Abstract
Background: Fine-needle aspiration (FNA) has become an essential, critical test for breast masses. This study aimed to determine the value of diastase-resistant periodic acid-Schiff (DPAS) staining in the detection of malignant breast cells.
Methods: This prospective cross-sectional study was conducted in Khartoum state (Sudan) among Sudanese women who suffered from breast lumps. FNA samples were collected from each patient, and the material was simultaneously smeared onto two labeled glass slides. The DPAS score and aspiration cytology (AC) grade are expressed as mean +/- SD, and the 95% confidence intervals of the means were calculated.
Results: The findings revealed the following DPAS score frequencies among the studied women: negative (+/-) (28, 13.9%), one plus (+) (114, 56.7%), two plus (++) (27, 13.4%), and three-plus (+++) (32, 15.9%). Comparison of DPAS scores with the cytological categories (cytology results) revealed that DPAS positivity (++, +++) correlated best with malignancy. Of the 201 patients, the AC grades according to the International Academy of Cytology (IAC) system were: AC2 (30, 14.9%), AC3 (112, 55.7%), AC4 (27, 13.4%), and AC5 (32, 15.9%).
Conclusions: DPAS positivity in atypical cells in FNA aspirates may assist in upgrading from a suspicious to a malignant diagnosis in women with breast lumps.
Introduction
Breast cancer is prevalent common across the globe1; 1 in every 9 women in developed countries and 1 in every 20 women in less developed areas have a risk of breast cancer2. Breast cancer is the most common human female cancer worldwide. Its incidence is rising at approximately 2% per year in all populations3. Worldwide, approximately one million women are newly diagnosed with breast cancer each year4. In the United Kingdom, cancer accounts for about 25% of all deaths5, and breast cancer accounts for 20% of all forms of malignancies in females6.
The age-adjusted incidence rate of breast cancer rose rapidly in several Asian countries (e.g., Japan) that previously had the lowest incidence rates7. According to GLOBOCAN 2012, prevalence estimates for 2012 revealed that there were 32.6 million people over the age of 15 years who had a cancer diagnosis in the previous 5 years8.
In Sudan, breast cancer is the most frequent hospital‐treated malignancy, accounting for about 16% (4,005/25,064) of all reported cancer cases. In Sudan, precise clinical data are lacking, making it difficult to determine clinicopathologic correlations and to compile databases and registries9, 10, 11.
Female breast cancer is the leading cancer in the Sudan and has been recognized as an important health problem, being associated with a high rate of mortality and morbidity. The highest rate was reported in 1998 (38.4% of all female cancers) by the Radiation Isotope Center Khartoum12, 13, 14. Most diseases of the breast present as palpable masses, painful lesions, nipple discharge, or mammography changes15.
Many risk factors for breast cancer have been identified, such as age, locality, early menarche, late menopause, age at first pregnancy, family history of breast cancer, previous benign breast disease, radiation, lifestyle, oral contraceptive use, hormone replacement therapy, and socioeconomic class16.
Recently, the accuracy of diagnosing breast tumors has been improved by fine needle aspiration (FNA) cytology17. FNA has become an important preoperative and screening test for breast masses18.
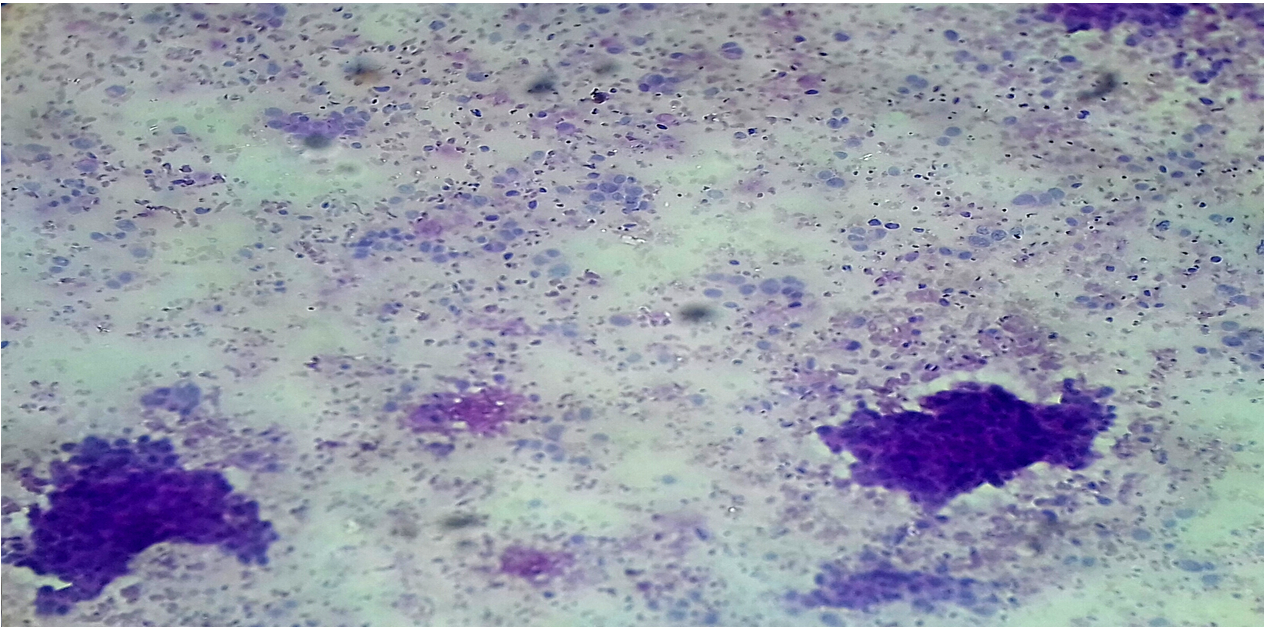
Diastase-resistant periodic acid-Schiff (DPAS) is a stain often used by pathologists as an ancillary investigation when making a histological diagnosis on paraffin-embedded tissue specimens19. Strong intracytoplasmic PAS-positive, diastase-resistant (DPAS) staining within atypical cells has been used as a marker for carcinoma in breast aspirates in previous studies. Furthermore, there is a correlation between cytological intracellular DPAS positivity and subsequent malignant histology20, 21.
In a country like Sudan, where resource management is critical, it is crucial to adopt low-cost techniques. The diagnostic power of FNA cytology, specifically in breast samples, can be improved by following validated procedures and scientific standardization of simple low-cost techniques, like DPAS, to replace costly advanced techniques. However, adapting low-cost techniques alone is not enough and should be accompanied by strict evaluation and reliability of these techniques. This study evaluated simple, low-cost techniques to determine whether they were applicable in the FNA cytological diagnosis of breast lesions. This study aimed to determine the value of DPAS in the detection of malignant breast cells.
Methods
A prospective cross-sectional study was conducted in Khartoum state (Sudan) on 201 Sudanese women who suffered from breast lumps.
Tissues obtained via FNA were used to prepare two direct smears. One of the direct smears was immediately fixed in 95% ethyl alcohol and was wet for the subsequent Pap staining, while the other direct smear was allowed to air dry and was then fixed in methanol for subsequent May–Grunwald–Giemsa (MGG) staining.
DPAS staining was performed on unstained or MGG-destined slides after cytological assessment. The slides were covered with a damp filter paper, we applied fresh saliva to the filter paper, and the slide was incubated for 30 minutes at 37°C. The slides were then rinsed in water, covered with 1% periodic acid (BDH-Merck Ltd, Lutterworth, UK) for 8 minutes, rinsed in distilled water, covered with Schiff’s reagent (BDH-Merck Ltd) for 30 minutes, then washed in running water for 30 minutes and counterstained with hematoxylin21.
Atypia was assessed cytologically by using the standard criteria described by Ahmed and Elemirri22. The criteria of atypia included the presence of major malignant features, including nuclear enlargement associated with increased nuclear-cytoplasmic ratio, hyperchromatism, chromatin clumping with moderately prominent nucleolation and irregular nuclear borders, bi- or multinucleation, scantiness of the cytoplasm, and variations in size and/or shape of the cells and nuclei. The criteria of atypia included the presence of the significant malignant features23.
The C1–5 grading system was used to determine an aspiration cytology (AC) grade between 0 and 5, as described by Johnson and Wadehra21:
AC0 is an inadequate specimen containing no breast duct epithelial cells or just one group; AC1 is also inadequate, containing less than six groups of epithelial cells; AC2 is an adequate sample containing at least six groups (at least 12 duct epithelial cells in each group) of benign cells (with an additional diagnosis when appropriate; e.g., fibrocystic change or fibroadenoma); AC3 is a sample with atypia that is probably benign; AC4 reflects atypia that is probably malignant (suspicious of carcinoma); and AC5 is diagnostic of carcinoma (with a type and grade given whenever possible; e.g., ductal, lobular, mucinous).
For intracellular DPAS positivity to be considered relevant, we required staining that produced a definite magenta color, fully within the cytoplasm, and round in shape with a well-defined, crisp edge. Intracellular DPAS staining was recorded semi-quantitatively: negative; ±, equivocal staining (taken as negative when assessing results); +, occasional cells with definite staining; ++, an intermediate number of cells with definite staining; or +++, numerous positive cells or particularly strong staining21.
Statistical analysis
Data were analyzed using Statistical Package for Social Science (SPSS) software version 20 (IBM Corp, Armonk, NY, USA).
DPAS scores are expressed as mean ± SD and the 95% confidence intervals (CIs) of the calculated means. The χ2 test was used to compare the differences in categorical variables between the different tests. Relationships between variables were analyzed using Pearson’s correlation analysis. p<0.05 was considered statistically significant. Chi-square analysis was used to obtain all the p values in this study24, 25, 26.
Ethical approval and consent to participate
All participants were fully informed about the aims and outcomes of the study, and they were asked to sign a written consent form before the specimen was obtained by the pathologist in-charge. The results were presented to and discussed with the patients. Ethical approval was obtained from the National Ribat University Ethical Research Committee in accordance with the Declaration of Helsinki principles, and consent was obtained from all patients before sample and data collection. The patient’s information was highly secured and not used for purposes other than scientific inquiry. Risk and benefits for the patients from the outcomes of the research was ensured.
Approval reference number: NRU-REC/05-021./07
Approval date: 26/5/2021
| Age group | Cytological diagnosis | ||||||
| Benign lump | Inflammation | Suspicious of malignancy | Malignant | Total | |||
| N (%) | N (%) | N (%) | N (%) | N (%) | Chi | p | |
| < 15 years | 2 (7.4%) | 10 (8.9%) | 1 (5.9%) | 1 (2.2%) | 14 (7.0%) | 27.2 | 0.011* |
| 15 - 25 years | 4 (14.8%) | 37 (33.0%) | 3 (17.6%) | 6 (13.3%) | 50 (24.9%) | ||
| 26 - 35 years | 10 (37.0%) | 39 (24.8%) | 6 (35.3%) | 23 (51.1%) | 78 (38.8%) | ||
| 36 - 45 years | 10 (37.0%) | 14 (12.5%) | 2 (11.8%) | 6 (13.3%) | 32 (15.9%) | ||
| > 45 years | 1 (3.7%) | 12 (10.7%) | 5 (29.4%) | 9 (20.0%) | 27 (13.4%) | ||
| Total | 27 (100.0%) | 112 (100.0%) | 17 (100.0%) | 45 (100.0%) | 201 (100.0%) | ||
| Cytological diagnosis | |||||||
| Benign lump | Inflammation | Suspicious of malignancy | Malignant | Total | Chi | P | |
| Co-morbidity | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| None | 6 (22.2%) | 32 (28.6%) | 2 (11.8%) | 3 (6.7%) | 43 (21.4%) | 22.4 | 0.010* |
| Diabetes | 6 (22.2%) | 34 (30.4%) | 6 (35.3%) | 22 (48.9%) | 68 (33.8%) | ||
| Hypertension | 10 (37.0%) | 39 (34.8%) | 9 (52.9%) | 19 (42.2%) | 77 (38.3%) | ||
| Anemia | 5 (18.5%) | 7 (6.3%) | 0 (0.0%) | 1 (2.2%) | 13 (6.5%) | ||
| Total | 27 (100.0%) | 112 (100.0%) | 17 (100.0%) | 45 (100.0%) | 201 (100.0%) | ||
| Family history of breast cancer | Cytological diagnosis | ||||||
| Benign lump | Inflammation | Suspicious of malignancy | Malignant | Total | |||
| N (%) | N (%) | N (%) | N (%) | N (%) | Chi | P | |
| Yes | 1 (3.7%) | 4 (3.6%) | 10 (58.8%) | 43 (95.6%) | 58 (28.9%) | 59.1 | 0.001* |
| No | 26 (96.3%) | 108 (96.4%) | 7 (41.2%) | 2 (4.4%) | 143 (71.1%) | ||
| Total | 27 (100.0%) | 112 (100.0%) | 17 (100.0%) | 45 (100.0%) | 201 (100.0%) | ||
| Affected family member | |||||||
| Mother | 1 (100.0%) | 3 (75.0%) | 2 (20.0%) | 21 (48.8%) | 27 (46.6%) | 5.9 | 0.210** |
| Sister | 0 (0.0%) | 1 (25.0%) | 8 (80.0%) | 21 (48.8%) | 30 (51.7%) | ||
| Aunt (father side) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.4%) | 1 (1.7%) | ||
| Total | 1 (100.0%) | 4 (100.0) | 10 (100.0%) | 43 (100.0%) | 58 (100.0%) | ||
| Consanguinity | |||||||
| First degree | 1 (100.0%) | 4 (100.0) | 10 (100.0%) | 42 (97.7%) | 57 (98.3%) | 3.0 | 0.734** |
| Second degree | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (102%) | 1 (1.7%) | ||
| Total | 1 (100.0%) | 4 (100.0) | 10 (100.0%) | 43 (100.0%) | 58 (100.0%) | ||
| DPAS score | Frequency N | Percentage % |
|---|---|---|
| ± Negative | 28 | 13.9 |
| + | 114 | 56.7 |
| ++ | 27 | 13.4 |
| +++ | 32 | 15.9 |
| Total | 201 | 100.0 |
| Cytological assessment | DPAS score | ||||
| ± Negative | + | ++ | +++ | Total | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Benign lump | 27 (96.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 27 (13.4%) |
| Inflammation | 1 (3.6%) | 110 (96.5%) | 1 (3.7%) | 0 (0.0%) | 112 (55.7%) |
| Suspicious of malignancy | 0 (0.0%) | 4 (3.5%) | 12 (44.4%) | 1 (3.1%) | 17 (8.5%) |
| Malignant | 0 (0.0%) | 0 (0.0%) | 14 (51.9%) | 31 (96.9%) | 45 (22.4%) |
| Total | 28 (100.0%) | 114 (100.0%) | 27 (100.0%) | 32 (100.0%) | 201 (100.0%) |
| AC grade | N | % |
|---|---|---|
| AC2 | 30 | 14.9 |
| AC3 | 112 | 55.7 |
| AC4 | 27 | 13.4 |
| AC5 | 32 | 15.9 |
| Total | 201 | 100.0 |
| Cytological assessment | AC grade | ||||
| AC2 | AC3 | AC4 | AC5 | Total | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Benign lump | 27 (90.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 27 (13.4%) |
| Inflammation | 3 (10.0%) | 108 (96.4%) | 1 (3.7%) | 0 (0.0%) | 112 (55.7%) |
| Suspicious of malignancy | 0 (0.0%) | 4 (3.6%) | 12 (44.4%) | 1 (3.1%) | 17 (8.5%) |
| Malignant | 0 (0.0%) | 0 (0.0%) | 14 (51.9%) | 31 (96.9%) | 45 (22.4%) |
| Total | 30 (100.0%) | 112 (100.0%) | 27 (100.0%) | 32 (100.0%) | 201 (100.0%) |
Results
In this study, the mean age was 33.6 ± 5.9 years, and the most prevalent age group was 26 – 35 years (78, 38.8%). There was a significant association between age group and malignant findings (P = 0.011).
In the medical history, 77 (38.3%) patients had hypertension, 68 (33.8%) had diabetes, and 13 (6.5%) had anemia. It should be noted that 43 (21.4%) of the women had no medical history. There was a significant association between co-morbidity and malignancy (P = 0.010).
Family history of breast cancer was reported in 58 (28.9%) women: 30 (51.7%) reported breast cancer in a sister, 27 (46.6%) in their mother, and 1 (1.7%) in an aunt from the father’s side. There was a significant association between family history of breast cancer and malignant findings (P = 0.001); however, there was no significant correlation between malignancy and having an affected family member with breast cancer (P = 0.210) or consanguinity (relative degree) (P = 0.734).
The DPAS scores were: negative (±) (28, 13.9%), one plus (+) (114, 56.7%), two plus (++) (27, 13.4%), and three plus (+++) (32, 15.9%).
There was a significant association between DPAS score and cytological assessment category; malignant findings were significantly associated with a DPAS score of three plus (+++) (P = 0.017).
Of the 201 women, the AC grades were: AC2 (30, 14.9%), AC3 (112, 55.7%), AC4 (27, 13.4%), and AC5 (32, 15.9%).
Comparison of the AC grades among the studied women according to the International Academy of Cytology (IAC) system with their cytology results revealed that AC2 was observed in 27 (90%) women in the benign group and 3 (10%) women in inflammation group, AC3 was observed in 108 (96.4%) women in the inflammation group and 4 (3.6%) women in the suspicious of malignancy group, AC4 was observed in 14 (51.9%) women in the malignant group and 12 (44.4%) women in the suspicious of malignancy group, and AC5 was observed in 31 (96.6%) women in the malignant group and 1 (3.1%) woman in the suspicious of malignancy group.
We upgraded 11 cases from suspicious of malignancy in AC4 to malignant in AC5. Furthermore, there was a significant association between AC grade and cytology result; malignant findings were significantly associated with AC5 (P = 0.014).
Discussion
The age of the patients ranged from younger than 15 years to older than 45 years. The mean age was 33.6 ± 5.9 years, and the most prevalent age group was 26–35 years (78, 38.8%).
Previous studies found the most prevalent age range to be between 30 and 60 years; however, other studies suggested that the increase in incidence was directly proportional to age, but that the disease was uncommon under 30 years old27. Patient age was associated with method of breast cancer detection28.
Regarding medical history, 77 (38.3%) women had hypertension, 68 (33.8%) had diabetes, and 13 (6.5%) had anemia. It should be noted that 43 (21.4%) of the women had no medical history.
In accordance with our findings, a meta-analysis study demonstrated that hypertension was associated with increased risk of breast cancer, especially among postmenopausal women29. Another study mentioned that some chronic conditions were considered risk factors for cancer, including as hypertension and diabetes30.
Family history of breast cancer was reported by 58 (28.9%) women; 30 (51.7%) reported it in a sister, 27 (46.6%) in their mother, and 1 (1.7%) in an aunt from the father’s side. There was a significant association between family history of breast cancer and malignant findings (P = 0.001).
In accordance with our findings Barnard et al. (2015) reported that the risk of developing breast cancer in those who had a family history of breast cancer was high, especially in those who had a first-degree relative with breast cancer31.
The DAPS scores were: negative (±) (28, 13.9%), one plus (+) (114, 56.7%), two plus (++) (27, 13.4%), and three plus (+++) (32, 15.9%).
Analysis of the DPAS scores versus the cytological categories (cytology results) revealed that negative score (±) was reported in 27 (96.4%) women in the benign group and 1 (3.6%) woman in the inflammation group, one plus score (+) was reported in 110 (96.5%) women in the inflammation group and 4 (3.5%) women in the suspicious of malignancy group, two plus score (++) was reported in 14 (51.9%) women in the malignant group and 12 (44.4%) women in the suspicious of malignancy group, and three plus score (+++) was reported in 31 (96.9%) women in the malignant group and 1 (3.1%) women in the suspicious of malignancy group. DPAS positivity (++, +++) was correlated with malignancy.
Eleven cases were reliably upgraded from suspicious of malignancy DPAS positivity two plus (++) to diagnostic of malignancy based on DPAS positivity three plus (+++). DPAS positivity in atypical cells in FNA aspirates may assist in upgrading from a suspicious to a malignant diagnostic result.
There was a significant association between DPAS score and cytological assessment category; malignant findings were significantly associated with DPAS score three plus (+++) (P = 0.017), which was in line with results obtained by Johnson and Wadehra21.
Intracytoplasmic PAS-D-positive globules may be helpful in differentiating between benign and malignant lesions of the breast; a higher grade of PAS-D positivity has been shown to correlate well with malignancy32.
The results of the previous study was in line with our findings. Our results revealed that 11 cases were reclassified from suspicious of malignancy to malignancy after using DPAS.
AC grades among the studied women according to IAC system were: AC2 (30, 14.9%), AC3 (112, 55.7%), AC4 (27, 13.4%), and AC5 (32, 15.9%). Furthermore, there was a significant association between AC grade and cytology results; malignant findings were significantly associated with AC5 (P = 0.014).
Our findings were in line with the reports of the IAC33 and Johnson and Wadehra21.
Limitations of the study
The study did not report on women who had breast cancer in previous years. The entire study population was from a residential area in Khartoum State; therefore, the study is likely not representative of other states in Sudan.
Conclusions
DPAS positivity (++, +++) was correlated with malignancy. Eleven cases were reliably upgraded from suspicious of malignancy DPAS positivity two plus (++) to final reports diagnostic of malignancy based on DPAS positivity three plus (+++). DPAS positivity within atypical cells in FNA aspirates may assist in upgrading lesions from suspicious of malignancy to a malignant diagnostic result. There was a significant association between DPAS score and cytological assessment category; malignant findings were significantly associated with DPAS score three plus (+++).
Abbreviations
AC: Aspiration cytology, CIs: Confidence intervals, DCIS: Ductal carcinoma in situ, DPAS: Diastase-Resistant Periodic Acid Schiff, FNA: Fine-Needle Aspiration, H&E: Hematoxylin and Eosin, IAC: International Academy of Cytology, MGG: May Grunwald Giemsa, Pap: Papanicolaou
Acknowledgments
Thanks for all participants involved in this research.
Author’s contributions
AAI and AAM conceived the design and carried out the experiments. NAO obtained, analyzed and interpreted the data. NAO and EAA wrote and revised the manuscript. AAI provides financial support for all experiments. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Bray
F.,
Ferlay
J.,
Soerjomataram
I.,
Siegel
R.L.,
Torre
L.A.,
Jemal
A.,
Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians.
2018;
68
(6)
:
394-424
.
View Article PubMed Google Scholar -
Dolatkhah
R.,
Somi
M.H.,
Jafarabadi
M.A.,
Hosseinalifam
M.,
Sepahi
S.,
Belalzadeh
M.,
Breast Cancer Survival and Incidence: 10 Years Cancer Registry Data in the Northwest, Iran. International Journal of Breast Cancer.
2020;
2020
:
1963814
.
View Article PubMed Google Scholar -
Bray
F.,
Colombet
M.,
Mery
L.,
Piñeros
M.,
Znaor
A.,
Zanetti
R.,
Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer. IARC Cancer Base No. 14. 2017:62-68.ISBN-978-92-832-0452-7..
.
-
Hanf
V.,
Gonder
U.,
Nutrition and primary prevention of breast cancer: foods, nutrients and breast cancer risk. European Journal of Obstetrics, Gynecology, and Reproductive Biology.
2005;
123
(2)
:
139-49
.
View Article PubMed Google Scholar -
Garfinkel
L.,
Boring
C.C.,
Heath
C.W.,
Changing trends. An overview of breast cancer incidence and mortality. Cancer.
1994;
74
(1)
:
222-7
.
View Article PubMed Google Scholar -
Malone
K.E.,
Daling
J.R.,
Thompson
J.D.,
O'Brien
C.A.,
Francisco
L.V.,
Ostrander
E.A.,
BRCA1 mutations and breast cancer in the general population: analyses in women before age 35 years and in women before age 45 years with first-degree family history. Journal of the American Medical Association.
1998;
279
(12)
:
922-9
.
View Article PubMed Google Scholar -
Kelsey
J.L.,
Breast cancer epidemiology: summary and future directions. Epidemiologic Reviews.
1993;
15
(1)
:
256-63
.
View Article PubMed Google Scholar -
Parkin
D.M.,
Is the recent fall in incidence of post-menopausal breast cancer in UK related to changes in use of hormone replacement therapy?. European Journal of Cancer (Oxford, England).
2009;
45
(9)
:
1649-53
.
View Article PubMed Google Scholar -
Mariani-Costantini
R.,
Elhassan
M.M.,
Aceto
G.M.,
Mohamedani
A.A.,
Awadelkarim
K.D.,
Epidemiology, Pathology, Management and Open Challenges of Breast Cancer in Central Sudan: A Prototypical Limited Resource African Setting. In: Pham, P. V. , editor. Breast Cancer - From Biology to Medicine [Internet]. London: IntechOpen; 2017 [cited 2022 Aug 31]. Available from: https://www.intechopen.com/chapters/53860 doi: 10.5772/67175.
.
-
Elbasheer
M.M.,
Alkhidir
A.G.,
Mohammed
S.M.,
Abbas
A.A.,
Mohamed
A.O.,
Bereir
I.M.,
Spatial distribution of breast cancer in Sudan 2010-2016. PLoS One.
2019;
14
(9)
:
e0211085
.
View Article PubMed Google Scholar -
Elgaili
M. Elgaili,
Dafalla
O. Abuidris,
Rahman
M.,
Michalek
A. M.,
Mohammed
S. I.,
Breast cancer burden in central Sudan. Int J Womens Health.
2010;
2
:
77-82
.
View Article Google Scholar -
Ahmed
H.G.,
Impact of Implementing Grading Fine Needle Aspiration Cytology in Diagnosis of Breast Cancer amongst Sudanese Women. Oman Medical Journal.
2011;
26
(2)
:
99-103
.
View Article PubMed Google Scholar -
Awadelkarim
K.D.,
Arizzi
C.,
Elamin
E.O.,
Hamad
H.M.,
De Blasio
P.,
Mekki
S.O.,
Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: implications for breast cancer in Africa. Histopathology.
2008;
52
(4)
:
445-56
.
View Article PubMed Google Scholar -
Saeed
I.E.,
Weng
H.Y.,
Mohamed
K.H.,
Mohammed
S.I.,
Cancer incidence in Khartoum, Sudan: first results from the Cancer Registry, 2009-2010. Cancer Medicine.
2014;
3
(4)
:
1075-84
.
View Article PubMed Google Scholar -
Donegan
W.L.,
Spratt
J.S.,
Cancer of the BreastElsevier Science Ltd.: London, UK; 2002.
Google Scholar -
McPherson
K.,
Steel
C.M.,
Dixon
J.M.,
ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ (Clinical Research Ed.).
2000;
321
(7261)
:
624-8
.
View Article PubMed Google Scholar -
Yamashita
A.,
Sakuma
K.,
Shiina
Y.,
Standardization of fine needle aspiration cytology of the breast - comparison of Auto Cyto Fix and conventional smears. Cytopathology.
2003;
14
(2)
:
79-83
.
View Article PubMed Google Scholar -
Kaufman
Z.,
Shpitz
B.,
Shapiro
M.,
Rona
R.,
Lew
S.,
Dinbar
A.,
Triple approach in the diagnosis of dominant breast masses: combined physical examination, mammography, and fine-needle aspiration. Journal of Surgical Oncology.
1994;
56
(4)
:
254-7
.
View Article PubMed Google Scholar -
Bancroft
J.D.,
Gamble
M.,
Theory and Practice of Histological TechniquesChurchill Livingstone Elsevier: Philadelphia; 2008.
Google Scholar -
Nijhawan
R.,
Rajwanshi
A.,
Gautam
U.,
Gupta
S.K.,
Cytoplasmic vacuolation, intracytoplasmic lumina, and DPAS staining in ductal carcinoma of the breast. Diagnostic Cytopathology.
2003;
28
(6)
:
291-4
.
View Article PubMed Google Scholar -
Johnson
S.J.,
Wadehra
V.,
The importance of intracytoplasmic DPAS positivity in fine needle aspirates of breast lesions. Journal of Clinical Pathology.
2001;
54
(2)
:
146-51
.
View Article PubMed Google Scholar -
Ahmed
H.G.,
Elemirri
D.A.,
Assessment of oral cytological changes associated with exposure to chemotherapy and/or radiotherapy. Cytojournal.
2009;
6
:
8
.
View Article PubMed Google Scholar -
Costa
A.L.,
Araújo
N.S. de,
Pinto
D.S.,
Araújo
V.C. de,
PCNA/AgNOR and Ki-67/AgNOR double staining in oral squamous cell carcinoma. Journal of Oral Pathology & Medicine.
1999;
28
(10)
:
438-41
.
View Article PubMed Google Scholar -
Argyrous
G.,
Statistics for Research: With a Guide to SPSSSAGE: London; 2005.
Google Scholar -
Bryman
A.,
Cramer
D.,
Quantitative Data Analysis with IBM SPSS 17, 18 and 19: A Guide for Social ScientistsRoutledge: New York; 2011.
Google Scholar -
Levesque
R.,
SPSS Programming and Data Management: A Guide for SPSS and SAS UsersSPSS Inc.: Chicago (Illinois); 2007.
Google Scholar -
Kerlikowske
K.,
Barclay
J.,
Grady
D.,
Sickles
E.A.,
Ernster
V.,
Comparison of risk factors for ductal carcinoma in situ and invasive breast cancer. Journal of the National Cancer Institute.
1997;
89
(1)
:
76-82
.
View Article PubMed Google Scholar -
Thind
A.,
Diamant
A.,
Hoq
L.,
Maly
R.,
Method of detection of breast cancer in low-income women. Journal of Women's Health.
2009;
18
(11)
:
1807-11
.
View Article PubMed Google Scholar -
Han
H.,
Guo
W.,
Shi
W.,
Yu
Y.,
Zhang
Y.,
Ye
X.,
Hypertension and breast cancer risk: a systematic review and meta-analysis. Scientific Reports.
2017;
7
(1)
:
44877
.
View Article PubMed Google Scholar -
Perrine
C.G.,
Nelson
J.M.,
Corbelli
J.,
Scanlon
K.S.,
Lactation and maternal cardio-metabolic health. Annual Review of Nutrition.
2016;
36
(1)
:
627-45
.
View Article PubMed Google Scholar -
Barnard
M.E.,
Boeke
C.E.,
Tamimi
R.M.,
Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochimica et Biophysica Acta.
2015;
1856
(1)
:
73-85
.
PubMed Google Scholar -
Panicker
N.,
Jariwala
P.,
Buch
A.,
Joshi
M.,
The utility of periodic acid schiff with diastase and alcian blue stains on fine needle aspirates of breast and salivary gland neoplasms. Journal of Cytology / Indian Academy of Cytologists.
2012;
29
(4)
:
221-5
.
View Article PubMed Google Scholar -
Panwar
H.,
Ingle
P.,
Santosh
T.,
Singh
V.,
Bugalia
A.,
Hussain
N.,
FNAC of Breast Lesions with Special Reference to IAC Standardized Reporting and Comparative Study of Cytohistological Grading of Breast Carcinoma. Journal of Cytology / Indian Academy of Cytologists.
2020;
37
(1)
:
34-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 8 (2022)
Page No.: 5224-5232
Published on: 2022-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3720 times
- PDF downloaded - 1435 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress