Abstract
Background: The prevalence of patients with heart failure with mid-range ejection fraction (HFmrEF) remains unchanged regardless of healthcare strategies. HFmrEF has mixed characteristics of heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). The treatment of HFmrEF was recommended to be similar to the treatment of HFpEF in the 2016 guidelines of the European Society of Cardiology (ESC) but was changed to those of HFrEF in the 2021 version. Objective: To describe the clinical characteristics of inpatients with HFmrEF with a focus on treatment practices.
Methods: A prospective study was conducted on patients diagnosed with HFmrEF who were admitted to the cardiology department of Nhan Dan Gia Dinh hospital from July 2019 to July 2020. Rehospitalization and mortality were followed up by telephone after 1 year.
Results: Of a total of 529 heart failure cases admitted to the hospital during the study period, there were 73 patients (13.8%) with HFmrEF. The mean age was 68 years and males comprised 53%. The average hospitalization stay length was 6.7 days. The most common precipitating factors of heart failure were non-adherence (42.5%) and infection (39.7%) followed by hypertensive crisis (8.2%), anemia (6.8%), acute myocardial infarction (5.5%), arrhythmia (4.1%), and hyperthyroidism (2.7%). Ischemic heart disease was the leading cause (61.6%) of HFmrEF, followed by hypertension (12.3%). The rates of HFmrEF patients treated with beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and aldosterone receptor antagonists were 76.1%, 76.71%, 37.73%, and 47.95%, respectively. There were no statistically significant differences in the guideline-directed medical therapy (GDMT) medications administered between HFmrEF due to ischemic and non-ischemic causes. After 1-year follow-up, the combined outcome of rehospitalization and mortality in HFmrEF was 24.7%.
Conclusion: The treatment of HFmrEF was similar to that of HFrEF between 2019 and 2020 at Nhan Dan Gia Dinh hospital and was in accordance with the 2021 ESC guidelines.
Introduction
Diagnosis and treatment of heart failure are mainly based on objective assessment of left ventricular ejection fraction (LVEF). Previously, heart failure had been classified into two groups according to LVEF: heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). Recommendations from the American Heart Association/American College of Cardiology (AHA/ACC) and the European Heart Association (ESC) defined HFrEF as heart failure with an LVEF < 40%, while HFpEF was heart failure with LVEF ≥ 50%. This definition created an intermediate group that was not well studied. The 2016 ESC guidelines considered classifying the group with LVEF between 40% and 49% as heart failure with mid-range ejection fraction (HFmrEF)1. Based on current studies, the prevalence of HFmrEF in the heart failure population ranges from 13% to 24%2, 3, 4. Trend analysis in the Get With the Guidelines — Heart Failure (GWTG-HF) registry showed that, although the percentage of HFpEF increased (33% to 39%) and HFrEF decreased (52% to 47%) between 2005 and 2010, the rate of HFmrEF remained relatively stable (13–15%)5.
Because it represents the "gray zone” of the heart failure population, more evidence is needed to better understand and manage HFmrEF. The 2016 ESC guidelines also emphasized further investigation in this group. There are very few studies on HFmrEF in the Asian population. Therefore, our study was conducted to investigate the clinical characteristics and evaluate the treatment practices of HFmrEF at a tertiary hospital in Vietnam.
Methods
Study design
A prospective study was conducted at the cardiology department of Nhan Dan Gia Dinh hospital, Ho Chi Minh City, Vietnam, from July 2019 to July 2020. Hospitalized HFmrEF patients ≥ 18 years old who provided consent to participate in the study were recruited. Pregnant or breastfeeding women were excluded from the study.
Sample size
Because the treatment practice was considered the main objective, the sample size was estimated based on the following formula:
N = p.(1-p).(1.96/m)2
in which, p was obtained from a meta-analysis study by Lauritsen in which the prescribing prevalence of angiotensin-converting enzyme inhibitors (ACEis)/angiotensin II receptor blockers (ARBs), beta-blockers (BBs), and mineralocorticoid receptor antagonists (MRAs) were 79.6%, 82%, and 20.3%, respectively6. The m value was chosen to be 10%. Therefore, the minimum sample size was 63 patients from the three results of 62.3, 56.7, and 62.3, respectively.
For the estimation of 1-year prognosis, the same sample formula was used with p = 22.5% of the combined outcome of rehospitalization and mortality in the Chronic Heart Failure Analysis and Registry in the Tohoku District 2 (CHART-2) study, and m = 10%. The minimum sample was 67 patients4.
Variable definition
Heart failure with mid-range ejection fraction
The diagnosis was made with all of the following criteria (1):
— Symptoms ± signs of heart failure
— NT pro-BNP >125 pg/mL
— Echocardiography
+ LVEF 40–49%
+ At least one additional criterion: (1) relevant structural heart disease (left ventricular hypertrophy and/or left atrial enlargement) and (2) diastolic dysfunction.
Medications in guideline-directed medical therapy
ACEis, ARBs, sacubitril/valsartan (angiotensin receptor-neprilysin inhibitor [ARNI]), BBs, and MRAs in the guideline-directed medical therapy (GDMT) of HFrEF were the targets of the investigation.
Measurement of left ventricular ejection fraction
Echocardiography was performed by certified specialists who had more than 5 years of experience. LVEF was measured by the biplane Simpson method using a Phillips Affinity 50 device.
Follow-up protocol
The outcomes of rehospitalization and mortality were collected every 3 months or whenever information was provided by patients and/or their families for up to 1 year. The follow-up was conducted via telephone.
Statistical analysis
Data were analyzed using STATA version 16.0. Categorical variables are presented as frequencies and percentages, and continuous variables are presented as mean and standard deviation or median and interquartile values based on the distribution. The Chi-square test was used to analyze the relationship between the GDMT medications between the ischemic and non-ischemic groups. p < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study sample
From July 2019 to July 2020, 529 patients with a diagnosis of heart failure were admitted to the cardiology department at Nhan Dan Gia Dinh hospital. The study recruited 73 patients diagnosed with HFmrEF; therefore, the prevalence of HFmrEF in this study was 13.8% of the inpatient heart failure population.
The mean age was 68.25 ± 12.21 years, and more than half of the participants were male. Most patients with HFmrEF were diagnosed with heart failure NYHA III (47.9%) or NYHA II (41.1%). Ischemic heart disease accounted for the most common (61.6%) cause of heart failure, followed by hypertension (12.3%). The most common precipitating factor of heart failure in HFmrEF was non-adherence (42.5%) followed by infection (39.7%). Hypertension was the leading comorbidity. The high values of NT-proBNP and LVMi were compatible with HFmrEF diagnosis (Table 1).
| Male | 39 (53) |
| Age (years) | 68 (61 – 78) |
| BMI (kg/m 2 ) | 22.5 ± 3.0 |
| Length of hospitalization (days) | 6 (3.5 – 8.0) |
| Cause of heart failure | |
| Ischemic heart disease | 45 (61.6) |
| Hypertension | 9 (12.3) |
| Cardiomyopathy | 8 (11.0) |
| Valvular heart disease | 8 (11.0) |
| Others | 3 (4.1) |
| NYHA class | |
| I | 2 (2.7) |
| II | 30 (41.1) |
| III | 35 (47.9) |
| IV | 6 (8.2) |
| Precipitating factors | |
| Infection | 29 (39.7) |
| Acute myocardial infarction | 4 (5.5) |
| Hypertensive crisis | 6 (8.2) |
| Non-adherence | 31 (42.5) |
| Hyperthyroidism | 2 (2.7) |
| Arrhythmias | 3 (4.1) |
| Anemia | 5 (6.8) |
| Concomitant conditions | |
| Hypertension | 50 (68.5) |
| Diabetes | 23 (31.5) |
| Dyslipidemia | 18 (24.7) |
| Atrial fibrillation | 21 (28.8) |
| Previous stroke | 6 (8.2) |
| Chronic pulmonary disease | 5 (6.8) |
| Chronic kidney disease | 19 (26.0) |
| Hyperthyroidism | 2 (2.7) |
| Cancer | 9 (12.3) |
| Obesity | 17 (23.3) |
| Smoking | 10 (13.7) |
| Alcohol abuse | 4 (5.5) |
| Laboratory results | |
| NT-proBNP (ng/L) | 3767 (1072 – 6679) |
| hsTnT (ng/L) | 28.0 (14.0 – 52.5) |
| Creatinine ( μ mol/L) | 94.5 (77.5 – 128) |
| Na (mEq/L) | 138 (135 – 140) |
| Kali (mEq/L) | 3.76 ± 0.51 |
| Echocardiography | |
| LVEF (%) | 44.0 ± 3.0 |
| LVMi (g/m 2 ) | 121 (96 – 162) |
| Total (73) | Ischemic (45) | Non-ischemic (28) | p-value | |
|---|---|---|---|---|
| BBs | 56 (76.7) | 37 (82.2) | 19 (67.9) | 0.158 |
| ACEis/ARBs | 56 (76.7) | 35 (77.8) | 21 (75.0) | 0.785 |
| MRAs | 35 (48.0) | 21 (46.7) | 14 (50.0) | 0.782 |
| Mortality | 8 (11.0) |
| Rehospitalization | 10 (13.7) |
| Combined mortality and rehospitalization | 18 (24.7) |
Medications in the guideline-directed medical therapy in HFmrEF
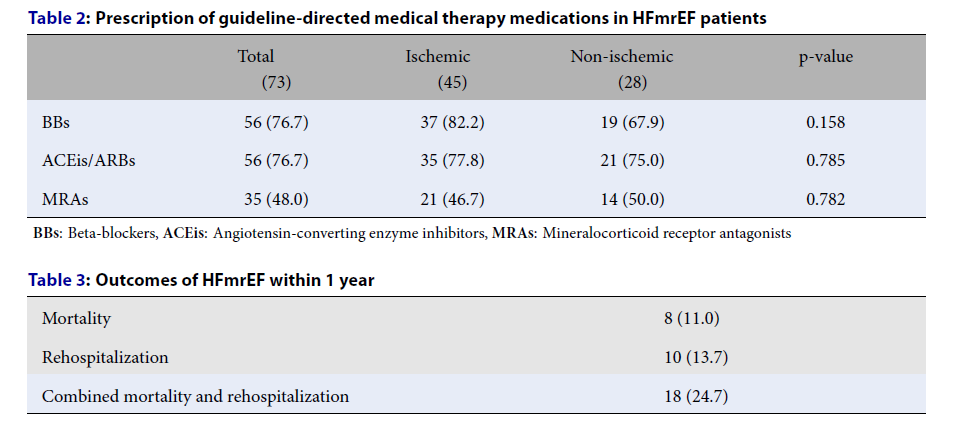
The rates of BB and ACEi/ARB prescription were higher than MRA prescription. No ARNIs were prescribed during the study period. There was no difference between the ischemic and non-ischemic groups in terms of BBs, ACEis/ARBs, or MRAs prescribed (Table 2).
Rehospitalization and mortality within 1 year
One in four patients with HFmrEF had an unfavorable outcome at 1 year. The rates of mortality and rehospitalization were comparable in our study (Table 3).
Discussion
The rate of HFmrEF in the heart failure population at Nhan Dan Gia Dinh hospital was 13.6%, which was in line with other studies4, 7, 8. The average age of our participants was 68.25 years, which was consistent with previous reports4, 9, 10, 11, 12. The proportion of men being higher than women in our study was also observed in recent investigations4, 8, 13. These results indicated that Asian HFmrEF patients had similar demographic characteristics to Western populations.
Ischemic heart disease was the leading cause of heart failure in our study, which is in line with other investigations4, 8, 9. Previous data suggested that a significant proportion of HFmrEF patients may be in transition between HFpEF and HFrEF and that ischemic pathophysiology could be an important mediator in LVEF deterioration14. The two most common precipitating factors of decompensated heart failure were infection (39.7%) and non-adherence (42.5%). In the investigation by Kapoor and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) study, pneumonia was the most common factor in heart failure exacerbations and non-adherence was not prominent3, 15. The rate of comorbidities in the HFmrEF group was similar to that of the HFpEF group, which was similar to other reports7, 16.
Initially, data from the OPTIMIZE-HF registry and the Acute Decompensated Heart Failure National Registry (ADHERE) suggested that the clinical characteristics, treatment, and outcomes of the HFmrEF population might be closer to that of the HFpEF population9, 17. Similar results were observed in more than 40,000 Medicare patients hospitalized for heart failure in the GWTG-HF registry7. In contrast, the European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT) study of 2,212 patients in 2017 reported that the clinical characteristics of the HFmrEF patients tended to be more comparable to those of the HFrEF group. These features included male, younger age, coronary artery disease as a prominent cause of heart failure, low incidence of atrial fibrillation, and few comorbidities12. Some of these characteristics were consistent with the Swedish Heart Failure Registry (SwedeHF) study from 2017 and the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) study from 201810, 11. It is worth noting that the HFmrEF group can include both patients with HFrEF with improved LVEF and HFpEF with reduced LVEF; this may explain why the HFmrEF patients had clinical characteristics of both these groups. The results of our study revealed a similar situation.
The 2016 ESC guidelines did not make any recommendations that were shown to improve outcomes in HFmrEF. Treatment was recommended to focus on comorbidities, risk factors, and symptoms to minimize heart failure exacerbations and improve quality of life (i.e. similar to HFpEF management)1. However, in the following years, several studies revealed that the HFrEF treatment strategy might be beneficial in the HFmrEF group. The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial in patients with an LVEF of 45% found that spironolactone had no effect on the primary outcome but noted a reduction in hospitalization in the treatment group (HR: 0.83). Furthermore, there was a significant interaction between treatment and LVEF, with greater benefit in the LVEF range of 45 — 50%18. Similarly, the CHARM-Preserved trial investigated the efficacy of candesartan in heart failure with LVEF > 40% and reported a beneficial effect on hospitalization (HR: 0.84). However, in the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE), irbesartan was investigated in heart failure with LVEF ≥ 45% and did not produce a significant benefit; however, the mean LVEF was higher (59%) than that of the CHARM-Preserved group (54%)19. In the CHART-2 study, BB treatment in the HFmrEF group was shown to improve mortality4. Moreover, in the Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction (PARAGON-HF) trial, patients with LVEF ≤57% who received an ARNI had a 22% reduction in mortality and cardiovascular hospitalization when compared with the valsartan group. Moreover, when the data of the PARAGON-HF and Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trials were combined, they revealed that ARNIs helped to reduce the hospitalization rate, especially in the HFmrEF group20, 21.
In the past, the prescription rates of BBs, MRAs, and ACEis/ARBs in HFmrEF have been negligible22, 23. Nevertheless, BBs, ACEis/ARBs, MRAs, and ARNIs were recommended to reduce hospitalization and mortality in HFmrEF in the 2021 ESC guidelines for heart failure24. There was a high rate of prescription of BBs, ACEis/ARBs, and MRAs in HFmrEF in our study. There was an overlap in the treatment of ischemic heart disease regarding these GDMT medications; however, a comparative analysis of the non-ischemic and ischemic groups of HFmrEF revealed no significant difference. ARNIs were not widely available and their high cost during the study period may explain the zero rate of prescription.
The 1-year outcomes in our study were similar to those of CHART-2 (2017), but much lower than OPTIMIZE-HF (2007). Of note, the rates of GDMT medication prescriptions in our study were similar to CHART-2, while they were less often used in OPTIMIZE-HF. Our target was not to investigate the risk factors of poor outcomes in HFmrEF; however, our results indicated the usage of GDMT medications in HFmrEF. The treatment of patients with HFmrEF in our hospital was comparable to the treatment of HFrEF based on evidence from studies published following publication of the 2016 ESC guideline. This rational practice was subsequently confirmed in the 2021 version of the ESC recommendations.
Our study provided results that could be representative of the HFmrEF population in tertiary centers like Nhan Dan Gia Dinh hospital. There were several limitations in our study. First, we only described LVEF cross-sectionally, while it might show longitudinal alterations. Second, there was no direct comparison between HFmrEF and the other two groups of heart failure. Third, we were unable to collect detailed information about diagnosis and treatment by telephone follow-up. More prospective multicenter studies with larger samples are needed to investigate this group, especially LVEF changes and risk factors related to rehospitalization and mortality.
Conclusions
HFmrEF was treated in the same way as HFrEF at our center between 2019 and 2020 based on the available evidence. The significant prevalence and high rate of poor outcomes in our study could be used as preliminary data for more in-depth investigations of HFmrEF.
Abbreviations
ACEi: Angiotensin-converting enzyme inhibitor, ADHERE: The Acute Decompensated Heart Failure National Registry, AHA/ACC: The American Heart Association/American College of Cardiology, ARB: Angiotensin II receptor blocker, ARNI: Sacubitril/valsartan, BB: Beta blocker, BMI: Body mass index, CHARM: The Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity trial, CHART-2: The Chronic Heart Failure Analysis and Registry in the Tohoku District 2 study, ESC: The European Society of Cardiology, ESC-HF-LT: The European Society of Cardiology Heart Failure Long-Term Registry, GDMT: Guideline-directed medical therapy, GWTG-HF: Get With the Guidelines – Heart Failure registry, HFmrEF: Heart failure with mid-range ejection fraction/Heart failure with mildly reduced ejection fraction, HFpEF: Heart failure with preserved ejection fraction, HFrEF: Heart failure with reduced ejection fraction, I-PRESERVE: The Irbesartan in Heart Failure with Preserved Ejection Fraction Study, LVEF: Left ventricular ejection fraction, LVMi: Left ventricular mass index, MRA: Mineralocorticoid receptor antagonist, NT-proBNP: NT-pro B type natriuretic peptide, NYHA: New York Heart Association, OPTIMIZE-HF: The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure registry, PARADIGM-HF: Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure, PARAGON-HF: Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction trial, SwedeHF: The Swedish Heart Failure Registry TOPCAT: The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist trial
Acknowledgments
None.
Author’s contributions
Si Van Nguyen devised the project, the main conceptual ideas, and the proof outline. Truong Xuan Tran and Tung Thien Nguyen analyzed the data. Thuc Minh Do and Dung Truong My Pham wrote the manuscript. All the authors took part in data collection and patient follow-up. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent. This study was approved by the Ethics Committee of Nhan Dan Gia Dinh hospital (number: 56-2019/NDGĐ-HĐĐĐ)
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Ponikowski
P.,
Voors
A.A.,
Anker
S.D.,
Bueno
H.,
Cleland
J.G.,
Coats
A.J.,
Scientific Document Group
ESC,
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal.
2016;
37
(27)
:
2129-200
.
View Article PubMed Google Scholar -
Coles
A.H.,
Tisminetzky
M.,
Yarzebski
J.,
Lessard
D.,
Gore
J.M.,
Darling
C.E.,
Magnitude of and Prognostic Factors Associated With 1-Year Mortality After Hospital Discharge for Acute Decompensated Heart Failure Based on Ejection Fraction Findings. J Am Heart Assoc.
2015;
4
(12)
:
e002303
.
View Article PubMed Google Scholar -
Kapoor
J.R.,
Kapoor
R.,
Ju
C.,
Heidenreich
P.A.,
Eapen
Z.J.,
Hernandez
A.F.,
Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC. Heart Failure.
2016;
4
(6)
:
464-72
.
View Article PubMed Google Scholar -
Tsuji
K.,
Sakata
Y.,
Nochioka
K.,
Miura
M.,
Yamauchi
T.,
Onose
T.,
Investigators
CHART-2,
Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. European Journal of Heart Failure.
2017;
19
(10)
:
1258-69
.
View Article PubMed Google Scholar -
Steinberg
B.A.,
Zhao
X.,
Heidenreich
P.A.,
Peterson
E.D.,
Bhatt
D.L.,
Cannon
C.P.,
Get With the Guidelines Scientific Advisory Committee
Investigators
Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation.
2012;
126
(1)
:
65-75
.
View Article PubMed Google Scholar -
Lauritsen
J.,
Gustafsson
F.,
Abdulla
J.,
Characteristics and long-term prognosis of patients with heart failure and mid-range ejection fraction compared with reduced and preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Fail.
2018;
5
(4)
:
685-694
.
View Article PubMed Google Scholar -
Cheng
R.K.,
Cox
M.,
Neely
M.L.,
Heidenreich
P.A.,
Bhatt
D.L.,
Eapen
Z.J.,
Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. American Heart Journal.
2014;
168
(5)
:
721-30
.
View Article PubMed Google Scholar -
Rickenbacher
P.,
Kaufmann
B.A.,
Maeder
M.T.,
Bernheim
A.,
Goetschalckx
K.,
Pfister
O.,
Investigators
TIME-CHF,
Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). European Journal of Heart Failure.
2017;
19
(12)
:
1586-96
.
View Article PubMed Google Scholar -
Fonarow
G.C.,
Stough
W.G.,
Abraham
W.T.,
Albert
N.M.,
Gheorghiade
M.,
Greenberg
B.H.,
Investigators
OPTIMIZE-HF,
Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology.
2007;
50
(8)
:
768-77
.
View Article PubMed Google Scholar -
Lund
L.H.,
Claggett
B.,
Liu
J.,
Lam
C.S.,
Jhund
P.S.,
Rosano
G.M.,
Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. European Journal of Heart Failure.
2018;
20
(8)
:
1230-9
.
View Article PubMed Google Scholar -
Koh
A.S.,
Tay
W.T.,
Teng
T.H.,
Vedin
O.,
Benson
L.,
Dahlstrom
U.,
A comprehensive population-based characterization of heart failure with mid-range ejection fraction. European Journal of Heart Failure.
2017;
19
(12)
:
1624-34
.
View Article PubMed Google Scholar -
Chioncel
O.,
Lainscak
M.,
Seferovic
P.M.,
Anker
S.D.,
Crespo-Leiro
M.G.,
Harjola
V.P.,
Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. European Journal of Heart Failure.
2017;
19
(12)
:
1574-85
.
View Article PubMed Google Scholar -
He
K.L.,
Burkhoff
D.,
Leng
W.X.,
Liang
Z.R.,
Fan
L.,
Wang
J.,
Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40%. The American Journal of Cardiology.
2009;
103
(6)
:
845-51
.
View Article PubMed Google Scholar -
Gerber
Y.,
Weston
S.A.,
Berardi
C.,
McNallan
S.M.,
Jiang
R.,
Redfield
M.M.,
Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. American Journal of Epidemiology.
2013;
178
(8)
:
1272-80
.
View Article PubMed Google Scholar -
Fonarow
G.C.,
Abraham
W.T.,
Albert
N.M.,
Stough
W.G.,
Gheorghiade
M.,
Greenberg
B.H.,
Investigators
OPTIMIZE-HF,
Hospitals
Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Archives of Internal Medicine.
2008;
168
(8)
:
847-54
.
View Article PubMed Google Scholar -
Q. Zhou,
P. Li,
H. Zhao,
X. Xu,
S. Li,
J. Zhao,
D. Xu,
Q. Zeng,
Heart failure with mid-range ejection fraction: a distinctive subtype or a transitional stage?. Frontiers in Cardiovascular Medicine.
2021;
8
:
678121
.
View Article PubMed Google Scholar -
Sweitzer
N.K.,
Lopatin
M.,
Yancy
C.W.,
Mills
R.M.,
Stevenson
L.W.,
Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. The American Journal of Cardiology.
2008;
101
(8)
:
1151-6
.
View Article PubMed Google Scholar -
Solomon
S.D.,
Claggett
B.,
Lewis
E.F.,
Desai
A.,
Anand
I.,
Sweitzer
N.K.,
Investigators
TOPCAT,
Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. European Heart Journal.
2016;
37
(5)
:
455-62
.
View Article PubMed Google Scholar -
Yusuf
S.,
Pfeffer
M.A.,
Swedberg
K.,
Granger
C.B.,
Held
P.,
McMurray
J.J.,
Investigators
CHARM,
Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet.
2003;
362
(9386)
:
777-81
.
View Article PubMed Google Scholar -
Solomon
S.D.,
Vaduganathan
M.,
L Claggett
B.,
Packer
M.,
Zile
M.,
Swedberg
K.,
Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation.
2020;
141
(5)
:
352-61
.
View Article PubMed Google Scholar -
Solomon
S.D.,
McMurray
J.J.,
Anand
I.S.,
Ge
J.,
Lam
C.S.,
Maggioni
A.P.,
Investigators
PARAGON-HF,
Committees
Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. The New England Journal of Medicine.
2019;
381
(17)
:
1609-20
.
View Article PubMed Google Scholar -
Lam
C.S.,
Solomon
S.D.,
The middle child in heart failure: heart failure with mid-range ejection fraction (40-50%). European Journal of Heart Failure.
2014;
16
(10)
:
1049-55
.
View Article PubMed Google Scholar -
Hsu
J.J.,
Ziaeian
B.,
Fonarow
G.C.,
Heart Failure With Mid-Range (Borderline) Ejection Fraction: Clinical Implications and Future Directions. JACC. Heart Failure.
2017;
5
(11)
:
763-71
.
View Article PubMed Google Scholar -
McDonagh
T.A.,
Metra
M.,
Adamo
M.,
Gardner
R.S.,
Baumbach
A.,
Böhm
M.,
Scientific Document Group
ESC,
2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal.
2021;
42
(36)
:
3599-726
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 8 (2022)
Page No.: 5209-5215
Published on: 2022-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4886 times
- PDF downloaded - 1558 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress


