Abstract
Background: Papillary renal cell carcinoma (PRCC), the second most common type of renal cancer, is a heterogeneous disease with diverse molecular and clinical characteristics. Involvement of the inferior vena cava (IVC) is a predictor of poor prognosis; however, literature is scarce in this regard.
Case presentation: We present a case with a large PRCC and extension to the IVC without metastasis to nodes or other organs that was successfully treated with radical nephrectomy and resection of the IVC.
Conclusion: It is necessary to pay greater attention to diagnosis and appropriate treatment of PRCC extending to the IVC.
Introduction
Kidney cancer is among the top 10 cancers worldwide, and papillary renal cell carcinoma (PRCC) accounts for about 15% of all cases of kidney cancers1. From a histopathological perspective, PRCC has two major subtypes (type I and II), and the more favorable prognosis of type I PRCC is due to the genetic basis of the subtypes2. In addition to subtype, tumor grade, TNM stage, and tumor necrosis are important predictors of mortality and metastasis3. Definite diagnosis and differentiation of the subtypes are based on histopathological examination of the resected specimen; however, imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), can also aid in pre-operative differentiation, indicating hypovascular and homogenous lesions on CT and hypointense lesions on T2-weighted MRI4. Atypical imaging findings, such as necrosis, hemorrhage, and calcification, have also been reported, especially in lesions with a diameter > 4 cm5. Tumor size, reported as mean diameter of 7 cm, is also associated with prognosis6; therefore, it is necessary to consider the tumor size. It has been reported that involvement of the inferior vena cava (IVC), which forms a venous tumor thrombus (VTT), in patients with PRCC has a negative impact on cancer-related survival7, mainly due to the aggressive nature and nodal or remote metastases8. However, due to the scarcity of available data, further studies are required in this regard. We present a case of a large PRCC with extension to the IVC without metastasis to nodes or other organs that was successfully treated with radical nephrectomy and resection of the IVC.
| Value | Unit | reference range | |
|---|---|---|---|
| White blood cell | 3.7 x 10 3 | /µl | 4 - 10 x 10 3 |
| Hemoglobin | 9.3 | gr/dl | 13.5 - 17.5 |
| Platelet count | 212 x 10 3 | /µl | 150 - 450 x 10 3 |
| Serum urea | 17 | mg/dl | 10 - 20 |
| Serum creatinine | 1.2 | mg/dl | 0.84 - 1.21 |
| Serum potassium | 3.6 | mEq/l | 3.7 - 5.2 |
| Prothrombin time | 17.5 | seconds | 11 - 13.5 |
| International normalized ratio | 1.7 | - | 0.8 - 1.1 |
| Partial thromboplastin time | >120 | seconds | 60 - 70 |
Case presentation
A 72-year-old man was referred due to gross hematuria, urinary tract symptoms, weight loss, and anorexia for 2 months. Past medical history was unremarkable except for right inguinal herniorrhaphy 20 years ago. Physical examination was unremarkable except for a right flank mass. The results of blood and serum analysis are shown in Table 1. As indicated, the patient had anemia, decreased white blood cell (WBC) count, and increased PTT (the patient received heparin and warfarin).
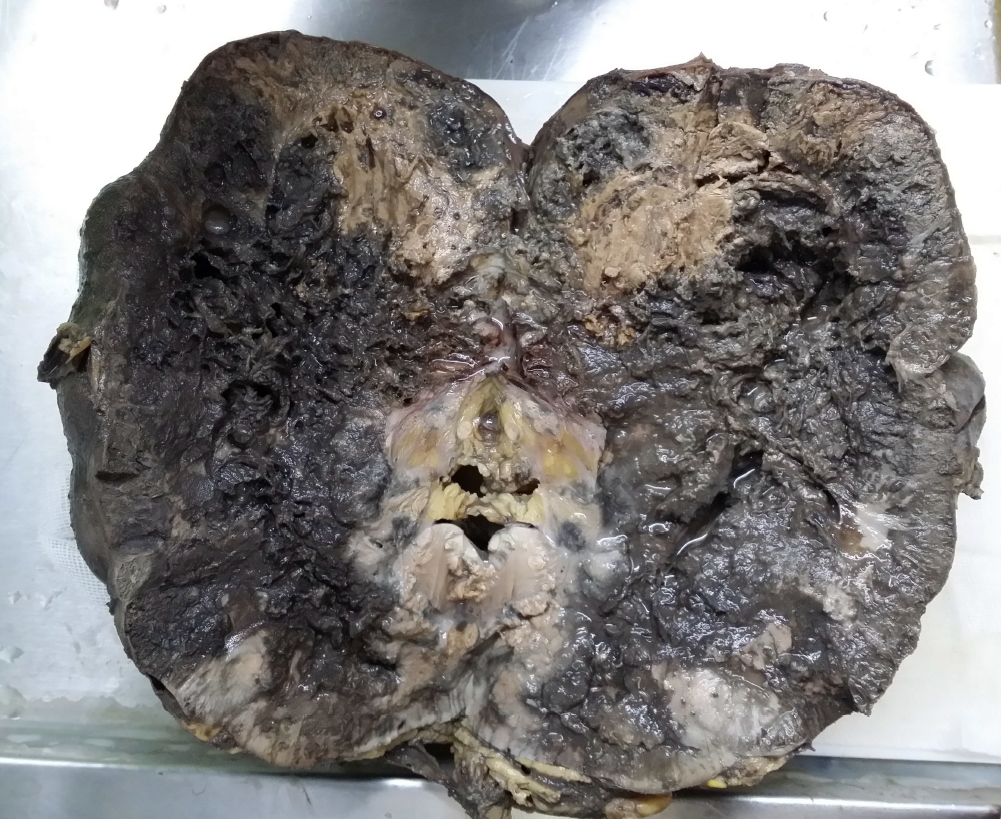
Ultrasound examination revealed a lobulated, heterogeneous, hypervascular mass in the lower pole of the right kidney measuring 100 × 130 mm with involvement of the lower and middle sinuses. CT revealed a 155-mm heterogeneous mass compressing the IVC without any calcification or fatty component. Right radical nephrectomy with IVC thrombectomy was performed. The specimen was sent to the pathology department in two containers: one container contained the right kidney measuring 20 × 10 × 9 cm and included a mass with necrosis and hemorrhagic areas occupying the kidney on cut section (Figure 1); the second container contained the resected IVC, which showed thrombosis of the IVC and several gray pieces measuring 6 × 5 × 4 cm in total on macroscopic examination.
The pathology report revealed type II PRCC, nuclear grade 3/4, with vascular, periureteral, and perirenal fat involvement (Figure 2). The tumor had a papillary structure, and the papillae contained pseudostratified epithelium composed of cells with abundant eosinophilic cytoplasm. The greatest diameter of the tumor was 20 cm, with necrosis on 20% of the surface area. The adrenal gland was free of tumor, and tumor invasion to the IVC was confirmed. The patient was discharged in good condition. After 3 weeks, the patient received the pathology report and a physician’s visit revealed that the patient was in good condition. No further follow-up is available.
Discussion
This case involved a large PRCC (20 cm) with necrosis and invasion of the IVC resulting in thrombosis of the IVC. Hematuria and anorexia were the only symptoms of the patient, and timely diagnosis by imaging and appropriate surgery saved the patient’s life. The literature is scarce on the presenting symptoms of PRCC with extension to the IVC, and its appropriate management remains under debate. A report of one case of a pregnant woman diagnosed with a rapidly growing PRCC in the first trimester of pregnancy that was complicated by IVC thrombosis after surgery emphasizes the importance of this condition9.
Tumor invasion of the IVC has been previously associated with poor prognosis in patients with renal cell carcinoma (RCC)7. Among 413 patients with RCC with invasion of the IVC, 29 had PRCC, and evaluation of the consistency of the venous tumor thrombosis revealed 11 cases with friable IVC and 18 with solid IVC; poorer prognosis has been observed in cases with friable IVC7. Comparison of 68 patients with RCC and IVC thrombosis who underwent radical nephrectomy and IVC thrombectomy showed that the papillary subtype was an important predictor of poor prognosis, while patients with clear cell subtype had better cancer-specific survival8. Of the 12 patients with PRCC and IVC involvement (all had type II PRCC), type II PRCC was a strong predictor of poor prognosis and resulted in a 2-year survival rate of 28% and a 5-year survival rate of 0% after surgery10. A study by Kondo and colleagues reported that the papillary subtype is an aggressive disease, and the median survival time after surgery in patients with PRCC, IVC involvement, and nodal or remote metastases was reduced to just 5.2 months8. Therefore, it has been suggested that these patients may not benefit from surgical treatment8. Some have suggested the use of anti-programmed cell death 1 antibody drugs, like nivolumab, in inoperable patients with type II PRCC and IVC involvement for safe nephrectomy and thrombectomy11. Therefore, the most appropriate management of these patients is yet to be determined.
Another notable finding in our case was the large tumor size. PRCC is considered a heterogeneous tumor, and it has been previously reported that atypical imaging findings are more commonly observed in large lesions with a diameter > 4 cm5. A review of 13 cases of PRCC revealed a mean diameter of 7 cm (6.92 ± 3.06 cm in type I and 7.27 ± 3.10 cm in type II PRCC), and there was no significant difference in tumor size among the PRCC types6. In another study on 577 patients with PRCC, median tumor size was reported to be 4 cm (maximum of 6 cm)12. However, the tumor size of our study (20 cm) was significantly larger than the reported mean sizes in these studies6, 12. To our knowledge, such a large PRCC tumor has not been previously reported, especially in association with IVC involvement; it is necessary to take into consideration the combination of factors affecting prognosis when deciding the best treatment approach for the patient.
Conclusions
The present case involved the rare phenomenon of IVC involvement in an extremely large PRCC tumor, which draws the attention of physicians to this condition. The mechanism of this concurrence and the most appropriate treatment of these patients should be further investigated.
Abbreviations
CT: Computed tomography, IVC: Inferior vena cava, MRI: Magnetic resonance imaging, PRCC: Papillary renal cell carcinoma, VTT: Venous tumor thrombus
Acknowledgments
The authors would like to thank the Clinical Research Development Center of Imam Reza Hospital for Consulting Services and MS. Sholeh Akradi for providing data.
Author’s contributions
M.R. and F.A. conceived of the presented idea. K.M. and F.A. contributed to sample preparation. M.A. wrote the manuscript in consultation with M.R. M.S. contributed to the interpretation of the results and designed the figures. M.R. supervised the work. All authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Inamura
K.,
Renal cell tumors: understanding their molecular pathological epidemiology and the 2016 WHO classification. International Journal of Molecular Sciences.
2017;
18
(10)
:
2195
.
View Article PubMed Google Scholar -
Linehan
W.M.,
Spellman
P.T.,
Ricketts
C.J.,
Creighton
C.J.,
Fei
S.S.,
Davis
C.,
Cancer Genome Atlas Research Network
Comprehensive molecular characterization of papillary renal-cell carcinoma. The New England Journal of Medicine.
2016;
374
(2)
:
135-45
.
View Article PubMed Google Scholar -
Pichler
M.,
Hutterer
G.C.,
Chromecki
T.F.,
Jesche
J.,
Kampel-Kettner
K.,
Rehak
P.,
Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. American Journal of Clinical Pathology.
2012;
137
(2)
:
283-9
.
View Article PubMed Google Scholar -
Couvidat
C.,
Eiss
D.,
Verkarre
V.,
Merran
S.,
Corréas
J.M.,
Méjean
A.,
Renal papillary carcinoma: CT and MRI features. Diagnostic and Interventional Imaging.
2014;
95
(11)
:
1055-63
.
View Article PubMed Google Scholar -
Muglia
V.F.,
Prando
A.,
Renal cell carcinoma: histological classification and correlation with imaging findings. Radiologia Brasileira.
2015;
48
(3)
:
166-74
.
View Article PubMed Google Scholar -
Liu
K.,
Ren
Y.,
Pang
L.,
Qi
Y.,
Jia
W.,
Tao
L.,
Papillary renal cell carcinoma: a clinicopathological and whole-genome exon sequencing study. International Journal of Clinical and Experimental Pathology.
2015;
8
(7)
:
8311-35
.
PubMed Google Scholar -
Mager
R.,
Daneshmand
S.,
Evans
C.P.,
Palou
J.,
Martínez-Salamanca
J.I.,
Master
V.A.,
International Renal Cell Carcinoma-Venous Thrombus Consortium
Renal cell carcinoma with inferior vena cava involvement: prognostic effect of tumor thrombus consistency on cancer specific survival. Journal of Surgical Oncology.
2016;
114
(6)
:
764-8
.
View Article PubMed Google Scholar -
Kondo
T.,
Ikezawa
E.,
Takagi
T.,
Kobayashi
H.,
Hashimoto
Y.,
Iizuka
J.,
Negative impact of papillary histological subtype in patients with renal cell carcinoma extending into the inferior vena cava: single-center experience. International Journal of Urology.
2013;
20
(11)
:
1072-7
.
View Article PubMed Google Scholar -
Boukhannous
I.,
Mhanna
T.,
El Houmaidi
A.,
Aynaou
M.,
Chennoufi
M.,
Barki
A.,
Fast growing papillary renal cell carcinoma in first trimester pregnancy with postoperative inferior vena cava thrombosis: A case report. Urology Case Reports.
2020;
33
:
101292
.
View Article PubMed Google Scholar -
Kim
K.H.,
You
D.,
Jeong
I.G.,
Kwon
T.W.,
Cho
Y.M.,
Hong
J.H.,
Type II papillary histology predicts poor outcome in patients with renal cell carcinoma and vena cava thrombus. BJU International.
2012;
110
(11 Pt B)
:
673-8
.
View Article PubMed Google Scholar -
T. Shinagawa,
H. Ito,
Y. Sakai,
Remarkable effect of presurgical nivolumab on originally inoperable papillary renal cell carcinoma with tumor thrombus in inferior vena cava. Int Cancer Conf J; 2019: Springer..
.
-
Zucchi
A.,
Novara
G.,
Costantini
E.,
Antonelli
A.,
Carini
M.,
Carmignani
G.,
Prognostic factors in a large multi-institutional series of papillary renal cell carcinoma. BJU International.
2012;
109
(8)
:
1140-6
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 8 (2022)
Page No.: 5196-5200
Published on: 2022-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3515 times
- PDF downloaded - 1380 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress



