Abstract
Introduction: Paracetamol overdose potentially causes liver injury. This study aimed to evaluate the impact of morin against paracetamol overdose-induced hepatotoxicity.
Methods: Thirty albino rats weighing 185 ± 5 g were randomly selected from five groups: group I: orally administered with 1% Tween 80; group II: administered with 1 g paracetamol; group III: administered with 1 g paracetamol and 50 mg morin; group IV: administered with 100 mg paracetamol and morin; and group V: administered with 100 mg paracetamol and silymarin, with all treatments administered for 14 days.
Results: Morin and silymarin significantly protected the rats against induced alterations in the plasma total cholesterol, triacylglycerol, and HDL-C levels as well as the liver ALT, AST, ALP, LDH, protein thiol, GSH, SOD, CAT, MDA, and tumor necrosis factor-alpha levels. Furthermore, morin significantly inhibited the expression of NF-κB, NADPH oxidase-2, and interleukin-6 and induction of heme oxygenase-1 compared with paracetamol. The histological results indicated that morin protected the liver tissues against the toxic effect of paracetamol.
Conclusion: Morin significantly depletes the side effects of paracetamol and protects the liver tissue from the resulting free radicals.
Introduction
Paracetamol is often used to treat mild aches and pains, including headaches. In high doses, it is specifically toxic to the liver and generally causes liver necrosis, renal injury, or death in humans and laboratory animals1, 2 . Paracetamol is bio-transformed in the liver into the N-acetyl-para-benzoquinone imine (NAPQI) metabolite, which causes liver damage3.
The resulting free radical scavengers have a wide spectrum of biological activity4, 5, 6, 7. In the scientific literature, many hepatoprotective biologically active compounds exist, including natural and synthetic compounds8, 9, 10, 11, 12.
Several antioxidant-rich alternatives decrease the liver injury caused by paracetamol in animal models13. A variety of plant extracts containing saponins, polyphenols, and flavonoids have been utilized to treat a wide range of disorders10, 11, 12, 13. Nutraceuticals and herbal medications are important owing to their low toxicity, availability, and less side effects compared with synthetic drugs4, 5, 6, 7, 10, 11, 12, 13.
Morin is a flavanol that has been isolated from a variety of plants14, 15. It has been demonstrated to have free radical scavenging15, antioxidant16, 17, and anti-inflammatory18, 19 effects in numerous diseases, including liver20 and lung diseases20, 21, diabetes22, myocardial infarction23, and cancer24, 25, 26. The pharmacological properties of morin are mediated by modulation of various cellular cytokine signaling pathways27, 28, 29, 30, 31. Many reports have proven the biological importance of natural products, including morin, in medicine and pharmaceutical applications10, 11, 12, 13. In this study, we evaluated the pharmaceutical impact of morin on paracetamol-induced liver toxicity in rats.
Methods
Chemicals
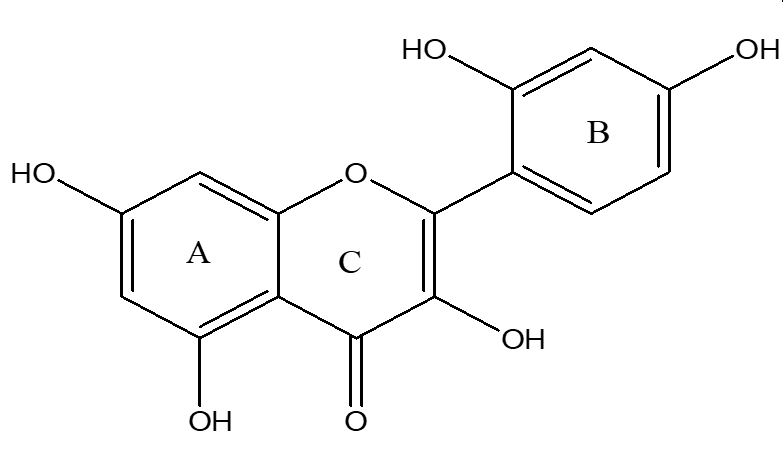
Morin (98%) (Figure 1), paracetamol (99%), and silymarin (98%) were purchased from Sigma-Aldrich (Germany).
Animals
Adult albino rats weighing roughly 185 ± 5 g were obtained from the animal house at the National Institute of Cancer and maintained in an observational environment unders daily observation. They were housed in cages and provided free access to food and water.
| Groups | Group name | Treatment description |
|---|---|---|
| I | Normal 1 % tween 80 | 3 mL of 1 % tween 80, orally for 14 days |
| II. | Control Paracetamol | 3 mL of 1 % tween 80, orally for 14 days |
| III | Paracetamol + Morin | Rats treated with Morin (50 mg/kg.b.w.) suspended in 1 % tween 80 by oral gavages for 14 days. |
| IV | Paracetamol + Morin | Rats treated with Morin (100 mg/kg.b.w.) suspended in 1 % tween 80 by oral gavages for 14 days. |
| V | Paracetamol + Silymarin | Rats treated with silymarin (100 mg/kg b.w.) suspended in 1 % tween 80 by oral gavages for 14 days. |
Experimental setup
The hepatoprotective activity of morin when administered for 2 weeks against paracetamol-induced hepatotoxicity was evaluated. The Animal Care and Use Committee of the Faculty of Applied Health Sciences Technology at O6U developed the criteria for this experiment. Table 1 illustrates the experimental study design.
The animals in all groups fasted for 18 hours on day 13, the day before the last treatment. Those categorized into groups II, III, IV, and V were administered with paracetamol (1 g/kg body weight) 1 hour after the last dose of morin therapy on day 1432.
Blood sample treatment
Blood was drawn from the retroorbital vein of each animal and then placed in heparin-containing tubes. The heparinized blood was centrifuged at 1000 × g for 20 minutes, and the cholesterol33, triglyceride34, and HDL-C35 levels were measured in the separated plasma.
Liver sample preparation
The rats were sacrificed, and their livers were collected to assess the biochemical parameters. The liver homogenate was prepared using 25% W/V saline and glass homogenizer. Four aliquots of the homogenate were made.
The first aliquot was used to calorimetrically estimate the ALT36, AST36, ALP37, LDH38, tumor necrosis factor-alpha (TNF-α)39, and protein thiol40 levels. The GSH level was calorimetrically measured in the second aliquot41 at 412 nm. The third aliquot was used to calculate the liver MDA level42 at 535 nm against a blank containing all reagents, except for the tissue homogenate. The fourth aliquot was minced with physiological saline and rinsed in ice-cold water. The supernatant was used to evaluate the SOD43 and CAT44 levels.
| Genes | Primer sequences |
|---|---|
| NF-κB | F: 5′-CATGAAGAGAAGACACTGACCATGGAAA-3’ |
| R: 5′-TGGATAGAGGCTAAGTGTGACACG-3’ | |
| NOX-2 | F: 5′-TGAACAACAGCACTCACCAATGCC-3′ |
| R: 5′-AGTTGTTGAACCAGGCAAAGGCAC-3′ | |
| IL-6 | F: 5'-GCCCTTCAGGAACAGCTATGA-3' |
| R:5'-TGTCAACAACATCAGTCCCAAGA-3' | |
| HO-1 | F:5′-TGCTAGCCTGGTGCAAGATA-3′ |
| R:5′-GCCAACAGGAAGCTGAGAGT-3′ | |
| β-Actin (internal control for qRT-PCR) | F: 5′-GGCTGTATTCCCCTCCATCG-3’ |
| R:5′-CCAGTTGGTAACAATGCCATGT-3’ |
Real-time PCR
The total liver RNA was obtained using the TRIzol technique in accordance with the manufacturer’s instructions (Life Technologies Corp., Grand Island, NY). Thereafter, 1 µg RNA was combined with 0.5 mmol/L dNTP, 10 nmol/L dithiothreitol, 25 pg primer oligo (dT), and 200 units reverse RNase H superscript II in reaction buffer. The reactions were incubated for one cycle at 42°C for 2 minutes and then for another 50 minutes at 42°C, after which they were heated for 15 minutes at 70°C and chilled at 4°C. Table 2 illustrates the primers for NF-κB, NADPH oxidase-2 (NOX-2), interleukin-6 (IL-6), and heme oxygenase-1 (HO-1). β-actin mRNA was employed as a housekeeping gene45.
Histological assessment
The liver tissues were divided into very thin slices (4 μm) and then fixed in 10% formaldehyde solution46. A light microscope was used to evaluate the histological alterations on H&E-stained slides.
Statistical analysis
The results were reported as means ± SDs. All data were statistically analyzed using SPSS version 1847 and one-way analysis of variance, with the statistical significance level set at P < 0.001.
| No. | Groups | Cholesterol (mg/dl) | Triglycerides (mg/dl) | HDL-C (mg/dl) |
|---|---|---|---|---|
| (I) | Normal 1 % tween 80 | 153.2 ± 13.50 | 101.3 ± 6.85 | 34.12 ± 3.90 |
| (II) | Control Paracetamol (1 g/kg.b.w) | 261.7 ± 14.50 a | 144.2 ± 9.61 a | 23.29± 4.97 a |
| (III) | Paracetamol + Morin (50 mg/kg.b.w.) | 184.2 ± 8.63 ab | 121.2 ± 7.47 ab | 29.48 ± 3.99 ab |
| (IV) | Paracetamol + Morin (100 mg/kg.b.w.) | 165.8 ± 14.95 b | 110.9 ± 11.55 b | 31.86± 3.67 b |
| (V) | Paracetamol + Silymarin (100 g/kg b.w.) | 157.6 ± 17.65 bc | 108.5 ± 8.27 b | 33.03± 3.43 b |
| No. | Groups | ALT (U/g tissue) | AST (U/g tissue) | ALP (U/g tissue) | LDH (U/g tissue) |
|---|---|---|---|---|---|
| (I) | Normal 1 % tween 80 | 63.26 ± 6.24 | 129.0 ± 6.15 | 206.08 ± 12.77 | 312.1 ± 19.25 |
| (II) | Control Paracetamol (1 g/kg.b.w) | 103.1 ± 9.29 a | 183.4 ± 8.40 a | 276.89 ± 10.45 a | 422.3 ± 14.19 a |
| (III) | Paracetamol + Morin (50 mg/kg.b.w.) | 82.26 ± 7.13 b | 137.19 ± 9.01 b | 225.02 ± 8.06 ab | 340.6 ± 15.22 b |
| (IV) | Paracetamol + Morin (100 mg/kg.b.w.) | 68.58 ± 4.33 bc | 134.5 ± 5.18 b | 211.99 ± 12.53 b | 311.9 ± 20.65 b |
| (V) | Paracetamol + Silymarin (100 g/kg b.w.) | 64.62 ± 5.86 bc | 129.8 ± 11.41 b | 208.53 ± 8.92 b | 308.8 ± 16.89 bc |
| No. | Groups | Protein thiols (nmol sulfhydryl/mg protein) | GSH (nmol/gm tissue) | SOD (U/gm tissue) | CAT (U/g tissue) |
|---|---|---|---|---|---|
| (I) | Normal 1 % tween 80 | 78.96 ± 5.57 | 26.41 ± 3.09 | 30.53 ± 5.65 | 99.81 ± 8.27 |
| (II) | Control Paracetamol (1 g/kg.b.w) | 52.65 ± 7.57 a | 11.75 ± 1.68 a | 10.86 ± 2.49 a | 35.92 ± 4.46 a |
| (III) | Paracetamol + Morin (50 mg/kg.b.w.) | 64.75 ± 5.47 ab | 20.88 ± 2.63 ab | 24.29 ± 3.42 ab | 79.95 ± 7.23 ab |
| (IV) | Paracetamol + Morin (100 mg/kg.b.w.) | 73.44 ± 6.99 b | 22.37 ±3.13 b | 27.36 ± 3.27 b | 88.98 ± 8.44 b |
| (V) | Paracetamol + Silymarin (100 g/kg b.w.) | 79.36 ± 7.41 bc | 24.11 ±2.99 b | 29.13 ±2.63 b | 92.26 ±11.50 b |
| No. | Groups | Hepatic MDA (nmole/g tissue) | Plasma TNF-α (Pg/mL) |
|---|---|---|---|
| (I) | Normal 1 % tween 80 | 73.82 ± 6.93 | 36.64 ± 4.97 |
| (II) | Control Paracetamol (1 g/kg.b.w) | 154.0 ± 11.84 a | 122.8 ± 10.28 a |
| (III) | Paracetamol + Morin (50 mg/kg.b.w.) | 81.56 ± 7.92 b | 50.20± 6.0 ab |
| (IV) | Paracetamol + Morin (100 mg/kg.b.w.) | 76.07 ± 7.68 b | 43.25± 4.38 b |
| (V) | Paracetamol + Silymarin (100 g/kg b.w.) | 72.53 ± 5.10 b | 40.91± 5.61 b |
| No. | Groups | Congestion | Lymphatic infiltration | Necrosis | Degenerated hepatocytes | Pyknosis, karyolysis | Fatty degeneration (steatosis) |
|---|---|---|---|---|---|---|---|
| (I) | Normal (1 % tween 80) | - | - | - | - | - | - |
| (II) | Control Paracetamol (1 g/kg.b.w) | ++ | ++ | ++ | +++ | + | +++ |
| (III) | Paracetamol + Morin (50 mg/kg.b.w.) | + | + | + | ++ | + | ++ |
| (IV) | Paracetamol + Morin (100 mg/kg.b.w.) | + | + | - | + | - | - |
| (V) | Paracetamol + Silymarin (100 g/kg b.w.) | + | + | - | + | + | - |
Results
The plasma TC, TG, and HDL-C levels in the different groups of treated rats are shown in Table 3. The plasma TC and TG levels increased significantly by 70.82% and 42.34%, respectively, while the plasma HDL-C level decreased significantly by 31.74% in the paracetamol-treated rats compared with that in the control rats. Furthermore, morin treatment (50 mg) significantly reduced (P < 0.001) the plasma TC and TG levels by 36.64% and 15.95%, respectively, and significantly increased (P < 0.001) the plasma HDL-C level by 26.57%. Meanwhile, compared with paracetamol treatment, morin treatment (100 mg) significantly reduced the plasma TC and TG levels by 36.64% and 23.09%, respectively, and significantly increased the plasma HDL-C level by 36.76%. Silymarin treatment (100 mg) significantly decreased the plasma TC and TG levels by 39.77% and 24.75%, respectively, and significantly increased the plasma HDL-C level by 41.82% compared with paracetamol treatment (P < 0.001). Compared with the control treatment, oral paracetamol (1 g) administration substantially increased the liver ALT, AST, ALP, and LDH levels by 62.97%, 42.17%, 34.36%, and 35.31%, respectively, indicating the presence of acute liver injury (P < 0.001). Compared with paracetamol treatment, morin treatment (50 mg) significantly reduced the liver ALT, AST, ALP, and LDH levels by 20.24%, 25.19%, 18.73%, and 19.35%, respectively, while morin treatment (100 mg) significantly reduced the liver ALT, AST, ALP, and LDH levels by 33.48%, 26.66%, 23.46%, and 26.14%, respectively (Table 4).
Silymarin treatment (100 mg/kg body weight) reduced the liver AST, ALT, and ALP levels by 37.32%, 29.23%, 24.68%, and 26.87%, respectively, compared with paracetamol treatment.
Table 5 shows that the liver protein thiol, GSH, SOD, and CAT levels significantly decreased (P < 0.001) by 33.32%, 55.50%, 64.42%, and 64.01%, respectively, in the paracetamol (1 g)-treated rats compared with those in the control rats. Meanwhile, the liver protein thiol, GSH, SOD, and CAT levels increased by 22.98%, 77.70%, 104.81%, and 122.58%, respectively, in the paracetamol (1 g)- and morin (50 mg)-treated rats compared with those in the paracetamol-treated rats (P < 0.001). Morin treatment (100 mg) among the paracetamol-treated rats significantly increased the liver protein thiol, GSH, SOD, and CAT levels by 39.48%, 90.38%, 151.93%, and 147.72%, respectively, compared with paracetamol treatment alone (P < 0.001). Silymarin treatment (100 mg) significantly increased the liver protein thiol, GSH, SOD, and CAT levels by 50.73%, 105.19%, 160.23%, and 156.85%, respectively, compared with paracetamol treatment.
Table 6 shows the effect of morin and silymarin on the liver malondialdehyde (MDA) and plasma TNF-α levels among the rats. The liver MDA and TNF-α levels (P < 0.001) in the paracetamol (1.0 g)-treated rats significantly increased by 108.62% and 235.15%, respectively, compared with those in the control rats. Further, morin treatment (50 mg) significantly decreased the liver MDA and TNF-α levels by 47.03% and 59.12%, respectively, compared with paracetamol treatment (P < 0.001).
The liver MDA and TNF-α levels significantly decreased by 50.60% and 64.78%, respectively, in the morin-treated rats compared with those in the paracetamol-treated rats (P < 0.001).
However, silymarin administration significantly decreased the liver MDA and TNF-α levels by 52.90% and 66.69%, respectively, compared with paracetamol administration alone (P < 0.001).
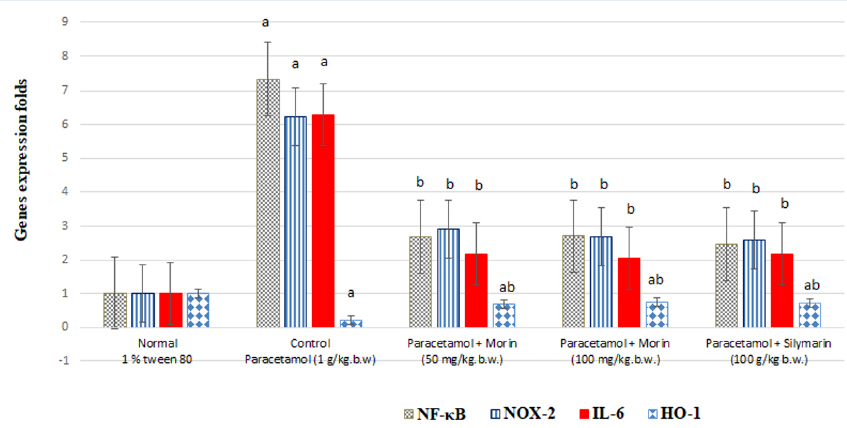
Figure 3 shows significantly increased (P < 0.001) liver NF-κB, NOX-2, and IL-6 levels (by 618.6%, 516.83%, and 523.76%, respectively) as well as a significantly decreased (P < 0.001) liver HO-1 level (by 77.23%) in the paracetamol (1 g)-treated rats compared with those in the control rats (P < 0.001), suggesting the presence of acute liver inflammation. Morin administration at 50 mg/kg body weight significantly decreased the expression of liver NF-κB, NOX-2, and IL-6 by 63.44%, 53.29%, and 65.5%, respectively, and significantly increased (P < 0.001) that of liver HO-1 by 204.34% compared with paracetamol administration (P < 0.001).
Morin administration at 100 mg significantly decreased the expression of liver NF-κB, NOX-2, and IL-6 by 63.16%, 57.14%, and 67.31%, respectively, and significantly increased (P < 0.001) that of liver HO-1 by 226.09% compared with paracetamol administration (P < 0.001).
Further, the silymarin (100 mg)-treated rats showed significantly decreased liver NF-κB, NOX-2, and IL-6 levels (by 66.30%, 58.42%, and 65.39%, respectively) and significantly increased (P < 0.001) liver HO-1 levels (by 208.70%) compared with the paracetamol-treated rats (P < 0.001).
The results of the agarose gel electrophoresis of NF-κB, NOX-2, IL-6, HO-1, and β-action via real-time PCR are presented in Figure 3.
In the histopathological evaluation of the liver sections, the control rats had a typical central vein and hepatocyte arrangement as well as a normal structure with no histological abnormalities. The nuclei of the hepatocytes were visible as dark red structures within the cells, whereas the cytoplasm was stained red. The sinusoids and typical portal area as well as the hepatic strands were observed from the margin of the lobule to the central vein. The number of binucleated and Kupffer cells was evaluated (Table 7 and Figure 4a).
The liver sections of the paracetamol-treated rats showed an altered lobular shape and a nuclear disintegration in certain locations as well as disarrangement of normal hepatic cells, necrosis, and fatty degeneration. Enlargement and congestion of the hepatic central vein were observed, and lymphocyte infiltration was found in the portal area (Table 7 and Figure 4b). Furthermore, the hepatocytes revealed some pathological alternation; moderate hepatocyte deterioration was observed. Moderate fatty degeneration was seen, along with mild mononuclear cell invasion and pyknosis/necrotic alterations in the paracetamol- and morin (50 mg/kg body weight)-treated rats compared with that in the paracetamol-treated control rats (Table 7 and Figure 4 c).
Histological examination of the hepatocytes of the groups showed signs of progress. The active euchromatic nuclei in the hepatocytes appeared to be in outstanding condition, while the hepatocytes and portal components appeared to be in good condition. Mild infiltrations of inflammatory cells were identified adjacent to the portal triads in the hepatic sections. The observed congestion was mild, and no evidence of fatty degeneration was found. The paracetamol- and morin-treated rats and silymarin-treated rats showed no necrotic changes compared with the paracetamol-treated rats (Table 7 and Figure 4d). The hepatic histology appeared normal, with the hepatic cords correctly positioned around the sinusoids. The observed congestion was minimal, and there was no evidence of fatty degeneration, necrosis, or invasion of mononuclear cells. There were a few liver cells that had deteriorated. The blood vessels were blocked to a modest degree in the silymarin-treated rats compared with those in the paracetamol-treated rats (Table 7 and Figure 4 e).
Discussion
Paracetamol promotes hepatotoxicity by activating metabolic pathways. It is bio-transformed into NAPQI by the cytochrome P2E1 system in the endoplasmic reticulum. When NAPQI is combined with cellular lipids and proteins, mitochondrial dysfunction, lipid peroxidation, and oxidative stress occur48. The loss of Ca2+ homeostasis eventually leads to cell death. The potential of a drug to suppress the aromatase activity of cytochrome P450, leading to liver regeneration, is known as a hepatoprotective action49, 50. In our study, oral paracetamol administration significantly increased the TC and TG levels and decreased the plasma HDL-C level, indicating the presence of liver toxicity. Morin and silymarin treatment reduced the plasma TC and TG levels while considerably increasing the HDL-C level. Generally, lipid deposition in the liver can occur because of an excessive supply of lipids or lipid deposition interference. Lipid-lowering drugs have been reported to have antioxidant properties that prevent lipid peroxidation, TC and TG elevation, and HDL-C induction by paracetamol51. Omidi et al. suggested polyphenols as beneficial agents in improving the lipid profile levels52.
In the present study, the liver ALT, AST, ALP, and LDH levels dramatically increased in the paracetamol-treated rats. This elevation may be attributed to the disruption of the liver cells as a result of necrosis53.
The liver cell damage induced by paracetamol overdose leads to NAPQI overproduction, hepatocyte necrosis, and liver enzyme level elevation54.
Our results indicate that morin and silymarin significantly reverse the enhancement of liver enzyme activity. The hepatoprotective activity of morin has been identified in certain toxicant in vitro and in vivo models of induced liver fibrosis55, 56, 57, while that of polyphenols is common in experimental and clinical studies. Morin and silymarin are used as a hepatoprotective and antioxidant drug58.
Our study findings agree with those of Asmah et al.59 that polyphenols have a hepatoprotective impact and considerably reduce the liver enzyme levels in animal models.
The current work investigated paracetamol-induced liver necrosis in vivo. Endogenous reactive oxygen species are produced by NAPQI linked to the thiol group of GSH, resulting in lipid peroxidation and cell death60. Our analyses revealed that the paracetamol-treated rats had lower protein thiol, GSH, SOD, and CAT levels.
Oral morin treatment significantly improved the liver protein thiol, GSH, SOD, and CAT levels. Morin has a wide range of antioxidant activities because it has a hydroxyl group (-OH) and a double bond between the C2 and C3 atoms that activates the double bond61. Its anti-lipid peroxidation potential is caused by the presence of 2 -OH at the 2′ and 4′ locations of the B ring62, 63, 64. Furthermore, the antiradical action of related polyphenols is thought to be predominantly due to -OH61.
The free radical scavenging activity of morin against ABTS+ and cytoprotective activity against ABTS+ radicals in tissue fibroblasts were evaluated and found to increase the superoxide dismutase activity and reduce intracellular ROS generation.
Herein, paracetamol-induced lipid peroxidation was detected in the rats, as demonstrated by the increased MDA levels and decreased GSH, SOD, and CAT levels in the liver tissues, all of which are key antioxidant indicators. MDA immunohistochemistry was found to be associated with hepatocyte necrosis in the central zone at early time points. Attenuation of inflammatory markers is mediated by a similar mechanism65. We found an elevation of the liver TNF-α levels that indicated the presence of oxidative stress and inflammation in the liver of the paracetamol-treated rats. However, oral morin and silymarin administration to the paracetamol-treated rats significantly decreased the MDA and TNF-α levels compared with paracetamol administration only. The current results on morin and silymarin treatment could be attributed to their antioxidant and membrane-stabilizing capabilities as well as a probable influence on paracetamol metabolism, as suggested by our in vivo findings on phase I bio-transformation enzymes involved in paracetamol bioactivation60, 61.
Morin enhanced the hemodynamic profile and inhibited the inflammatory marker pathways in a dose-dependent manner66.
We observed that the paracetamol-treated rats showed increased NF-κB, NOX-2, and IL-6 levels and decreased HO-1 levels. However, morin and silymarin administration depleted the expression of NF-κB, NOX-2, and IL-6 and alleviated the significant increase in the OH-1 level compared with paracetamol administration only.
Morin downregulated HO-1, which is linked to decreased Nrf2 expression and phosphorylation and improved Keap1 expression67. Morin administration in chemotherapy also had cytoprotective effects68.
The effect of morin in suppressing LXR signaling may help reduce liver steatosis and other metabolic disorders69. Morin can also improve the degree of gene expression of inflammatory mediators and controlled liver function induced by anticancer medication70. All biochemical parameters, especially NF-κB, NOX-2, IL-6, and HO-1, proved the antioxidant and anti-inflammatory mechanism of morin in the amelioration of paracetamol-induced toxicity.
To our knowledge, our study may be the first to evaluate the regulatory activity of morin for NF-κB, NOX-2, IL-6, and HO-1 in a model of paracetamol-induced liver fibrosis.
Conclusions
Morin protects against paracetamol-induced liver fibrosis by ameliorating the antioxidant and inflammatory biomarker levels. Its scavenging activity improves the expression of NF-κB, NOX-2, IL-6, and HO-1 in the liver.
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Yoon
E.,
Babar
A.,
Choudhary
M.,
Kutner
M.,
Pyrsopoulos
N.,
Acetaminophen-induced hepatotoxicity: a comprehensive update. Journal of Clinical and Translational Hepatology.
2016;
4
(2)
:
131-42
.
View Article Google Scholar -
Hinson
J.A.,
Roberts
D.W.,
James
L.P.,
Mechanisms of acetaminophen-induced liver necrosis. Handbook of Experimental Pharmacology.
2010;
196
(196)
:
369-405
.
View Article Google Scholar -
Attia
S.M.,
Deleterious effects of reactive metabolites. Oxidative Medicine and Cellular Longevity.
2010;
3
(4)
:
238-53
.
View Article Google Scholar -
Hussein
M.A.,
El-Gizawy
H.A.,
Gobba
N.A.,
Mosaad
Y.O.,
Synthesis of Cinnamyl and caffeoyl derivatives of cucurbitacin-e-glycoside isolated from Citrullus colocynthis fruits and their structures antioxidant and anti-inflammatory activities relationship. Current Pharmaceutical Biotechnology.
2017;
18
(8)
:
677-93
.
View Article Google Scholar -
Abdel-Gawad
S.M.,
Ghorab
M.M.,
El-Sharief
A.M.,
El-Telbany
F.A.,
Abdel-Alla
M.,
Design, synthesis, and antimicrobial activity of some new pyrazolo[3,4-d]pyrimidines. Heteroatom Chemistry.
2003;
14
(6)
:
530-4
.
View Article Google Scholar -
El Gizawy
H.A.,
Hussein
M.A.,
Abdel-Sattar
E.,
Biological activities, isolated compounds and HPLC profile of Verbascum nubicum. Pharmaceutical Biology.
2019;
57
(1)
:
485-97
.
View Article Google Scholar -
Boshra
S.A.,
Hussein
M.A.,
Cranberry extract as a supplemented food in treatment of oxidative stress and breast cancer induced by N-Methyl-N-Nitrosourea in female virgin rats. International Journal of Phytomedicine.
2016;
8
(2)
:
217-27
.
-
Abdel Maksoud
H.A.,
Elharrif
M.G.,
Mahfouz
M.K.,
Omnia
M.A.,
Abdullah
M.H.,
Eltabey
M.E.,
Biochemical study on occupational inhalation of benzene vapours in petrol station. Respiratory Medicine Case Reports.
2019;
27
:
100836
.
View Article Google Scholar -
Ghorab
M.M.,
Ismail
Z.H.,
Abdalla
M.,
Synthesis and biological activities of some novel triazoloquinazolines and triazinoquinazolines containing benzenesulfonamide moieties. Arzneimittel-Forschung.
2010;
60
(2)
:
87-95
.
View Article Google Scholar -
Borik
R.M.,
Hussein
M.A.,
Synthesis, Molecular Docking, Biological Potentials and Structure Activity Relationship of New Quinazoline and Quinazoline-4-one Derivatives. Asian Journal of Chemistry.
2021;
33
(2)
:
423-38
.
View Article Google Scholar -
Hussein
M.A.,
Synthesis of some novel triazoloquinazolines and triazinoquinazolines and their evaluation for anti-inflammatory activity. Medicinal Chemistry Research.
2012;
21
(8)
:
1876-86
.
View Article Google Scholar -
Elgizawy
H.A.,
Ali
A.A.,
Hussein
M.A.,
Resveratrol: Isolation, and Its Nanostructured Lipid Carriers, Inhibits Cell Proliferation, Induces Cell Apoptosis in Certain Human Cell Lines Carcinoma and Exerts Protective Effect Against Paraquat-Induced Hepatotoxicity. Journal of Medicinal Food.
2021;
24
(1)
:
89-100
.
View Article Google Scholar -
El Gizawy
Heba A,
Abo-Salem
Heba M,
Ali
Ali A,
Hussein
Mohammed A,
Phenolic Profiling and Therapeutic Potential of Certain Isolated Compounds from Parkia roxburghii against AChE Activity as well as GABAA $α$5, GSK-3$β$, and p38$α$ MAP-Kinase Genes. ACS omega.
2021;
6
(31)
:
20492-20511
.
-
Caselli
A.,
Cirri
P.,
Santi
A.,
Paoli
P.,
Morin: a promising natural drug. Current Medicinal Chemistry.
2016;
23
(8)
:
774-91
.
View Article Google Scholar -
Lee
M.H.,
Cha
H.J.,
Choi
E.O.,
Han
M.H.,
Kim
S.O.,
Kim
G.Y.,
Antioxidant and cytoprotective effects of morin against hydrogen peroxide‑induced oxidative stress are associated with the induction of Nrf‑2‑mediated HO‑1 expression in V79‑4 Chinese hamster lung fibroblasts. International Journal of Molecular Medicine.
2017;
39
(3)
:
672-80
.
View Article Google Scholar -
Rizvi
F.,
Mathur
A.,
Krishna
S.,
Siddiqi
M.I.,
Kakkar
P.,
Suppression in PHLPP2 induction by morin promotes Nrf2-regulated cellular defenses against oxidative injury to primary rat hepatocytes. Redox Biology.
2015;
6
:
587-98
.
View Article Google Scholar -
Hyun
H.B.,
Lee
W.S.,
Go
S.I.,
Nagappan
A.,
Park
C.,
Han
M.H.,
The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. International Journal of Oncology.
2015;
46
(6)
:
2670-8
.
View Article Google Scholar -
Yu
S.,
Liu
X.,
Yu
D.,
Changyong
E.,
Yang
J.,
Morin protects LPS-induced mastitis via inhibiting NLRP3 inflammasome and NF-κB signaling pathways. Inflammation.
2020;
43
(4)
:
1293-303
.
View Article Google Scholar -
Sinha
K.,
Ghosh
J.,
Sil
P.C.,
Morin and its role in chronic diseases. Anti-inflammatory nutraceuticals and chronic diseases.
2016;
:
453-471
.
View Article Google Scholar -
Li
X.,
Yao
Q.,
Huang
J.,
Jin
Q.,
Xu
B.,
Chen
F.,
Morin hydrate inhibits TREM-1/TLR4-mediated inflammatory response in macrophages and protects against carbon tetrachloride-induced acute liver injury in mice. Frontiers in Pharmacology.
2019;
10
:
1089
.
View Article Google Scholar -
Cai
B.,
Gan
X.,
He
J.,
He
W.,
Qiao
Z.,
Ma
B.,
Morin attenuates cigarette smoke-induced lung inflammation through inhibition of PI3K/AKT/NF-κB signaling pathway. International Immunopharmacology.
2018;
63
:
198-203
.
View Article Google Scholar -
Tianzhu
Z.,
Shihai
Y.,
Juan
D.,
The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation.
2014;
37
(6)
:
1976-83
.
View Article Google Scholar -
Paoli
P.,
Cirri
P.,
Caselli
A.,
Ranaldi
F.,
Bruschi
G.,
Santi
A.,
The insulin-mimetic effect of Morin: a promising molecule in diabetes treatment. Biochimica et Biophysica Acta.
2013;
1830
(4)
:
3102-11
.
View Article Google Scholar -
Verma
V.K.,
Malik
S.,
Narayanan
S.P.,
Mutneja
E.,
Sahu
A.K.,
Bhatia
J.,
Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Molecular Biology Reports.
2019;
46
(1)
:
1139-48
.
View Article Google Scholar -
Nandhakumar
R.,
Salini
K.,
Niranjali Devaraj
S.,
Morin augments anticarcinogenic and antiproliferative efficacy against 7,12-dimethylbenz(a)-anthracene induced experimental mammary carcinogenesis. Molecular and Cellular Biochemistry.
2012;
364
(1-2)
:
79-92
.
View Article Google Scholar -
Sivaramakrishnan
V.,
Niranjali Devaraj
S.,
Morin regulates the expression of NF-kappaB-p65, COX-2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chemico-Biological Interactions.
2009;
180
(3)
:
353-9
.
View Article Google Scholar -
Sivaramakrishnan
V.,
Shilpa
P.N.,
Praveen Kumar
V.R.,
Niranjali Devaraj
S.,
Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel flavonol-Morin. Chemico-Biological Interactions.
2008;
171
(1)
:
79-88
.
View Article Google Scholar -
Verma
V.K.,
Malik
S.,
Narayanan
S.P.,
Mutneja
E.,
Sahu
A.K.,
Bhatia
J.,
Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Molecular Biology Reports.
2019;
46
(1)
:
1139-48
.
View Article Google Scholar -
Wang
J.,
Guo
C.,
Wei
Z.,
He
X.,
Kou
J.,
Zhou
E.,
Morin suppresses inflammatory cytokine expression by downregulation of nuclear factor-κB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. Journal of Dairy Science.
2016;
99
(4)
:
3016-22
.
View Article Google Scholar -
Gupta
S.C.,
Phromnoi
K.,
Aggarwal
B.B.,
Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochemical Pharmacology.
2013;
85
(7)
:
898-912
.
View Article Google Scholar -
Chung
S.S.,
Oliva
B.,
Dwabe
S.,
Vadgama
J.V.,
Combination treatment with flavonoid morin and telomerase inhibitor MST‑312 reduces cancer stem cell traits by targeting STAT3 and telomerase. International Journal of Oncology.
2016;
49
(2)
:
487-98
.
View Article Google Scholar -
Luo
Z.,
Harada
T.,
London
S.,
Gajdusek
C.,
Mayberg
M.R.,
Antioxidant and iron-chelating agents in cerebral vasospasm. Neurosurgery.
1995;
37
(6)
:
1154-8
.
View Article Google Scholar -
Fossati
P.,
Prencipe
L.,
Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry.
1982;
28
(10)
:
2077-80
.
View Article Google Scholar -
Allain
C.C.,
Poon
L.S.,
Chan
C.S.,
Richmond
W.,
Fu
P.C.,
Enzymatic determination of total serum cholesterol. Clinical Chemistry.
1974;
20
(4)
:
470-5
.
View Article Google Scholar -
Burstein
M.,
Scholnick
H.R.,
Morfin
R.,
Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. Journal of Lipid Research.
1970;
11
(6)
:
583-95
.
View Article Google Scholar -
Reitman
S.,
Frankel
S.,
A colorimetric method for the determination of plasma oxaloacetic acid and glutamic pyruvic transaminases. American Journal of Clinical Pathology.
1957;
28
:
56-63
.
View Article Google Scholar -
Kind
P.R.,
King
E.J.,
Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. Journal of Clinical Pathology.
1954;
7
(4)
:
322-6
.
View Article Google Scholar -
Buhl
S.N.,
Jackson
K.Y.,
Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate to pyruvate to lactate reactions in human plasma at 25, 30 and 37 oC. Clinical Chemistry.
1978;
2415
:
828
.
View Article Google Scholar -
Beyaert
R.,
Fiers
W.,
Tumor necrosis factor and lymphotoxin. In: Mire-Sluis AR, Thorpe R, editors. Cytokines. San Diego, CA, USA: Academic Press; 1998. p. 335–60.
.
-
Sedlak
J.,
Lindsay
R.H.,
Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical Biochemistry.
1968;
25
(1)
:
192-205
.
View Article Google Scholar -
Owen
J. B.,
Butterfield
D .A.,
Measurement of oxidized/reduced glutathione ratio. Methods Mol Biol.
2010;
648
:
269-277
.
View Article Google Scholar -
Mihara
M.,
Uchiyama
M.,
Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry.
1978;
86
(1)
:
271-8
.
View Article Google Scholar -
Marklund
S.,
Marklund
G.,
Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry.
1974;
47
(3)
:
469-74
.
View Article Google Scholar -
Glorieux
C.,
Zamocky
M.,
Sandoval
J.M.,
Verrax
J.,
Calderon
P.B.,
Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med.
2015;
87
:
84-97
.
View Article Google Scholar -
Clotman
F.,
Lannoy
V.J.,
Reber
M.,
Cereghini
S.,
Cassiman
D.,
Jacquemin
P.,
The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development (Cambridge, England).
2002;
129
(8)
:
1819-28
.
View Article Google Scholar -
Alturkistani
H.A.,
Tashkandi
F.M.,
Mohammedsaleh
Z.M.,
Histological Stains: A Literature Review and Case Study. Global Journal of Health Science.
2015;
8
(3)
:
72-9
.
View Article Google Scholar -
SPSS. 2012. (SPSS 15), Inc., Chicago, IL, USA..
.
-
Buwa
S.,
Patil
S.,
Kulkarni
P.H.,
Kanase
A.,
Hepatoprotective action of abhrak bhasma, an ayurvedic drug in albino rats against hepatitis induced by CCl4. Indian Journal of Experimental Biology.
2001;
39
(10)
:
1022-7
.
-
Knight
T.R.,
Fariss
M.W.,
Farhood
A.,
Jaeschke
H.,
Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicological Sciences.
2003;
76
(1)
:
229-36
.
View Article Google Scholar -
Polizio
A.H.,
Peña
C.,
Effects of angiotensin II type 1 receptor blockade on the oxidative stress in spontaneously hypertensive rat tissues. Regulatory Peptides.
2005;
128
(1)
:
1-5
.
View Article Google Scholar -
Yousef
M.I.,
Omar
S.A.,
El-Guendi
M.I.,
Abdelmegid
L.A.,
Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food and Chemical Toxicology.
2010;
48
(11)
:
3246-61
.
View Article Google Scholar -
Omidi
A.,
Ansari nik
H.,
Ghazaghi
M.,
Prosopis farcta beans increase HDL cholesterol and decrease LDL cholesterol in ostriches (Struthio camelus). Tropical Animal Health and Production.
2013;
45
(2)
:
431-4
.
View Article Google Scholar -
Singh
S.,
Singh
S.K.,
Kumar
M.,
Chandra
K.,
Singh
R.,
Ameliorative potential of quercetin against paracetamol-induced oxidative stress in mice blood. Toxicology International.
2011;
18
(2)
:
140-5
.
View Article Google Scholar -
Giannini
E.G.,
Testa
R.,
Savarino
V.,
Liver enzyme alteration: a guide for clinicians. Canadian Medical Association Journal.
2005;
172
(3)
:
367-79
.
View Article Google Scholar -
Li
X.,
Yao
Q.,
Huang
J.,
Jin
Q.,
Xu
B.,
Chen
F.,
Morin hydrate inhibits TREM-1/TLR4-mediated inflammatory response in macrophages and protects against carbon tetrachloride-induced acute liver injury in mice. Frontiers in Pharmacology.
2019;
10
:
1089
.
View Article Google Scholar -
Bachewal
P.,
Gundu
C.,
Yerra
V.G.,
Kalvala
A.K.,
Areti
A.,
Kumar
A.,
Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. BioFactors (Oxford, England).
2018;
44
(2)
:
109-22
.
View Article Google Scholar -
Komirishetty
P.,
Areti
A.,
Sistla
R.,
Kumar
A.,
Morin mitigates chronic constriction injury (CCI)-induced peripheral neuropathy by inhibiting oxidative stress induced PARP over-activation and neuroinflammation. Neurochemical Research.
2016;
41
(8)
:
2029-42
.
View Article Google Scholar -
Reid
A.B.,
Kurten
R.C.,
McCullough
S.S.,
Brock
R.W.,
Hinson
J.A.,
Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. The Journal of Pharmacology and Experimental Therapeutics.
2005;
312
(2)
:
509-16
.
View Article Google Scholar -
Asmah
H.,
Siti
B.B.,
Rafidah
A.P.,
Pakri
M.,
Noraida
A.M.,
Yuliani
Y.,
Role of oxidative stress in the protective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced hepatotoxicity in Sprague-Dawley rats. Australian Journal of Basic and Applied Sciences.
2011;
5
(8)
:
1519-25
.
-
Borik
R.M.,
Hussein
M.A.,
A Novel Quinazoline-4-one Derivatives as a Promising Cytokine Inhibitors: Synthesis, Molecular Docking, and Structure-activity Relationship. Current Pharmaceutical Biotechnology.
2022;
23
(9)
:
1179-203
.
View Article Google Scholar -
Hussein
M.A.,
Ismail
N.E.,
Mohamed
A.H.,
Borik
R.M.,
Ali
A.A.,
Mosaad
Y.O.,
Plasma Phospholipids: A Promising Simple Biochemical Parameter to Evaluate COVID-19 Infection Severity. Bioinformatics and Biology Insights.
2021;
15
:
11779322211055891
.
View Article Google Scholar -
Wolfe
K.L.,
Liu
R.H.,
Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. Journal of Agricultural and Food Chemistry.
2008;
56
(18)
:
8404-11
.
View Article Google Scholar -
Farkas
O.,
Jakus
J.,
Héberger
K.,
Quantitative structure-antioxidant activity relationships of flavonoid compounds. Molecules (Basel, Switzerland).
2004;
9
(12)
:
1079-88
.
View Article Google Scholar -
Mendoza-Wilson
A.M.,
Santacruz-Ortega
H.,
Balandrán-Quintana
R.R.,
Spectroscopic and computational study of the major oxidation products formed during the reaction of two quercetin conformers with a free radical. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy.
2011;
81
(1)
:
481-8
.
View Article Google Scholar -
Morales
J.,
Günther
G.,
Zanocco
A.L.,
Lemp
E.,
Singlet oxygen reactions with flavonoids. A theoretical-experimental study. PLoS One.
2012;
7
(7)
:
e40548
.
View Article Google Scholar -
Chen
Y.,
Li
Y.,
Xu
H.,
Li
G.,
Ma
Y.,
Pang
Y.J.,
Morin mitigates oxidative stress, apoptosis and inflammation in cerebral ischemic rats. African Journal of Traditional, Complementary, and Alternative Medicines.
2017;
14
(2)
:
348-55
.
View Article Google Scholar -
Verma
V.K.,
Malik
S.,
Narayanan
S.P.,
Mutneja
E.,
Sahu
A.K.,
Bhatia
J.,
Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Molecular Biology Reports.
2019;
46
(1)
:
1139-48
.
View Article Google Scholar -
Lee
M.H.,
Cha
H.J.,
Choi
E.O.,
Han
M.H.,
Kim
S.O.,
Kim
G.Y.,
Antioxidant and cytoprotective effects of morin against hydrogen peroxide-induced oxidative stress are associated with the induction of Nrf-2‑mediated HO-1 expression in V79-4 Chinese hamster lung fibroblasts. International Journal of Molecular Medicine.
2017;
39
(3)
:
672-80
.
View Article Google Scholar -
El-Gizawy
H.A.,
Hussein
M.A.,
Fatty acids profile, nutritional values, anti-diabetic and antioxidant activity of the fixed oil of malvaparviflora growing in Egypt. International Journal of Phytomedicine.
2015;
7
:
219-30
.
-
Gobba
N.A.,
Ali
A. Hussein,
Sharawy
D.E. El,
Hussein
M.A.,
The potential hazardous effect of exposure to iron dust in Egyptian smoking and nonsmoking welders. Archives of Environmental & Occupational Health.
2018;
73
(3)
:
189-202
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 9 (2022)
Page No.: 5260-5271
Published on: 2022-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4779 times
- PDF downloaded - 1500 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress