Abstract
Introduction: Acute brain injury caused by cerebral ischemia, either due to stroke or ischemic hypoxic-ischemic encephalopathy (HIE), represents a major neurological cause of death and disability worldwide. Neuronal cell injury has been shown to correlate with a significant increase in neuron-specific enolase (NSE) levels in in vitro studies. This study aims to measure NSE levels in the blood serum of rats with HIE.
Methods: This study used an experimental post-test design in which occlusion of the right common carotid artery (CCA) was performed on Wistar rats, which were then placed in a hypoxic chamber and reperfused after 60 minutes (treatment group). Neurological scores were collected over the first 24 hours. After 48 hours, experimental animals were sacrificed and their serum NSE levels were measured using ELISA. Statistical analysis using a T-test was performed for independent samples.
Results: A total of 16 male rats were included in the study. Neurological scores indicated that all HIE group rats experienced hemiparesis to varying degrees. NSE levels in the treatment group were significantly higher than in the control group (p < 0.05). The mean NSE level in the treatment group was higher than that in the other group.
Conclusion: Consistent with in vitro studies, we found that NSE was higher in the HIE rat model than in controls. These data support future studies to assess the fitness of serum NSE as a biomarker of brain damage.
Introduction
Acute brain injury after cerebral ischemia, due to stroke or ischemic hypoxic-ischemic encephalopathy (HIE), remains a major cause of mortality and morbidity worldwide1. Stroke occurs in 13 per 100,000 children over the age of one month every year, whereas in newborns, 25–40 children per 100,000 experience a stroke each year2. Similarly, HIE occurs in 2–9 per 1,000 babies per year in both developed and developing countries3. Most affected children sustain lasting physical and/or neurological disabilities4, 5.
The prognosis after brain damage can be assessed using clinical, neuroimaging and electrophysiological methods. However, applying these methods together is often challenging. Biochemical markers and particularly markers that could be sampled from blood could offer a widely accessible alternative. Enolase is a glycolytic enzyme that converts 2-phosphoglycerate to phosphoenolpyruvate6. Previous research has demonstrated that neuron-specific enolase (NSE), which can be measured in cerebrospinal fluid, is a sensitive biomarker of neuronal injury, making it a useful tool for nerve and cerebral injury prognoses7, 8. Greater levels of NSE have been observed in adult gray matter, whereas decreased levels of NSE have been found in white matter. The interaction of NSE with many other molecules in the central nervous system, including membrane-bound and cytoplasmic molecules, also increases the likelihood that NSE helps to activate glial cells during neuronal injury9, 10. We sought to measure the level of NSE in the serum of HIE rats, to determine whether the elevation of soluble NSE that has been reported in CSF could also be detected in peripheral blood, which would have immediate clinical relevance.
Methods
Design
This was an experimental randomized posttest-only control group design.
Time and setting
Research activities were carried out at the Research and Development Centre for Stem Cells, Universitas Airlangga in July, and August 2021. The adaptation phase was carried out for one week, while the treatment and symptom observation phase lasted 48 hours.
Animal subjects
Experimental animals used were all healthy, six-month-old, male Wistar rats, a strain of Rattus norvegicus. Rats were divided into a control group and a treatment group. Animals in the control group underwent no experimental interventions. In the treatment group, the right common carotid artery (CCA) was ligated. The rats were then placed for 45 minutes in a hypoxic atmosphere chamber, with an ambient gas composition of 8% O2 and 92% N211, 12, 13. Following hypoxia, reperfusion was performed by opening the CCA ligation after 60 minutes. After 48 hours, the rats were sacrificed. NSE levels were measured in blood serum by ELISA, using NSE reagent E-EL0R0058 (Elabscience Biotechnology Inc., China). Plates were read using a SPECTROstar® Nano plate reader (BMG Labtech, Germany).
Hypoxic-ischemic rat model
We established a cerebral ischemia model according to the Vannucci method. Wistar rats were anaesthetized with xylazine 10%, injected intramuscularly. An incision was made in the midventral cervical area in the middle of the neck, on the upper edge of the sternum. This allowed access to the sternocleidomastoid muscle and the sternohyoid muscle. The sternocleidomastoid muscle deep inside the sternohyoid muscle was pulled slowly until the sternohyoid muscle appeared. The common carotid artery (CCA) is normally enwrapped in fibrous connective tissue, which contains vagus nerves. The CCA was carefully separated from the attached tissue using ophthalmic forceps. The unilateral CCA was then bound twice with two stitches with 4-0 silk, next to each other, and then the surgical wound was closed with a suture. Reperfusion was performed by opening of the CCA ligation for two hours.
Neurology Score
Clinical evidence of brain ischemia was assessed with a neurological examination, using a six-point Longa score scale as follows: 0: no neurological deficit; 1: left forefoot fails to fully extend, indicating a mild focal neurologic deficit; 2: turns left, meaning a moderate focal neurologic deficit; 3: falls to the left, indicating a severe focal neurologic deficit; 4: does not run spontaneously and exhibits a lower level of consciousness; 5: death due to brain ischemia. If the animal's score was 0 or 5, it was excluded from the study14.
Ethical clearance
All animal protocols were approved by the Committee of Animal Care and Use, Faculty of Veterinary Medicine, Airlangga University No: 2.KE.019.02.2018.
Statistics
Normality of the control and treatment group data distributions was tested using the Shapiro–Wilk test, followed by a t-test for independent sample analysis. The Statistical Package for the Social Sciences (SPSS) 21.0 software program was used for this analysis.
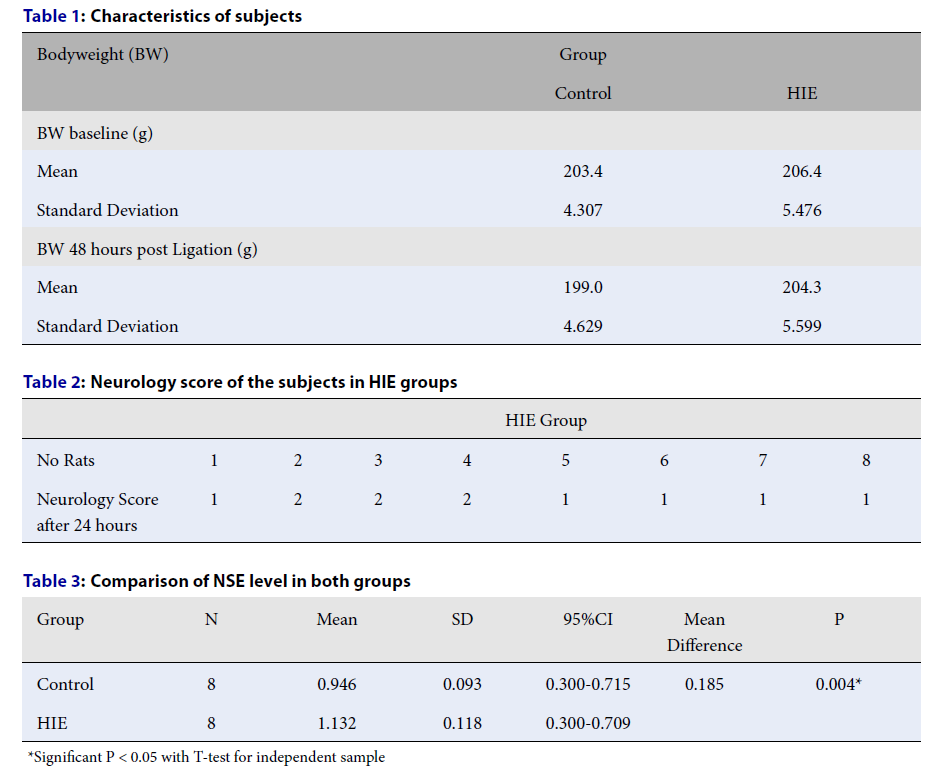
| Bodyweight (BW) | Group | |
|---|---|---|
| Control | HIE | |
| BW baseline (g) | ||
| Mean | 203.4 | 206.4 |
| Standard Deviation | 4.307 | 5.476 |
| BW 48 hours post Ligation (g) | ||
| Mean | 199.0 | 204.3 |
| Standard Deviation | 4.629 | 5.599 |
| HIE Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| No Rats | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Neurology Score after 24 hours | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| Group | N | Mean | SD | 95%CI | Mean Difference | P |
|---|---|---|---|---|---|---|
| Control | 8 | 0.946 | 0.093 | 0.300-0.715 | 0.185 | 0.004* |
| HIE | 8 | 1.132 | 0.118 | 0.300-0.709 |
Results
Twenty-two healthy male Wistar rats that had not been used in previous studies were selected for the experiment. The characteristics of these rats are shown in Table 1.
We evaluated animals phenotypically for evidence of brain ischemia by performing a neurological examination on each animal, using the six-point Longa score scale (see methods). We found that all HIE group rats experienced hemiparesis at different levels, as shown in Table 2.
The normality of the NSE variable data was tested using the Shapiro–Wilks test. NSE data in each group showed a normal distribution. Levene's test was used to test whether our two groups had homogeneous variances. We found that the NSE levels were significantly higher in the treatment group compared with the control group (Table 3).
It is worth noting that the mortality of rats during the experiment reached 27% (3 rats) each in the control group and treatment group. The exact cause of death is unknown, but a severe neurological disorder is suspected. A total of 16 rats completed the study.
Discussion
Enolase, or 2-phospho-D-glycerate hydrolase, is an essential metabolic enzyme and is found in the cytoplasm. Enolase is one of the enzymes of the glycolytic pathway that converts glucose into pyruvate. Brain enolase consists only of alpha and gamma subunits. Gamma enolase is categorized as neuron-specific enolase (NSE) because of the distinction of its neurons15.
Previous studies have shown that NSE is detectable at high levels in CSF after brain injury. One outstanding question not addressed by those studies is the level of NSE in the blood serum in brain injury. This study set out to answer that question by measuring NSE level from blood serum of HIE rats model. We used CCA ligation to induce hypoxic ischemia and measured NSE levels after reperfusion. We found that NSE levels were higher in the serum of the study group compared to controls. NSE is considered a precise marker for neuronal injury but not glial injury8, 16. It is also a good marker for brain ischemic animal models, including local infarct and global ischemia16, 17. Cerebrospinal fluid (CSF) NSE levels are correlated with ischemia duration and the size of cerebral infarct. We interpreted our serum results after ischemia to mean that the level of neuron death was higher in our experimental animals than in controls.
One previous study compared the NSE levels in mouse CSF before and until seven days after ligation. The NSE level was measured using a radioimmunoassay kit. In those experiments, consolidation of the damaged area on the frontal lobe was evaluated to measure the infarct volume. There was a correlation between CSF NSE levels on the third day postligation and the infarct volume18.
NSE has been used as a more global marker of ischemia in humans, e.g., after cardiac arrest, status epilepticus, or drowning16, 18. A second study reported that focal ischemia treatment efficacy can be evaluated using NSE as a marker. They investigated the correlation between NSE and infarct volume in test animals by ligating the carotid artery. Ten minutes after ligation, animals were given intraperitoneal nicardipine 1.2 mg/kg and then again at 8, 16 and 24 hours after ligation. The mice that were given nicardipine had a 19% smaller infarct volume than the ligated group that was not treated. When NSE levels were measured 24 hours after ligation, they found that NSE was three times higher in ligated animals than in a control group that was not ligated. Interestingly, animals that were ligated and also treated with nicardipine had a lower level of NSE than those that were ligated and untreated, with the difference being up to 50% lower at 24 hours, 42% at 48 hours and 59% at 72 hours after ligation. These data showed that NSE levels may be correlated with different outcomes from therapeutic intervention for stroke19, 20.
Earlier studies have reported that NSE can be measured not only from CSF but also from blood10. In one such study, serum NSE levels were measured in mice with status epilepticus (SE) induced by lithium pilocarpine, and in healthy mice at the ages of 1, 2, 3 and 4 weeks. Brain damage was defined using a scale of 0 for no damage and up to 5 for more than 50% cellular loss. The 1- and 2-week-old mice had higher baseline serum NSE (s-NSE) than the other mice. After induction of SE, the 1-week-old mice did not show an increase in s-NSE, and there was no histological evidence of damage. In the older mice, NSE increased between 18.9 ± 0.8 ng/ml in 2-week-old mice (vs 11.5 ± 0.5 for the control group) and 35.8 ± 2.1 ng/ml in 3-week-old mice (vs 12.1 ± 0.8 for the control group). After SE, the s-NSE of the mice also increased from 5.4 ± 0.4 for the control group to 30.4 ± 1.3. They found that serum NSE after HIE injury correlated with histological changes and suggested that further studies to validate the use of NSE as a peripheral readout for brain injury may be warranted21.
In the current study, we were unable to replicate their study parameters and assess the presence of neuronal damage in the brain, since visualization of damage to the brain could was constrained by the limitations of equipment at our location.
Conclusions
Induced HIE in a rat model led to detectable increase in serum levels of NSE. Our study suggests that serum NSE may be a promising biomarker of brain damage. However, due to the limitations of our study (sample size, brain damage histological correlation with serum findings), further exploration will be needed, in varied neuropathological conditions and with larger sample sizes.
Abbreviations
BW: body weight; CCA: common carotid artery; CSF: cerebro spinal fluid; HIE: hypoxic-ischemic encephalopathy; NSE: neuron-specific enolase; SE: status epilepticus; s-NSE: serum neuron-specific enolase
Acknowledgments
The authors wish to thank the Ministry of Education, Culture, Research and Technology Indonesian Republic for research support; Committee of Animal Care and Use, Faculty of Veterinary Medicine for the ethical approval; and Faculty of Medicine, Universitas Airlangga and endless support.
Author’s contributions
Gunawan design conceptual framework, data collection, analysis and interpretation, discussion and summary. Noviandi collected data, revised the manuscript and supervised. Samosir revised the manuscript, figures and tables editing and literature review. All authors discussed the results and to the final version of the manuscript.
Funding
This study was funded by the Ministry of Education, Culture, Research and Technology Indonesian Republic under a project of basic research programs in collaboration with Universitas Airlangga for research development.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Meloni
B.P.,
Pathophysiology and neuroprotective strategies in hypoxic-ischemic brain injury and stroke. Brain Sciences.
2017;
7
(8)
:
110
.
View Article PubMed Google Scholar -
Rivkin
M.J.,
Bernard
T.J.,
Dowling
M.M.,
Amlie-Lefond
C.,
Guidelines for urgent management of stroke in children. Pediatric Neurology.
2016;
56
:
8-17
.
View Article PubMed Google Scholar -
Eghbalian
F.,
Frequency of Hypoxic-Ischemic Encephalopathy among Hospitalized Neonates in West Iran. Iranian Journal of Pediatrics.
2010;
20
(2)
:
244-5
.
PubMed Google Scholar -
van Handel
M.,
Swaab
H.,
de Vries
L.S.,
Jongmans
M.J.,
Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. European Journal of Pediatrics.
2007;
166
(7)
:
645-54
.
View Article PubMed Google Scholar -
Prastiya
I.G.,
Risky
V.P.,
Mira
I.,
Retno
A.S.,
Darto
S.,
Erny
P.,
Risk factor of mortality in Indonesian children with cerebral palsy. The Journal of Medical Investigation.
2018;
65
(1.2)
:
18-20
.
View Article PubMed Google Scholar -
Yardimoğlu
M.,
Ilbay
G.,
Dalcik
C.,
Dalcik
H.,
Sahin
D.,
Ates
N.,
Immunocytochemistry of neuron specific enolase (NSE) in the rat brain after single and repeated epileptic seizures. The International Journal of Neuroscience.
2008;
118
(7)
:
981-93
.
View Article PubMed Google Scholar -
León-Lozano
M.Z.,
Arnaez
J.,
Valls
A.,
Arca
G.,
Agut
T.,
Alarcón
A.,
Cerebrospinal fluid levels of neuron-specific enolase predict the severity of brain damage in newborns with neonatal hypoxic-ischemic encephalopathy treated with hypothermia. PLoS One.
2020;
15
(6)
:
e0234082
.
View Article PubMed Google Scholar -
Morozova
A.Y.,
Arutjunyan
A.V.,
Milyutina
Y.P.,
Morozova
P.Y.,
Kozina
L.S.,
Zhuravin
I.A.,
Influence of prenatal hypoxia on the content on neuron specific enolase in the structures of the brain and blood serum of rats in early ontogeny. Neurochemical Journal.
2020;
14
(3)
:
290-4
.
View Article Google Scholar -
Lafon-Cazal
M.,
Bougault
I.,
Steinberg
R.,
Pin
J.P.,
Bockaert
J.,
Measurement of gamma-enolase release, a new method for selective quantification of neurotoxicity independently from glial lysis. Brain Research.
1992;
593
(1)
:
63-8
.
View Article PubMed Google Scholar -
Haque
A.,
Polcyn
R.,
Matzelle
D.,
Banik
N.L.,
New insights into the role of Neuron-Specific Enolase in neuro-Inflammation, neurodegeneration, and neuroprotection. Brain Sciences.
2018;
8
(2)
:
33
.
View Article PubMed Google Scholar -
Northington
F.J.,
Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR Journal.
2006;
47
(1)
:
32-8
.
View Article PubMed Google Scholar -
Wilson
M.D.,
Wilson. Animal models of cerebral palsy: hypoxic brain injury in the newborn. Iranian Journal of Child Neurology.
2015;
9
(2)
:
9-16
.
PubMed Google Scholar -
Vannucci
R.C.,
Vannucci
S.J.,
Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Developmental Neuroscience.
2005;
27
(2-4)
:
81-6
.
View Article PubMed Google Scholar -
Bachour
S.P.,
Hevesi
M.,
Bachour
O.,
Sweis
B.M.,
Mahmoudi
J.,
Brekke
J.A.,
Comparisons between Garcia, Modo, and Longa rodent stroke scales: optimizing resource allocation in rat models of focal middle cerebral artery occlusion. Journal of the Neurological Sciences.
2016;
364
(364)
:
136-40
.
View Article PubMed Google Scholar -
Yardimoğlu
M.,
Ilbay
G.,
Dalcik
C.,
Dalcik
H.,
Sahin
D.,
Ates
N.,
Immunocytochemistry of neuron specific enolase (NSE) in the rat brain after single and repeated epileptic seizures. The International Journal of Neuroscience.
2008;
118
(7)
:
981-93
.
View Article PubMed Google Scholar -
Zetterberg
H.,
Smith
D.H.,
Blennow
K.,
Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nature Reviews. Neurology.
2013;
9
(4)
:
201-10
.
View Article PubMed Google Scholar -
Palmio
J.,
Peltola
J.,
Vuorinen
P.,
Laine
S.,
Suhonen
J.,
Keränen
T.,
Normal CSF neuron-specific enolase and S-100 protein levels in patients with recent non-complicated tonic-clonic seizures. Journal of the Neurological Sciences.
2001;
183
(1)
:
27-31
.
View Article PubMed Google Scholar -
Hatfield
R.H.,
McKernan
R.M.,
CSF neuron-specific enolase as a quantitative marker of neuronal damage in a rat stroke model. Brain Research.
1992;
577
(2)
:
249-52
.
View Article PubMed Google Scholar -
Pratamastuti
D.,
Indra Gunawan
P.,
Saharso
D.,
Serum neuron specific enolase is increased in pediatric acute encephalitis syndrome. Korean Journal of Pediatrics.
2017;
60
(9)
:
302-6
.
View Article PubMed Google Scholar -
Kittaka
M.,
Giannotta
S.L.,
Zelman
V.,
Correale
J.D.,
DeGiorgio
C.M.,
Weiss
M.H.,
Attenuation of brain injury and reduction of neuron-specific enolase by nicardipine in systemic circulation following focal ischemia and reperfusion in a rat model. Journal of Neurosurgery.
1997;
87
(5)
:
731-7
.
View Article PubMed Google Scholar -
Sankar
R.,
Shin
D.H.,
Wasterlain
C.G.,
Serum neuron-specific enolase is a marker for neuronal damage following status epilepticus in the rat. Epilepsy Research.
1997;
28
(2)
:
129-36
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 7 (2022)
Page No.: 5161-5165
Published on: 2022-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3396 times
- PDF downloaded - 1187 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress


