
J.,
Urine collection and processing for protein biomarker discovery and quantification. Cancer Epidemiology and Prevention Biomarkers.
2010;
19
(4)
:
953-959
.
View Article PubMed Google Scholar -
Global Laboratory Initiative. Advancing TB diagnosis Mycobacteriology Laboratory Manual. Clinics in Chest Medicine.
2014;
1
:
1-77
.
-
Noussair
L.,
Bert
F.,
Leflon-Guibout
V.,
Gayet
N.,
Nicolas-Chanoine
M.H.,
Early diagnosis of extrapulmonary tuberculosis by a new procedure combining broth culture and PCR. Journal of Clinical Microbiology.
2009;
47
(5)
:
1452-7
.
View Article PubMed Google Scholar -
Rock
R.B.,
Olin
M.,
Baker
C.A.,
Molitor
T.W.,
Peterson
P.K.,
Central nervous system tuberculosis: pathogenesis and clinical aspects. Clinical Microbiology Reviews.
2008;
21
(2)
:
243-61
.
View Article PubMed Google Scholar -
Song
F.,
Sun
X.,
Wang
X.,
Nai
Y.,
Liu
Z.,
Early diagnosis of tuberculous meningitis by an indirect ELISA protocol based on the detection of the antigen ESAT-6 in cerebrospinal fluid. Irish Journal of Medical Science.
2014;
183
(1)
:
85-8
.
View Article PubMed Google Scholar -
Turbawaty
D.K.,
Sugianli
A.K.,
Soeroto
A.Y.,
Setiabudiawan
B.,
Parwati
I.,
Comparison of the Performance of Urinary Mycobacterium tuberculosis Antigens Cocktail (ESAT6, CFP10, and MPT64) with Culture and Microscopy in Pulmonary Tuberculosis Patients. International Journal of Microbiology.
2017;
2017
(2)
:
3259329
.
View Article PubMed Google Scholar -
Ulrichs
T.,
Kaufmann
S.H.,
Immunology and Persistence. In: Kaufmann SHE, Hahn H (eds). Mycobacteria and TB.Issues Infect Dis. Basel: Karger, 2003, vol 2:112-127.
.
-
Zuñiga
J.,
Torres-García
D.,
Santos-Mendoza
T.,
Rodriguez-Reyna
T.S.,
Granados
J.,
Yunis
E.J.,
Cellular and humoral mechanisms involved in the control of tuberculosis. Clinical & Developmental Immunology.
2012;
2012
:
193923
.
View Article PubMed Google Scholar -
Ahmad
S.,
Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clinical & Developmental Immunology.
2011;
2011
:
814943
.
View Article PubMed Google Scholar -
Smith
I.,
Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clinical Microbiology Reviews.
2003;
16
(3)
:
463-96
.
View Article PubMed Google Scholar -
Rao
M.,
Ippolito
G.,
Mfinanga
S.,
Ntoumi
F.,
Yeboah-Manu
D.,
Vilaplana
C.,
Latent TB Infection (LTBI) - Mycobacterium tuberculosis pathogenesis and the dynamics of the granuloma battleground. International Journal of Infectious Diseases.
2019;
80S
:
58-61
.
View Article PubMed Google Scholar -
Bulterys
M.A.,
Wagner
B.,
Redard-Jacot
M.,
Suresh
A.,
Pollock
N.R.,
Moreau
E.,
Point-of-care urine LAM tests for tuberculosis diagnosis: a status update. Journal of Clinical Medicine.
2019;
9
(1)
:
111
.
View Article PubMed Google Scholar -
Choudhry
V.,
Saxena
R.K.,
Detection of Mycobacterium tuberculosis antigens in urinary proteins of tuberculosis patients. European Journal of Clinical Microbiology & Infectious Diseases.
2002;
21
(1)
:
1-5
.
View Article PubMed Google Scholar -
Alexandra
I. S.,
Dorina
B.,
Gabriela
I. D.,
Eugenia
P.,
Irina
R.,
Manole
C.,
Immunologic Diagnosis of Neurotuberculosis. In: Cardona, P. , editor. Understanding Tuberculosis - Global Experiences and Innovative Approaches to the Diagnosis [Internet]. London: IntechOpen; 2012 [cited 2022 Jul 03]. Available from: https://www.intechopen.com/chapters/28537 doi: 10.5772/30523 .
Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 6 (2022)
Page No.: 5113-5120
Published on: 2022-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4992 times
- PDF downloaded - 1610 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress




