Abstract
The number of articles on tissue engineering and regenerative medicine has increased dramatically in the last decade; however, the number of clinically implemented techniques remains small. Possible reasons include insufficient investigation of immune reactions on implanted tissue-engineered grafts and cells or a lack of consensus regarding which immunological tests must be performed to evaluate immunological responses. To provide an example of insufficiency in the assessment of immunological reactions, we analyzed three papers published between 2020 and 2021 and discussed the possibility of creating a standardized assay palette for the assessment of immunological responses in different types of implants.
Introduction
Currently, despite significant progress in tissue engineering techniques, post-implantation outcomes remain unacceptable. In the last decade, chronic inflammation has been a key challenge in tissue engineering, leading to the lack of physiological relevance1. There is no unanimous understanding of the mechanisms of inflammation related to the implantation of tissue-engineered constructs; therefore, it is not always possible to identify precise reasons for implant failure.
Regeneration-associated immunological responses have not yet been described in detail. Immunity is recognized as a major player in tissue homeostasis. T-helper cells are involved in the activation and regulation of non-immune cells2. Macrophage responses, namely the equilibrium between M1-like versus M2-like, are capable of maintaining tissue homeostasis and/or providing pro- or anti-inflammatory signals depending on the tissue’s origin and microenvironment3. The most recent evidence suggests that cell-macrophage crosstalk determines the microenvironment and reparative processes in tissue-engineered bone grafts4. Furthermore, cytokines released from immune cells regulate proliferation and differentiation of mesenchymal stromal cells5.
However, many studies do not comprehensively assess immunological responses to implanted tissue-engineered grafts. We believe that the explanation may be the absence of a standardized approach to assessing immunological responses. This assumption is based on analysis of three papers published in the respectable journal of Lancet family, eBioMedicine, in recent years6, 7, 8. Here, we provide examples of the types of immunological tests that could be useful.
Schaefer et al. (2020) identified tissue- and organ-specific regulation of stem cell adhesion and migration through the vasculature. Cellular chemotaxis is inhibited by inflammation and the deposition of inflammatory cytokines. Therefore, analysis of the cytokine profile of blood serum before and after cell therapy may be of value. In the event of cell migration into target tissues, local immunohistochemistry (IHC) assays would allow for visualization of the increased levels of anti-inflammatory cytokines. Furthermore, enhanced assessment of mast cell activity would improve the therapeutic efficacy of stem cell transplantation.
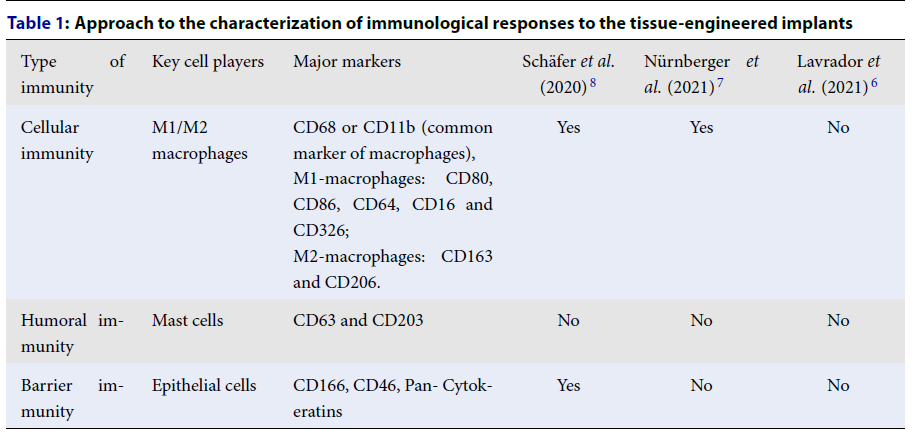
| Type of immunity | Key cell players | Major markers | Schäfer et al . (2020) 8 | Nürnberger et al . (2021) 7 | Lavrador et al . (2021) 6 |
|---|---|---|---|---|---|
| Cellular immunity | M1/M2 macrophages | CD68 or CD11b (common marker of macrophages), M1-macrophages: CD80, CD86, CD64, CD16 and CD326; M2-macrophages: CD163 and CD206. | Yes | Yes | No |
| Humoral immunity | Mast cells | CD63 and CD203 | No | No | No |
| Barrier immunity | Epithelial cells | CD166, CD46, Pan- Cytokeratins | Yes | No | No |
In an article by Nürnberger et al. (2021), cells were able to repopulate empty chondrocyte lacunae inside a scaffold matrix7. The IHC analysis conducted in the study revealed the presence of macrophages inside the notches of the scaffold. However, the presence of M1-macrophages does not exclude the presence of mast cells. Mast cells participate in the regulation of various physiological functions, including vasodilation and angiogenesis. Generally, tissue decellularization does not completely remove MHC I and II. Therefore, implanted decellularized scaffolds can modulate the macrophage response to an immunotolerant injury-response M2 type. We suggest that immunohistochemical assessment of mast cells would allow for identification of the bone marrow interaction with laser-treated cartilage and would add value to the study.
A study by Lavrador et al. (2021) provided an overview of research on the use of living materials as therapeutic platforms for tissue engineering; however, the study did not describe methods for evaluating immunological responses to biomaterials6. It is worth recalling that biological materials that are created de novo induce a response in untransformed human CD14+ monocytes characterized by gene expression and production of IL-1β (inflammatory cytokine) and IL-6 (acute phase reactant). The innate immune response to biological scaffolds can lead to increased apoptosis of macrophages adhering to the biomaterial resulting from in vivo interaction with the hydrophilic substrate.
Based on the triple classification of immunological responses by Tuzlak et al. (2021)2, we developed a standard approach for the characterization of immunological responses to tissue-engineered implants using the most affordable markers for IHC staining (Table 1).
Conclusions
While the use of assessment methods to confirm tissue function allows us to draw conclusions about the consistency of the implant, confirming biocompatibility is significantly more challenging. The definition of biocompatibility is not precise and more importantly, it may be tissue-specific and may depend on the location of the implant1, 9. Clinical manifestations of inadequate inflammatory response may develop after several months or even years. Insufficient immunological assessment can lead to post-publication revision and even retraction of articles10.
Insufficient assessment of the immunological response leads to misinterpretation of significant results in tissue engineering and regenerative medicine. However, immunological techniques require additional assay kits to ensure reasonable verification of host-implant interactions.
Abbreviations
CD: cluster of differentiation, IHC: immunohistochemistry, IL-1β: interleukin 1beta, IL-6: interleukin-6, MHC: major histocompatibility complex
Acknowledgments
None
Author’s contributions
Conceptualization, V.A.S. and I.D.K.; investigation, V.A.S, I.D.K., D.S.B.; writing—original draft preparation, V.A.S. and I.D.K., D.S.B., P.V.S., A.D.K.; writing—review and editing, I.D.K., P.V.S., A.D.K. All authors read and approved the final manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, Agreement No. 075-15-2021-1356 issued October 7, 2021 (15.CIN.21.0011, RF ID 0951.61321X0012).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Nürnberger
S.,
Schneider
C.,
Keibl
C.,
Schädl
B.,
Heimel
P.,
Monforte
X.,
Repopulation of decellularised articular cartilage by laser-based matrix engraving. EBioMedicine.
2021;
64
:
103196
.
View Article PubMed Google Scholar -
Chen
H.,
Agrawal
D.K.,
Thankam
F.G.,
Biomaterials-Driven Sterile Inflammation. Tissue Engineering. Part B, Reviews.
2022;
28
(1)
:
22-34
.
View Article PubMed Google Scholar -
Tuzlak
S.,
Dejean
A.S.,
Iannacone
M.,
Quintana
F.J.,
Waisman
A.,
Ginhoux
F.,
Repositioning TH cell polarization from single cytokines to complex help. Nature Immunology.
2021;
22
(10)
:
1210-7
.
View Article PubMed Google Scholar -
Wu
C.L.,
Harasymowicz
N.S.,
Klimak
M.A.,
Collins
K.H.,
Guilak
F.,
The role of macrophages in osteoarthritis and cartilage repair. Osteoarthritis and Cartilage.
2020;
28
(5)
:
544-54
.
View Article PubMed Google Scholar -
Zhao
Y.,
Chen
B.,
Progress of macrophage polarization in immunology of bone tissue engineering. Chinese Journal of Tissue Engineering Research.
2022;
26
(13)
:
2120-6
.
-
Chen
R.,
Hao
Z.,
Wang
Y.,
Zhu
H.,
Hu
Y.,
Chen
T.,
Mesenchymal Stem Cell-Immune Cell Interaction and Related Modulations for Bone Tissue Engineering. Stem Cells International.
2022;
2022
:
7153584
.
View Article PubMed Google Scholar -
Lavrador
P.,
Gaspar
V.M.,
Mano
J.F.,
Engineering mammalian living materials towards clinically relevant therapeutics. EBioMedicine.
2021;
74
:
103717
.
View Article PubMed Google Scholar -
Schäfer
R.,
Schwab
M.,
Siegel
G.,
von Ameln-Mayerhofer
A.,
Buadze
M.,
Lourhmati
A.,
Modulating endothelial adhesion and migration impacts stem cell therapies efficacy. EBioMedicine.
2020;
60
:
102987
.
View Article PubMed Google Scholar -
Williams
D.F.,
Biocompatibility pathways in tissue-engineering templates. Engineering (Beijing).
2018;
4
(2)
:
286-90
.
View Article Google Scholar -
Sjöqvist
S.,
Jungebluth
P.,
Lim
M.L.,
Haag
J.C.,
Gustafsson
Y.,
Lemon
G.,
Retraction: experimental orthotopic transplantation of a tissue-engineered oesophagus in rats. Nature Communications.
2017;
8
(1)
:
15077
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 11 (2022)
Page No.: 5384-5386
Published on: 2022-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3679 times
- PDF downloaded - 1412 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress


