Abstract
The simultaneous (synchronous) detection of 3 primary tumors is rare in clinical practice. Recognizing and differentiating metastatic malignancies have always been challenging for clinicians and pathologists; however, treatment outcomes and prognoses highly depend on this recognition. Here, we used immunostaining and a new approach that, to our knowledge, few people know about based on an understanding of exosomal microRNAs (miRNAs), phenotypic plasticity, and the tumor microenvironment to reach a final diagnosis of multiple primary neoplasms.
Introduction
When faced with a case of detection of multiple malignancies at different anatomical sites at the time of diagnosis (synchronous), a frequent question for the clinician and pathologist is whether they are multiple primary cancers (MPCs) or metastatic masses. The challenge is even greater in the presence of histological heterogeneity among these tumors. However, this distinction profoundly affects the choice of treatment method and the patient’s prognosis.
MPCs are rare in clinical practice, with studies reporting a prevalence between 0.7% and 11.7%1, 2. They are generally divided into two types: (1) synchronous, in which the cancers occur at the same time (within 2 months of each other), and (2) metachronous, in which the cancers occur in sequence (more than 2 months apart)3. In patients with cancer, the risk of developing a new malignancy is higher than in those without cancer4.
Histological heterogeneity is common in MPCs. In contrast, histological heterogeneity is rare in primary cancers and metastatic masses5, 6, 7. Therefore, it is easy to confuse the latter with multiple primary tumors. Several hypotheses have been proposed to explain the mechanism underlying histological heterogeneity between primary tumors and metastatic masses, including phenotypic plasticity, clonality, cancer stem cells, and the tumor microenvironment. These hypotheses are not mutually exclusive but complementary. Among them, the theory of phenotypic plasticity is of great interest8. Under the influence of tumor microenvironment factors, the phenotype and genotype of tumor cells can be altered9, similar to the Darwinian theory of evolution.
Phenotypic plasticity refers to the change in phenotype caused by environmental factors. In metastatic tumors, malignant cells exhibit phenotypic plasticity in that they can change their phenotype under the influence of new habitats to survive and grow10. This report aims to raise awareness of the distinction between MPCs and metastatic masses, especially in the presence of histological heterogeneity between primary cancers and their metastases. Updating the information on the phenotypic plasticity of cancer cells is necessary to avoid misinterpreting metastatic masses as MPCs.
CASE REPORT
A 65-year-old male patient was admitted to the hospital on August 16, 2022, due to acute pain in the right iliac fossa and unintentional weight loss (lost 16 kg in about 3 months). The patient had a history of gastric ulcers and heavy drinking many years ago. About 6 months before admission, the patient sometimes experienced the following symptoms, the severity of which gradually increased: tiredness, transient chest pain, poor appetite, vague discomfort in the abdomen, and fullness after eating only a small amount, without dysphagia. Then, about 1 week before admission, the patient experienced increased abdominal pain. These symptoms are often attributed to inflammatory diseases of the gastrointestinal tract and are not specific to cancers of the esophagus, stomach, and cecum.
Liver and kidney function tests, coagulation tests, and electrolyte levels were within normal limits. Complete blood cell analysis revealed marked changes in some indicators: RBC (2.63 T/L), HBG (70 g/l), HCT (0.254 L/L), MCV (96.4 fL), MCH (26.6 pg), MCHC (275 g/L), CHCM (270 g/L), PLT (920 G/L), RDW-CV (17.8% CV), and PDW (36 g/dL). An abdominal ultrasound showed that the hepatobiliary system, pancreas, spleen, kidney, bladder, and prostate were within normal limits. The rectovesical pouch was 80 mm thick. The cecum contained a mixed echogenic mass measuring 6 cm × 5 cm containing little fluid; the mass border was infiltrated. A CT scan of the abdomen showed that the liver, gallbladder, spleen, kidney, pancreas, and bladder were within normal limits, and the scan was negative for abdominal lymph nodes. The ileocecal angle was dilated, measuring 21 cm in length, with indistinct boundaries. A contrast-enhanced CT scan showed heterogeneous density after contrast administration, and the normal structure of the gastrointestinal tract wall was lost.
Endoscopy of the esophagus and stomach revealed the following: the esophageal mucosa had 2 protrusions, 1 cm and 2.2 cm in size, located 2.5 cm and 3.6 cm from the gastroesophageal junction, respectively (Figure 1). The body and fundus of the stomach had a flat lesion extending from the large curvature to the vertical part of the small curvature, with a rough surface and scattered mucosal corrosion with raised margins (Figure 2). The duodenum was within normal limits. A colonoscopy only detected cecal lesions comprising large, ulcerated areas covered with pseudomembranous membranes, with a rough surface and firm ulcer base (Figure 3).
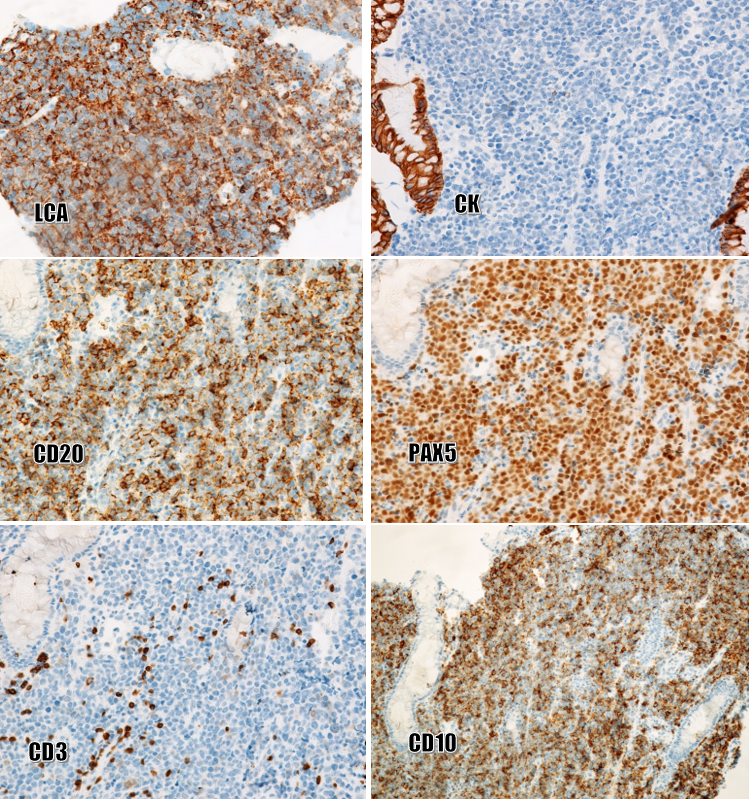
Biopsies of the esophagus, stomach, and cecum were performed. Histopathologically (Figure 4), squamous cell carcinoma was moderately differentiated and continuous with the esophageal mucosa. Several foci of moderate-grade dysplasia were seen in the esophageal squamous epithelium. Mixed-type gastric cancer consists of ductal glandular components and poorly adherent cell components. A mild dysplasia of the glandular epithelium was also present in the biopsies. The cecal tumor consisted of round, polymorphic cells that looked like poorly differentiated tumors. Immunostaining (Figure 5) was strong, diffuse-positive for LCA, CD20, PAX5, CD10, and Bcl6, and negative for CK, CD3, CD5, S100, vimentin, EBV, CD43, and MUM1. Final diagnosis: Multiple primary neoplasms including squamous cell carcinoma with moderate differentiation and moderate-grade dysplastic foci of squamous epithelium (in the esophagus), gastric adenocarcinoma, mixed type (in the stomach), and diffuse large B-cell lymphoma, germinal-center type (in the cecum).
The patient dropped out of the next course of treatment because his condition was very poor.
DISCUSSION
Squamous cell carcinoma is similar in histological structure in many different anatomical locations, so it is difficult to determine its primary location11. Thus, is esophageal squamous cell carcinoma the metastatic mass or the primary tumor in this case? Has the phenotypic plasticity of gastric adenocarcinoma cells been activated in the new metastatic microenvironment? Is this a morphological change to adapt to the new habitat? In our experience, two important criteria are used to distinguish primary and metastatic carcinomas: (1) reactive stroma is always present around the cluster of metastatic cells as opposed to the primary mass, and (2) some precancerous foci (epithelial dysplasia of different histological grades) are present in the primary tumor. In the present case, the reactive stroma around the squamous cell carcinoma was negative, while the precancerous lesion (squamous dysplasia) was positive. In gastric biopsies, similar lesions also presented as negative for reactive stroma and positive for mild dysplasia of the glandular epithelium.
In our experience, precancerous/dysplastic foci are often present in tissues or organs with primary cancer. Meanwhile, this phenomenon is rare in organs or tissues with secondary cancer. The known exosomal microRNAs (miRNAs) of cancer cells are short, non-coding RNA fragments functioning independently of the cancer cells. Some miRNAs, called oncomiRs, play an oncogenic role by impacting either oncogenes or tumor suppressor genes, while others, called metastamiRs, play a regulatory role in metastasis12, 13. Exosomes of cancer cells can communicate with adjacent healthy cells and pass oncomiRs and metastamiRs to them via pinocytosis13, 14. The long-term reception of cancer cell miRNAs leads to the progressive neoplastic transformation of healthy cells into precancerous lesions (i.e., dysplasia). Thus, the esophagus and stomach were the primary sites of squamous cell carcinoma and mixed-type adenocarcinoma, respectively, in this case.
The histological type of the primary gastric tumor was a mixture of ductal and poorly adherent structures, the latter consisting of rhabdoid and signet-ring cells. The histological type of the cecal tumor was round, polymorphic cells. The histological types of the 3 tumors in the present patient were completely different. Is the cecal tumor an independent primary mass or a metastatic mass of gastric or esophageal cancer?
The results of numerous studies comparing histological type between primary and secondary tumors indicate the common presence of histological homogeneity between the two sites. However, when comparing the histological type between the primary tumor and its corresponding metastatic tumor, rates of histological heterogeneity are not uncommon: 5% for renal cancer5, 9% for lung cancer6, and 22.7% and 14.3% for gastric cancer with liver metastasis and lymph node metastasis, respectively7.
To our knowledge, the histological type of the metastatic mass of differentiated squamous carcinoma is almost always the same as that of the primary tumor. Thus, the cecal tumor is not a metastasis of the squamous cell carcinoma of the esophagus. The possibility that the group of poorly adherent tumor cells of the gastric cancer metastasized to the cecum has not been ruled out yet. However, the morphological features of the tumor cells in the cecal tumors differed from those of the poorly adherent cells of the gastric cancer. In the present case, it is difficult to distinguish primary and secondary tumors by H&E staining alone because the two morphological criteria mentioned above cannot be assessed.
Many hypotheses can explain the histological heterogeneity between the two tumors of unknown origin, among which the theory of the phenotypic plasticity of tumor cells is of great interest. Under the influence of different environments, certain genotypes are formed in organisms or cells that can produce different phenotypes; this phenomenon is called phenotypic plasticity. Plasticity is the factor that causes the expression of a new phenotype induced by the environment and the process of selecting the expression of that phenotype in a new environment9.
Thus, under the influence of the tumor microenvironment, the phenotype of cancer cells is changed to different degrees; in some cases, the cell phenotype of the secondary mass is completely different from that of the primary mass15. In general, in malignancies, there is always an interaction between cancer cells and their microenvironment that affects the shaping of malignant behavior and promotes the progression of malignancies15.
In the present case, as the cecal tumor consisted of round, polymorphic cells, the following immunological markers were usually our first choice: CK, LCA, S100, and vimentin. The results showed positivity for LCA and negativity for CK, S100, and vimentin, so the malignancy of the cecum was determined to be non-Hodgkin lymphoma. Further immunomarker analysis was performed to reveal B- and T-cell lineage, germinal-center or non-germinal-center type, and the presence of EBV antigens, which revealed positivity for CD20, PAX5, CD10, and Bcl6 and negativity for CD3, CD5, CD43, MUM1, and EBV.
CONCLUSIONS
The patient, in this case, was ultimately diagnosed as having multiple primary tumors (synchronous) residing in 3 different anatomical sites: moderately differentiated squamous cell carcinoma (in the esophagus); gastric adenocarcinoma, mixed type (in the stomach); and diffuse large B-cell lymphoma, germinal-center type (in the cecum). A new approach with two important histological criteria, as described above, allowed us to identify the primary sites of the squamous cell carcinoma of the esophagus and the gastric adenocarcinoma, mixed type. Identifying the primary site of squamous cell carcinoma, a difficult problem for pathologists, was achieved. Based on our experience, this new approach may be the perfect complement to immunostaining for carcinomas derived from covering epithelia when the amount of tissue samples and the number of H&E-stained sections are satisfactory.
Abbreviations
Bcl6: B-cell lymphoma 6, CD10: Cluster of differentiation 10, CD20: Cluster of differentiation 20, CD3: Cluster of differentiation 3, CD43: Cluster of differentiation 43, CD5: Cluster of differentiation 5, CD79a: Cluster of differentiation 79a, CHCM: Cell hemoglobin concentration, CK: Cytokeratin, CT scan: computed tomography scan, EBV: Epstein-Barr virus, H&E: Hematoxylin and Eosin, HBG: Hemoglobin, HCT: Hematocrit, LCA: Leukocyte common antigen, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, MCV: Mean corpuscular volume, MUM1: Multiple myeloma, PAX5: Paired box 5, PDW: Platelet distribution width, PLT: Platelet count, RBC: Red blood cells, RDW-CV: Red blood cell distribution width — coefficient of variation, S100: Protein S100
Acknowledgments
We would like to thank the University for the study’s approval.
Author’s contributions
NVH and NTT: Conceptualization, Methodology, Writing-Original draft preparation; NTT, PDD, TNM: Visualization, Methodology, Software; TNM: Data curation, Writing-Original draft preparation; DTL: Validation, investigation, Supervision.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Coleman
M.P.,
Multiple primary malignant neoplasms in England and Wales, 1971-1981. The Yale Journal of Biology and Medicine.
;
59
(5)
:
517-531
.
PubMed Google Scholar -
Vogt
A.,
Schmid
S.,
Heinimann
K.,
Frick
H.,
Cerny
T.,
Multiple primary tumours: challenges and approaches, a review. ESMO Open.
;
2
(2)
:
e000172
.
View Article PubMed Google Scholar -
Howe
H.L.,
A review of the definition for multiple primary cancers in the United States. Workshop Proceedings from December 4-6, 2002, in Princeton, New Jersey.
2003
.
-
A. Luciani,
L. Balducci,
Multiple primary malignancies. Seminars in oncology.
;
31
(2)
:
264-273
.
View Article Google Scholar -
SP
Psutka,
JC
Cheville,
BA
Costello,
SB
Stewart-Merrill,
CM
Lohse,
BC
Leibovich,
SA
Boorjian,
RH
Thompson,
Concordance of Pathologic Features Between Metastatic Sites and the Primary Tumor in Surgically Resected Metastatic Renal Cell Carcinoma. Urology.
2016;
96
:
106-113
.
View Article PubMed Google Scholar -
N. Girard,
C. Deshpande,
L.A. Christopher,
D. Finley,
V. Rusch,
P.A. William,
W.D. Travis,
Comprehensive histologic assessment helps to differentiate multiple lung primary non-small cell carcinomas from metastases. The American journal of surgical pathology.
;
33
(12)
:
1752
.
View Article PubMed Google Scholar -
M
Mori,
H
Sakaguchi,
K
Akazawa,
M
Tsuneyoshi,
K
Sueishi,
K
Sugimachi,
Correlation between metastatic site, histological type, and serum tumor markers of gastric carcinoma. Human pathology.
;
26
(5)
:
504-508
.
View Article PubMed Google Scholar -
A. Marusyk,
K. Polyak,
Tumor heterogeneity: Causes and consequences. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer.
1805;
1805
(1)
:
105-117
.
View Article Google Scholar -
Fusco
G.,
Minelli
A.,
Phenotypic plasticity in development and evolution: facts and concepts. Philosophical Transactions of the Royal Society B: Biological Sciences.
2010;
365
(1540)
:
547-556
.
View Article Google Scholar -
Wei
X.-G.,
Bi
K.-W.,
Li
B.,
Phenotypic Plasticity Conferred by the Metastatic Microenvironment of the Brain Strengthens the Intracranial Tumorigenicity of Lung Tumor Cells. Frontiers in oncology.
2021;
11
:
637911
.
View Article PubMed Google Scholar -
Yan
W.,
Wistuba
I.I.,
Emmert-Buck
M.R.,
Erickson
H.S.,
Squamous cell carcinoma - similarities and differences among anatomical sites. American Journal of Cancer Research.
2011;
1
(3)
:
275-300
.
PubMed Google Scholar -
Kim
J.,
Yao
F.,
Xiao
Z.,
Sun
Y.,
Ma
L.,
MicroRNAs and metastasis: small RNAs play big roles. Cancer and Metastasis Reviews.
2016;
37
(1)
:
5-15
.
View Article PubMed Google Scholar -
S. El Andaloussi,
Mäger
I.,
Breakefield
X.O.,
M.J. Wood,
Extracellular vesicles: biology and emerging therapeutic opportunities. Nature reviews Drug discovery.
2013;
12
(5)
:
347-357
.
View Article PubMed Google Scholar -
Tkach
M.,
Théry
C.,
Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell.
2016;
164
(6)
:
1226-32
.
View Article PubMed Google Scholar -
Park
C.C.,
Bissell
M.J.,
Barcellos-Hoff
M.H.,
The influence of the microenvironment on the malignant phenotype. Molecular Medicine Today.
2000;
6
(8)
:
324-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 11 (2022)
Page No.: 5387-5393
Published on: 2022-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4414 times
- PDF downloaded - 1475 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress






