Abstract
The skin is an organ that performs complex functions of both the innate and adaptive immune systems. It serves as the first physical barrier to protect the body from environmental factors. Skin also carries aesthetic value in people's desire for eternal youth. Skin lesions are often unwanted, causing open wounds that can be contagious or permit infection of the body, scars, skin aging, and loss of skin function, with long-term psychological consequences. The application of stem cell therapy based on mesenchymal stem cells (MSCs) is constantly developing in the field of skin regeneration, which is highly regarded as a therapy for patients suffering skin lesions from burns, deep wounds, cosmetic surgery, or genetic diseases. MSC therapy has shed light on groundbreaking treatments in immune, anti-inflammatory, and regenerative medicine, including for skin diseases. Additionally, the development of stem cells seems to limit skin aging. It is gradually becoming an integral technique at hospitals as a regular therapy for illness, as well as a cosmetic intervention. This review seeks to introduce the skin system and its related disorders; highlight the common characteristics and mechanisms of MSCs; and analyze updated clinical applications and experiments to date in MSC therapy for regenerative biomedicine and skin diseases.
Introduction
During the past decade, mesenchymal stem cells (MSCs) have emerged as a promising therapy for the treatment of many pathologies. Clinical trials of MSCs to treat chronic wounds that do not heal are underway. There is also interest in their therapeutic potential to accelerate the closure of burn wounds and treat autoimmune disease damage. Many studies show the positive effects of MSC therapy. These positive results are not due to MSCs’ differentiation to replace damaged skin. Their therapeutic effects derive from the secretion of soluble factors that regulate cellular responses to skin injury.
Despite the potential of MSC therapy, the field remains in its infancy, with many challenges to be addressed before it can be used effectively in clinical practice. Studies to determine the interactions between MSCs and different cell types in wounds are urgently needed. Such studies must also identify the MSC-derived factors that are responsible for regulating local cellular responses to injury and how the wound environment affects MSCs. This paper will review the current understanding of MSCs for wound healing.
Skin and wound healing
Skin plays an exquisite, crucial role that involves different biological functions. It is the largest organ of the human body; in adults, it has an area of approximately 1.5 – 2 m2 and accounts for about 15% of body weight1, 2. The skin consists of different receptors to sense temperature, moisture, pain, and texture3. The skin regulates body temperature, stores water, and prevents dehydration to maintain the body’s internal balance and protect the body from negative conditions, such as extreme temperatures. The skin is also significant in metabolic processes, notably vitamin D synthesis and lipid storage2, 3. One of the most important functions of the skin system is to act as a protective barrier between the external and internal environment of the body2. Mechanically, the skin is constantly replenished to maintain a balance between cell death and regeneration. Sweat glands, sebaceous glands, and skin flora embedded within the skin layer also contribute greatly to the overall function of this organ4. The skin faces challenges due to aging (chronic) and wounds (acute).
Skin aging refers to the functional and aesthetic deterioration of the skin, diminishing its capacity to protect and regulate the body. Skin aging is characterized by the accumulation of damaged macromolecules in cells, diminution of regenerative capacity, and loss of physiological function5. Some discernible features of skin undergoing early again are thickening, deep wrinkles, spotting, and roughness6, 7.
Two types of skin aging occur simultaneously: aging over time and aging due to the effects of intrinsic factors (e.g., genetics, cell metabolism, hormones, etc.) and extrinsic factors (e.g., habitat, dust, radiation, toxins, chemicals, etc.). Over time, the accumulation of factors leads to molecular damage, including DNA mutation, telomere shortening, epigenetic alterations, etc., and cellular disorders, such as oxidative stress, cellular senescence, autophagy, proteostasis, inflammation, deficiency of the immune system, etc.8, 5. Thus, molecular changes due to genetic and epigenetic aging ultimately result in the aggregation of worn-out cells and the deterioration of tissue homeostasis and healing capacity9.
The extracellular components in the skin encompass collagen, elastin, fibrillin, and proteases. During aging, the ratio of type III collagen to type I collagen increases due to the loss of collagen I, which involves the down-regulation of the TGF-β/Smad signaling pathway and connective tissue growth factors; consequently, the architecture of the skin is poorly reconstructed10, 11. Moreover, the family of various matrix metalloproteinases (MMPs) exhibits a considerable impact on skin balance and anti-aging. MMPs originate from a common family of endopeptidases that decompose ECM proteins and, thus, promote the degradation of the skin12. This family has also been shown to increase with age, while endogenous MMP inhibitors decline correspondingly13, 14. Furthermore, reactive oxygen species (ROS) augment MMPs with age15. These elements could be synthesized internally as metabolized oxidizing precursors, or derive from external ultraviolet exposure. ROS activate the mitogen-activated protein kinase family (MAPK), which then induces MMP transcription factors16, 17. UV is associated with the NF-κB pathway, which is responsible for regulating MMP in skin fibroblasts16, 18.
Aging cells typically present a low expression of β-galactosidase, particularly those that are about to shift into senescence. Additionally, senescence-related gene expression, including CHEK1 and cyclin-dependent kinase inhibitor p16ink4a, is relatively down-regulated during aging9. Meticulous studies have revealed the dysregulation of microRNAs (miRNAs) as aging progresses. MiRNAs (short noncoding RNAs that bind to the 3’ untranslated region of target mRNA to prevent its gene expression) primarily serve as regulators for cell survival, proliferation, differentiation, and senescence19, 20. Age-dependent defectiveness restricts repair genes9.
The processes of skin regeneration and post-traumatic wound healing are indispensable, especially in the epidermal and dermal layers, as they help reduce the risk of infection that is associated with a high mortality rate21. This healing reaction occurs in three stages: the inflammatory phase, the proliferation phase, and the remodeling phase.
Skin injury triggers the inflammation response, which initiates coagulation, providing a fibrin network alongside vascular constriction to close the wound and preserve its integrity. This fibrin meshwork not only balances endogenous conditions and fights invading microorganisms, but also provides the site for cell migration and the collection of growth factors, including transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF)22. White blood cells are recruited into the site of injury within the first 24 hours and remain there for 2 to 5 days23, 24. White blood cells release protease and ROS to directly destroy bacteria, digest mortified tissue, and engulf the remnants of cell debris. Meanwhile, neutrophils appear to release cytokines, including necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, to amplify the inflammatory response25.
The proliferative phase occurs 48 after the wound occurs and can last for up to 14 days26 after the remission of inflammation, resulting in a miniature lesion when the contraction and formation of filaments induces horn cells26. This phase is characterized by granulation tissue formation to replace the provisional wound matrix and vascular network recovery of the sources of cytokines and growth factors, notably transforming growth factor-beta (TGF-beta, including TGF-β1, TGF-β2, and TGF-β3), interleukin (IL), and vascular formation factors27, 24. In response to tactile inhibition and the physical strain of endoplasmic lesions, horn cells and epithelial stem cells from hair follicles are triggered to migrate and proliferate to cover the wound margin. Furthermore, macrophages send nitric oxide28 signals or other cell types release epidermal growth factors (EGF), keratinocyte growth factors (KGF), insulin-like growth factor 1 (IGF-1), and neural growth factor (NGF), which appear to trigger the reepithelization process29, 30. Additionally, angiogenesis occurs to enable the transportation of nutrients and oxygen that favors wound healing. During the formation of new blood vessels, endothelial cells are activated by vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and thrombin serine protease31. The final step of this process is marked by the formation of granulation tissue leading to the infiltration and proliferation of fibroblasts into the wound edge. Granulation tissue consists of loosely organized fibroblasts, macrophages, capillaries, granular leukocytes, and bundles of type III collagen3, 32.
The remodeling phase completes the healing process, beginning within 2 to 3 weeks of the injury and potentially lasting for a year33. The process of controlling the balance between the degeneration and synthesis of new tissue is immensely rigorous and any disruption or error results in the formation of chronic wounds24, 32. TGF-β1 promotes fibroblast differentiation into myofibroblasts34. The extracellular matrix (ECM) is remodeled and synthesized to become more robust, characterized by the degeneration of type III collagen over time and its gradual replacement with type I collagen32. However, some skin components, such as hair follicles or sweat glands, cannot recover from severe skin damage35. Thus, wound healing terminates with the formation of scars, which are closely related to inflammation34.
Mesenchymal stem cells and their bioeffects on skin regeneration
Mesenchymal stem cells and their extracellular vesicles
MSCs have been found and isolated from various somatic locations36. Their biological functions have been assessed differently, as well as being isolated in different places with different methods; no distinct indication helped define the characterization of MSCs. To circumvent this problem, the International Society for Cellular Therapy suggested minimum standards to identify MSCs in humans37: First, MSCs must have a fibroblast shape, adhesive capacity, and maintain their shape under standard culture conditions. Second, MSCs must express CD73, CD90, and CD105 but not CD14, CD11b, or CD79α, CD19, CD34, CD45, and HLA-DR. Third, MSCs must differentiate into osteocytes, chondrocytes, and adipocytes in vitro37. Although scientists recently discovered that MSC populations do not thoroughly comply with these minimal criteria—for instance, some fail to express CD10538 —these rules are still ubiquitously used to compare and exchange data among MSC studies (Figure 1).
MSCs have been attested to work through the paracrine system via extracellular vesicles (EVs)39, 40. MSC-EVs comprise a variety of vesicles of varying sizes and are described as miniature prototypes of the cells that secrete them41. EVs can be divided into three main types by origin: apoptotic bodies (1000–5000 µm), microvesicles (1000 µm), and exosomes (30 – 150 µm)42. EVs seem to be more stable in the living body than foreign elements43. Immunoregulatory factors such as IL-10, TGF-β, INF- γ, IDO, and prostaglandin E2 persist within EVs44, 45. Their chief components include proteins, lipids, and nucleic acids. These components mediate many effects in recipient cells and are enclosed within a phospholipid bilayer. Furthermore, at least 730 different proteins have been shown to be enriched in exosomes derived from mesenchymal stem cells46. Additionally, 171 miRNAs have been pinpointed in EVs derived from MSCs47 and play pivotal roles in gene expression. For example, miR-130a-3p triggers cell proliferation, angiogenesis, the inhibition of programmed death, or the regulation of immune cell survival (https://www.ncbi.nlm.nih.gov/gene/406919). Exosomes have gradually revealed their potency and paved the way for the fourth generation of stem cell therapy.
MSCs’ bioeffects on skin
Immunomodulation
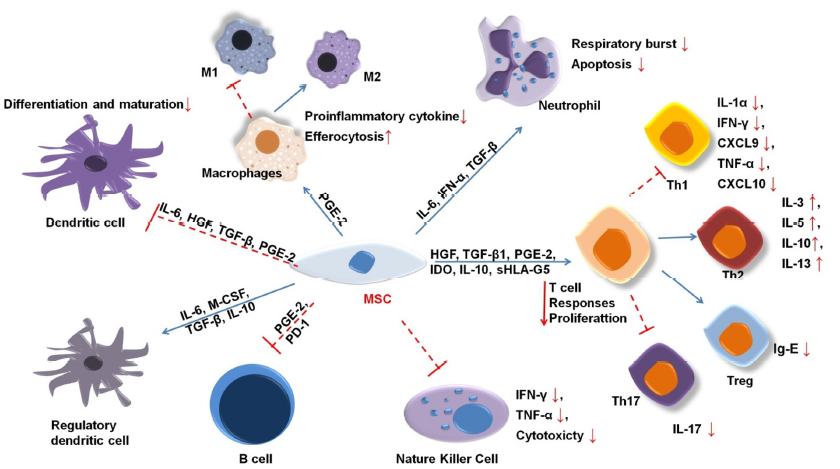
MSCs were first shown to regulate immunosuppressive activities in a mixture of lymphatic cells in vitro and persistent allograft in-vivo transplant rejection48. Since then, numerous studies have shown that MSCs mediate immunosuppression in both animal and human models. Their regulatory ability applies to both the innate and adaptive immune systems (Figure 2).
MSCs inhibit differentiation into type I macrophages49, 50, 51. IL-6 and insulin-like growth factor (IGF) prompt mononuclear cells to secrete IL-10, a strong anti-inflammatory cytokine that perpetuates osmotic balance, heals inflammatory tissue, and stimulates the transformation of macrophages into type 2 macrophages50. Additionally, MSC releases anti-ligands, such as interleukin 1 (IL1-RA) and PGE-2, which have similar effects52, 53.
MSC-derived IL-6 also inhibits apoptosis in neutrophils54. Neutrophils are the most common innate immune cells, appearing within hours in wounds. To date, the clinical associations between MSC and neutrophils are poorly understood. MSCs have been suggested to enhance neutrophil activity55, 56. MSCs also regulate a significant reduction in intracellular hydrogen peroxide, which affects neutrophils’ apoptosis process57. Neutrophils in environments with nutrient or serum deficiency can survive if cocultured with MSCs58.
Critically, MSC-secreted cytokines could inhibit pro-inflammatory T cells and stimulate the proliferation of regulatory T cells (Tregs)59, 60. Tregs are crucial for immune homeostasis because they prevent the autoimmune response. Growth factor B1 (TGFβ1), PGE2, and IL-10 innervate the increment of Tregs61, 62, 7. Although the mechanism of MSC immunomodulation remains largely unexplored, PGE2, IDO, HGF, and TGF-β1 have been strongly linked to the immunosuppression of T cells63. MSCs prohibit the production of IL-17, which suppresses the induction of Th1764, while the enhancement of IL-10 activates Tregs65. MSCs also secrete indoleamine 2,3-dioxygenase (IDO), an enzyme that decomposes tryptophan, which inhibits the growth of T cells by reducing tryptophan availability10. Several studies have suggested that a persistent decrease in tryptophan reduces the secretion of IL-4 in Th2 cells and the amount of Th1 released from IFN-gamma cells52, 66.
MSCs significantly enhance the immune regulation of B cells by mediating IL-1067, 68. One study demonstrated that the interaction of CD3+ T cells and MSCs inhibits the proliferation of plasma B cells69. At high IFN-γ concentrations, MSCs could activate programmed cell death receptors through direct contact and the PD-1/PD-L1 signaling pathway to hamper the growth and maturity of B cells70. Additionally, MSCs inhibit the CXCR4, CXCR5, and CCR7 molecules that directly affect B cells71.
MSCs inhibit the differentiation, maturity, and migration of DC cells72, 73. Specifically, IL-6 and IL-10 suppress the differentiation of mononuclear cells into DC cells74, 75. In addition, PGE2 is expected to demonstrate a similar inhibitory effect73. MSCs also promote the generation of regulatory DCs with immunomodulatory functions in mouse models76.
In addition, MSCs restrict NK cell proliferation, cytokine secretion, and cytotoxicity mediated by PGE2, IDO, HLA-5, or extracellular vesicles77, 12. This effect requires a higher ratio of MSCs to NK cells78. MSCs also regulate the CD73 expression of NK cells, which converts AMP to adenosine as an anti-inflammation inducer66, 79.
The effects that MSCs have on cells in the innate and adaptive immune systems are summarized in Table 1.
| Target cell | MSCs-derived cytokines |
|---|---|
| Tregs | IL-6, IL-10, TGFβ, IDO, VEGF |
| NK | TGFβ, Chemokines, PGE2, IDO |
| B | IL-6, TGFβ, IDO |
| Th1 | IL-10, CCL-5/RANTES, VEGF |
| Th2 | IL-6, CCL-2/MCP-1 |
| Th17 | IL-6, CCL-2/MCP-1, VEGF |
| DCs | IL-10, IL-6, TGFβ, IDO, VEGF, PGE2 |
| Neutrophils | IL-10, IL-6, CCL-5/RANTES |
| Monocytes | IL-6, CCL-5/RANTES, IDO, PGE2 |
MSC secretions in general and MSC-EVs, in particular, can modulate immunity and generate angiogenesis through their ability to regulate the proliferation and differentiation of cells. In the context of skin regeneration in general and chronic skin wounds, MSCs’ and MSC-EVs’ biological effects are apparent in infants: Early fetal skin wounds (< 3 months) heal and the skin regenerates without scarring81. The weak immune response found in fetal wounds reflects the low number of innate immune cells, such as neutrophils, macrophages, and mast cells, present82. This is characteristic of the difference between embryonic skin and adult skin.
The initial inflammatory process after the skin is wounded is very important, greatly affecting the skin composition, structure, and recovery through the subsequent processes. As Figure 2 shows, the products of MSCs exert regulatory effects on the cells of the innate and adaptive immune systems. They activate M2 macrophages and express signal transducers and activators of transcription 3 (STAT-3) concomitant with several transcription factors. These factors promote tissue regeneration and inhibit inflammation83, 84. In addition, MSCs increase the secretion of factors such as MCP-1, IL-6, and IL-8 to modulate inflammation85. Furthermore, MSCs inhibit T and B cell activation and the release of IL-10 and TGF-β—anti-inflammatory agents—and reduce IgE production86, 85.
Regeneration
The evidence suggests that MSCs and their products work to maintain skin homeostasis. In adult bodies, subcutaneous ADSCs are responsible for regulating and directing mature differentiated cells outside the epidermis, especially keratinocytes. These cells are responsible for regeneration and recovery from damage87. MSCs also release several factors that help modulate the expression of various pathways6, 88. For example, several miRNAs in MSC-EVs activate the signaling pathways AKT, ERK, and STAT-3, which are involved in many cellular processes including angiogenesis, proliferation, and cell migration89.
Keratinocytes and fibroblasts play major roles in normal proliferation and healing. Fibroblasts primarily produce ECM, cause the oral contraction of the wound, biosynthesize collagen, and regenerate tissue. Disordered or over-synthesized collagen in fibroblasts will cause scarring. MSC-EVs have been shown to influence the MAPK/ER pathway of fibroblasts, reducing scarring during the treatment of open wounds90. In addition, MSCs promote wound healing through the PI3K/AKT signaling pathway91. Fibroblast proliferation is influenced by FGF, EGF, PDGF, TGF-β, CTGF, and IGF-192.
The re-epithelialization of the skin depends on the proliferation and migration of keratinocytes. These are among the most important processes in wound healing. The EGF and TGF-β of MSCs play an important role in keratinocyte migration93. In addition, keratinocyte proliferation is stimulated by bFGF, IGF-1, and EGF93.
Mesenchymal stem cell transplantation: from animals to clinical trials
MSC therapy is promising as a fundamental therapy, with overwhelming advantages in regenerative capability, wound healing, and immunomodulation. Progressive effort has been made toward understanding the mechanisms by which MSCs promote skin wound healing. Studies of MSCs’ impact in animal models and their effect on skin lesions are presented in Table 2.
| Number | Sources | Model | Result | References |
|---|---|---|---|---|
| 1 | Adipose-derived stem cells | Burned skin, Wistar rats | + | 94 |
| 2 | Adipose-derived stem cells | 3rd-degree burns, BALB/c mice | + | 95 |
| 3 | Human amniotic mesenchymal stem cells | Burned skin, C57BL/6 mice | + | 96 |
| 4 | Human umbilical cord mesenchymal stem cells | Burned skin, C57BL/6 Mice | + | 97 |
| 5 | Adipose-derived stem cells | Surgical wound, Balb/c mice | + | 36 |
| 6 | Wharton’s jelly-derived mesenchymal stem cells | Radiation-induced skin wounds, rats | + | 98 |
| 7 | Human umbilical cord blood-derived mesenchymal stem cells | Imiquimod-induced psoriasis-like skin inflammation, C57/BL6 mice | + | 99 |
| 8 | Human umbilical cord-derived mesenchymal stem cells | Diabetic rats | + | 100 |
| 9 | Bone marrow mesenchymal stem cells | Wounds, Sprague-Dawley rats | + | 101 |
| 10 | Human umbilical cord Wharton’s jelly MSCs | Full-thickness skin defects, Balb/C mice | + | 102 |
| 11 | Umbilical cord and umbilical cord blood-derived mesenchymal stem cells | SKH-1 hairless mice | + | 103 |
| 12 | Human bone marrow and jaw bone marrow-derived mesenchymal stem cells | Full-thickness skin defects, C57BL/6J mice | + | 104 |
| Number | Type | Phase | Nation | Ref |
|---|---|---|---|---|
| 1 | Corlicyte® umbilical cord lining mesenchymal stem cells | Phase 1 | Usa | NCT04104451 |
| 2 | Placental mesenchymal stem cells | Phase 1 | China | NCT04464213 |
| 3 | Umbilical Cord Lining Stem Cells | Phase 1 | USA | NCT04723303 |
| 4 | Adipose-derived mesenchymal stem cells | Phase 2 | China | NCT04785027 |
| 5 | Adipose-derived stromal/stem cells | Phase 2 | France | NCT04356755 |
| 6 | Umbilical Cord Mesenchymal Stem Cells | Early phase 1 | China | NCT03765957 |
| 7 | Mesenchymal stem cells -derived Exosome | Phase 2 | USA | NCT04173650 |
| 8 | Adipose-derived mesenchymal stem cells | Phase 2 | Korea | NCT04137562 |
| 9 | Umbilical Cord Mesenchymal Stem Cells | Pha 2 | China | NCT03745417 |
| 10 | Hematopoietic stem cells | Phase 3 | Belgium, Croatia | NCT03754465 |
| 11 | Adipose-derived mesenchymal stem cells | Phase 2 | USA | NCT03754465 |
| 12 | Human bone marrow-derived mesenchymal stem cells | Phase 2 | Korea | NCT04179760 |
| Number | Name | Sources | Nation | Ref |
|---|---|---|---|---|
| 1 | XoGlo ® | Mesenchymal stem cell (MSC)-derived Exosome | USA | https://www.refineusa-exosomes.com/ |
| 2 | HematoPAC™-HSC-CB | Umbilical cord blood stem cells | USA | https://www.businesswire.com/news/home/20210311005075 |
| 3 | Venus Skin™ | Bone Marrow Stem Cell | USA | https://www.venustreatments.com/en-us/sct-serum.htm |
| 4 | AnteAGE Serum | Bone Marrow Stem Cell | USA | https://anteage.com/products/anteage-pro-serum-30ml |
| 5 | Exo skin simple | Adipose Stromal Cell-derived Exosome | USA | https://exoskinsimple.com/collections/products |
| 6 | ASCE+ skin | Adipose Stromal Cell-derived Exosome | KOREA | http://www.asceplus.co.kr/ |
| 7 | REBELLAXO | Umbilical cord blood- derived Exosome | USA | https://rebellabiologic.com/product/rebellaxo/ |
| 8 | InfiniVive Exosome Serum | Umbilical cord blood-derived Exosome | USA | https://infinivivemd.com/products/infinivive-exosome-serum |
| 9 | CELL PERFORMANCE SERUM | Bone Marrow Stem Cell | KOREA | https://ndclist.com/ndc/60949-080 |
| 10 | U Autologous TM | Stem cells derived from their | USA | https://www.leadingsalons.com/en/article/50/u-skincare |
| 11 | Dermaheal Stem C’rum | Adipose stem cell | KOREA | https://caregennordic.se/product/stem-crum/ |
| 12 | Cutisera™ | Bone marrow mesenchymal stem cell | INDIA | https://www.stempeutics.com/stempeucare.html |
| 13 | Adipose Stem Cell Growth Factor Anti-Aging Serum | Adipose Stem Cell | USA | https://www.amazon.com/Adipose-Hyaluronic-Matrixyl-Advanced-Anti-Aging/dp/B07SCPTSGN |
| 14 | Beautigenix Hydrating Mask | Adult Human Stem Cell | USA | https://incidecoder.com/products/beautygenix-hydrating-mask |
| 15 | ProPlus Eye Firming Complex | Peptides from Non-Embryonic Human Stem Cells | USA | https://lifelineskincare.com/products/proplus-eye-firming-complex |
Most studies of MSCs’ relationship to skin wounds on different model types report positive effects. This dynamic supports the application of MSCs to human skin lesions. To date, more than 400 clinical studies affirm the positive effects of MSCs on skin lesions (clinicaltrials.gov). These studies use mesenchymal stem cells from a variety of sources; MSC products, such as EVs, proteins, or miRNAs; and these agents together, along with a biomatrix (tissue engineering) to treat skin wounds. Some of the most up-to-date clinical trials are presented in Table 3.
In addition to the use of MSCs to heal ailments related to skin damage, MSCs and MSC products have been used for anti-aging cosmetic purposes. Research on animal models showed that exosomes from young mice could transfer miR-126b-5p to the tissues of old mice and reverse the expression of aging-related molecules such as p16, mTOR, and IGF-1R. They also affect the expression of telomerase-related genes, including Mre11a, Tep1, Terf2, Men1, Tert, and Tnks, in old mice105. If this effect occurs in humans, MSCs in general and MSCs originating from umbilical cords or placentas, in particular, will be a potential source of cosmetic pharmaceuticals, eliminating other cosmetics that currently dominate the market.
The market for exosome-related cosmetics is flourishing. Some MSCs and MSC products used in cosmetics are presented in Table 4.
Perspectives
Because MSCs and MSC exosomes manifest hypoimmunogenic properties, they are hopeful choices for treating chronic wounds, injuries, and plastic surgery incisions. This has huge potential in the field of tissue remodeling and engineering. Since the Physiology Nobel prize in 2013 was awarded to the three Laureates who presented insights into exosomes, studies of MSC exosomes have increased at a dizzying rate. According to Mordor Intelligence, the exosome market was reportedly worth 174.04 million USD in 2020 and is predicted to increase by 27.89% in this year (https://www.mordorintelligence.com/). The growing demand for anti-aging therapies drives much of the market. Furthermore, the availability of various methods for exosomal isolation and purification helps fuel further studies of exosome treatments.
Most research now focuses on proving the utility of MSC exosomes as a single treatment. Clinically, MSC exosomes should be used in combination with other therapies, such as laser, topical medication, surgery, etc. Consequently, more studies are needed to demonstrate the synergistic or inhibitory effects of MSC exosomes in combination with conventional treatments. Researchers are optimistic about the development of MSC therapy. Similarly, exosomes as a drug delivery system are a bright research path. For almost a decade, there has been tremendous progress in our understanding of all aspects of exosomes. Further improvements in drug-loading strategies and the optimization of these therapies are promising for future clinicians.
The limitations of MSC-EV use
The extracellular secretions of MSCs, especially the exosomes, are being intensively studied for their notable features. MSCs’ impact via exosomes is significant in tissue repair. MSC therapy is gaining popularity and is known as fourth-generation stem cell therapy—the new cell-free therapy106. MSCs not only play an essential role in the treatment of wounds, skin regeneration, and anti-aging but also contribute to the treatment of immune-related diseases, tumors, and neurological disorders and are the standard by which to diagnose and prevent disease. In addition, exosomes from MSCs can be used for drug delivery, encasing pharmaceuticals in a phospholipid bilayer that confers outstanding physiological advantages107.
In contrast, the considerations for using MSC exosomes include:
It is challenging to determine the composition of exosomes, such as their proteins, lipids, and, especially, miRNAs. All of these components derive from the cell culture process and are variable and heterogeneous. Qualitative studies generally seek to confirm whether an agent of interest persists within exosomes. The half-life of exosomes is also unknown. This is an important evaluation criterion to use exosomes for drug transport107. Drug efficacy is highly dependent on delivery time, so this is a major limitation of knowledge regarding the potential use of exosomes for transport.
Another important consideration is the exosome productivity of mesenchymal stem cells. The exosomes for this new cell-free therapy are usually obtained from MSC cultures. This means that cell therapy and exosome therapy are linked. A study has determined that producing sufficient exosomes to have the same effect as cell therapy requires 10 – 25 times the normal quantity of MSCs108. Significant development is needed before exosome therapy can be routinely applied. More research on a suitable culture medium to produce more extracellular vesicles is needed to address this limitation. The current cell culture abandonment medium does not meet the conditions required to develop a routine therapy.
There is also substantial concern about cell properties. Exosomes reflect the properties of their parent cells. Research shows that exosomes from young cells can reverse the aging of aging cells105. Therefore, more in-depth studies are needed on the relationship between MSC age and the exosomes they release. This greatly affects the potential of mesenchymal stem cells from different sites. If the assertions in105 are true, mesenchymal stem cells obtained from umbilical cords and placentas will be preferred.
Another problem in the production of exosomes is purity. MSCs do not express HLA-DR, so they should not induce an adverse immune response. However, an immune response has been observed when MSCs enter the body. This is associated with impurities in the injections. Ultracentrifugation is a standard method for exosome isolation and purification. It uses a density gradient, which may permit other agents or types of vesicles with the same density as the target components to remain. Developing an environment to eliminate, limit, or replace undesirable elements is one solution. However, the optimization of the culture and acquisition process is the ideal way to eliminate this problem.
Despite these limitations, the enormous potential of MSC exosomes is apparent. Exosomes have many advantages over mesenchymal stem cell therapy: They can be stored as proteins for short-term use without the concern of cell survival and using them after thawing is much easier. They are much smaller than MSCs and do not become trapped in small capillaries like cells do. One report shows that this is a serious limitation of cell therapy, as when intravenous administration results in pulmonary embolism109. However, exosome therapy cannot completely replace mesenchymal stem cell therapy because of their interrelationships.
Abbreviations
AMP: Adenosine monophosphate, bFGF: Basic fibroblast growth factor, CCL-2/ MCP1: Chemokine (C-C motif) ligand 2/ onocyte chemoattractant protein 1, CCL-5/ RANTES: Chemokine (C-C motif) ligand 5/ regulated on activation, normal T cell expressed and secreted, CXCR4 C-X-C: chemokine receptor type 4, CXCR5 C-X-C: chemokine receptor type 5, DCs: Dendritic cells, ECM: Extracellular matrix, EGF: Epidermal growth factor, EVs: Extracellular vesicles, HGF: Hepatocyte growth factor, HLA-5: Human Leukocyte Antigen - 5, HLA-DR: Human Leukocyte Antigen – DR isotype, IDO: Enzyme Indoleamine 2,3-dioxygenase, IGF-1: Insulin-like growth factor 1, IL: Interleukin, KGF: Keratinocyte Growth Factor, MAPK: Mitogen-activated protein kinase, MMP: Matrix metalloproteinase, MSC: Mesenchymal stem cell, NK cells: Natural killer cells, NGF: Nerve growth factor, PDGF: Platelet-derived growth factor, PGE-2: Prostaglandin E 2, ROS: Reactive oxygen species, TGF-β: Transforming growth factor beta, TGF-β1: Transforming growth factor beta 1, TGF-β2: Transforming growth factor beta 2, TGF-β3: Transforming growth factor beta 3, TNF: Tumor necrosis factor, Th1 cells: T helper cells, Th2 cells: T helper cells, Tregs: Regulatory T cells, VEGF: Vascular endothelial growth factor, IFN-γ: Interferons γ
Acknowledgments
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number 562-2020-18-03.
Author’s contributions
Phat Duc Huynh was responsible for the layout and content of the manuscript. Quynh Xuan Tran, Vy Quang Nguyen, and Sao Thi Nguyen equally contributed to this work. Ngoc Bich Vu conceptualized, coordinated and edited the article.
Funding
Vietnam National University Ho Chi Minh City (VNUHCM) under grant number 562-2020-18-03.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Richardson
M.,
Understanding the structure and function of the skin. Nursing Times.
2003;
99
(31)
:
46-8
.
PubMed Google Scholar -
Vig
K.,
Chaudhari
A.,
Tripathi
S.,
Dixit
S.,
Advances in Skin Regeneration Using Tissue Engineering. Int J Mol Sci.
2017;
18
(4)
:
789
.
View Article PubMed Google Scholar -
Agarwal
S.K.,
Skin. [Updated 2020 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. 2021
.
-
Sanford
J.A.,
Gallo
R.L.,
Functions of the skin microbiota in health and disease. Seminars in Immunology.
2013;
25
(5)
:
370-7
.
View Article PubMed Google Scholar -
Orioli
D.,
Dellambra
E.,
Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells.
2018;
7
(12)
:
268
.
View Article PubMed Google Scholar -
Ferreira
A. D. F.,
Gomes
D. A.,
Stem Cell Extracellular Vesicles in Skin Repair. Bioengineering (Basel).
2018;
6
(1)
:
4
.
View Article PubMed Google Scholar -
Luz-Crawford
P.,
Kurte
M.,
Bravo-Alegría
J.,
Contreras
R.,
Nova-Lamperti
E.,
Tejedor
G.,
Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Research & Therapy.
2013;
4
(3)
:
65
.
View Article PubMed Google Scholar -
Ferrucci
L.,
Gonzalez-Freire
M.,
Fabbri
E.,
Simonsick
E.,
Tanaka
T.,
Moore
Z.,
Measuring biological aging in humans: A quest. Aging Cell.
2020;
19
(2)
:
e13080
.
View Article PubMed Google Scholar -
Alt
E.U.,
Senst
C.,
Murthy
S.N.,
Slakey
D.P.,
Dupin
C.L.,
Chaffin
A.E.,
Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Research (Amsterdam).
2012;
8
(2)
:
215-25
.
View Article PubMed Google Scholar -
Perez-Sepulveda
A.,
Torres
M.J.,
Khoury
M.,
Illanes
S.E.,
Innate immune system and preeclampsia. Frontiers in Immunology.
2014;
5
:
244
.
View Article PubMed Google Scholar -
Quan
T.,
Shao
Y.,
He
T.,
Voorhees
J.J.,
Fisher
G.J.,
Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. The Journal of Investigative Dermatology.
2010;
130
(2)
:
415-24
.
View Article PubMed Google Scholar -
Qu
M.,
Cui
J.,
Zhu
J.,
Ma
Y.,
Yuan
X.,
Shi
J.,
Bone marrow-derived mesenchymal stem cells suppress NK cell recruitment and activation in PolyI:C-induced liver injury. Biochemical and Biophysical Research Communications.
2015;
466
(2)
:
173-9
.
View Article PubMed Google Scholar -
Qin
Z.,
Balimunkwe
R.M.,
Quan
T.,
Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. British Journal of Dermatology.
2017;
177
(5)
:
1337-48
.
View Article PubMed Google Scholar -
Quan
T.,
Little
E.,
Quan
H.,
Qin
Z.,
Voorhees
J.J.,
Fisher
G.J.,
Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. The Journal of Investigative Dermatology.
2013;
133
(5)
:
1362-6
.
View Article PubMed Google Scholar -
Quan
T.,
Fisher
G.J.,
Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology.
2015;
61
(5)
:
427-34
.
View Article PubMed Google Scholar -
Chiang
H.M.,
Chen
H.C.,
Chiu
H.H.,
Chen
C.W.,
Wang
S.M.,
Wen
K.C.,
Neonauclea reticulata (Havil.) Merr Stimulates Skin Regeneration after UVB Exposure via ROS Scavenging and Modulation of the MAPK/MMPs/Collagen Pathway. Evidence-Based Complementary and Alternative Medicine.
2013;
2013
:
324864
.
View Article PubMed Google Scholar -
Kim
J.,
Lee
C.W.,
Kim
E.K.,
Lee
S.J.,
Park
N.H.,
Kim
H.S.,
Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. Journal of Ethnopharmacology.
2011;
137
(1)
:
427-33
.
View Article PubMed Google Scholar -
Pittayapruek
P.,
Meephansan
J.,
Prapapan
O.,
Komine
M.,
Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int J Mol Sci.
2016;
17
(6)
:
868
.
View Article PubMed Google Scholar -
Ambros
V.,
The functions of animal microRNAs. Nature.
2004;
431
(7006)
:
350-5
.
View Article PubMed Google Scholar -
Bartel
D.P.,
MicroRNAs: target recognition and regulatory functions. Cell.
2009;
136
(2)
:
215-33
.
View Article PubMed Google Scholar -
Jeschke
M.G.,
Rehou
S.,
McCann
M.R.,
Shahrokhi
S.,
Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Research & Therapy.
2019;
10
(1)
:
337
.
View Article PubMed Google Scholar -
Vahabi
S.,
Vaziri
S.,
Torshabi
M.,
Rezaei Esfahrood
Z.,
Effects of Plasma Rich in Growth Factors and Platelet-Rich Fibrin on Proliferation and Viability of Human Gingival Fibroblasts. Journal of Dentistry (Tehran).
2015;
12
(7)
:
504-12
.
PubMed Google Scholar -
Gantwerker
E.A.,
Hom
D.B.,
Skin: histology and physiology of wound healing. Facial Plastic Surgery Clinics of North America.
2011;
19
(3)
:
441-53
.
View Article PubMed Google Scholar -
Wang
P.H.,
Huang
B.S.,
Horng
H.C.,
Yeh
C.C.,
Chen
Y.J.,
Wound healing. Journal of the Chinese Medical Association.
2018;
81
(2)
:
94-101
.
View Article PubMed Google Scholar -
Eming
S.A.,
Martin
P.,
Tomic-Canic
M.,
Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine.
2014;
6
(265)
.
View Article PubMed Google Scholar -
Li
J.,
Chen
J.,
Kirsner
R.,
Pathophysiology of acute wound healing. Clinics in Dermatology.
2007;
25
(1)
:
9-18
.
View Article PubMed Google Scholar -
Sorg
H.,
Tilkorn
D.J.,
Hager
S.,
Hauser
J.,
Mirastschijski
U.,
Skin Wound Healing: An Update on the Current Knowledge and Concepts. European Surgical Research.
2017;
58
(1-2)
:
81-94
.
View Article PubMed Google Scholar -
Witte
M.B.,
Barbul
A.,
Role of nitric oxide in wound repair. American Journal of Surgery.
2002;
183
(4)
:
406-12
.
View Article PubMed Google Scholar -
Barrientos
S.,
Stojadinovic
O.,
Golinko
M.S.,
Brem
H.,
Tomic-Canic
M.,
Growth factors and cytokines in wound healing. Wound Repair and Regeneration.
2008;
16
(5)
:
585-601
.
View Article PubMed Google Scholar -
Landén
N.X.,
Li
D.,
St\aahle
M.,
Transition from inflammation to proliferation: a critical step during wound healing. Cellular and Molecular Life Sciences.
2016;
73
(20)
:
3861-85
.
View Article PubMed Google Scholar -
Li
J.,
Zhang
Y.P.,
Kirsner
R.S.,
Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microscopy Research and Technique.
2003;
60
(1)
:
107-14
.
View Article PubMed Google Scholar -
Kangal
M.K. Ozgok,
Wound Healing. [Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535406/. 2021
.
-
Reinke
J.M.,
Sorg
H.,
Wound repair and regeneration. European Surgical Research.
2012;
49
(1)
:
35-43
.
View Article PubMed Google Scholar -
Broughton
G.,
Janis
J.E.,
Attinger
C.E.,
The basic science of wound healing. Plastic and Reconstructive Surgery.
2006;
117
(7)
:
12-34
.
View Article PubMed Google Scholar -
Schilling
J.A.,
Wound healing. The Surgical Clinics of North America.
1976;
56
(4)
:
859-74
.
View Article PubMed Google Scholar -
Luo
Y.,
Yi
X.,
Liang
T.,
Jiang
S.,
An Update on the Potential of Mesenchymal Stem Cell Therapy for Cutaneous Diseases. Stem Cells Int.
2019;
2021
:
8834590
.
View Article PubMed Google Scholar -
Dominici
M.,
Le Blanc
K.,
Mueller
I.,
Slaper-Cortenbach
I.,
Marini
F.,
Krause
D.,
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy.
2006;
8
(4)
:
315-7
.
View Article PubMed Google Scholar -
The subpopulation of CD105 negative mesenchymal stem cells show strong immunomodulation capacity compared to CD105 positive mesenchymal stem cells. Biomedical Research and Therapy.
2019;
6
(4)
:
3131-40
.
View Article Google Scholar -
Ranganath
S.H.,
Levy
O.,
Inamdar
M.S.,
Karp
J.M.,
Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell.
2012;
10
(3)
:
244-58
.
View Article PubMed Google Scholar -
Sajeesh
S.,
Broekelman
T.,
Mecham
R.P.,
Ramamurthi
A.,
Stem cell derived extracellular vesicles for vascular elastic matrix regenerative repair. Acta Biomaterialia.
2020;
113
:
267-78
.
View Article PubMed Google Scholar -
Worthington
E. N.,
Hagood
J. S.,
Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int J Mol Sci.
2020;
21
(7)
:
2318
.
View Article PubMed Google Scholar -
Jeppesen
D.K.,
Fenix
A.M.,
Franklin
J.L.,
Higginbotham
J.N.,
Zhang
Q.,
Zimmerman
L.J.,
Reassessment of Exosome Composition. Cell.
2019;
177
(2)
.
View Article PubMed Google Scholar -
Pluchino
S.,
Smith
J.A.,
Explicating Exosomes: Reclassifying the Rising Stars of Intercellular Communication. Cell.
2019;
177
(2)
:
225-7
.
View Article PubMed Google Scholar -
Crain
S.K.,
Robinson
S.R.,
Thane
K.E.,
Davis
A.M.,
Meola
D.M.,
Barton
B.A.,
Extracellular Vesicles from Wharton's Jelly Mesenchymal Stem Cells Suppress CD4 Expressing T Cells Through Transforming Growth Factor Beta and Adenosine Signaling in a Canine Model. Stem Cells and Development.
2019;
28
(3)
:
212-26
.
View Article PubMed Google Scholar -
Lopatina
T.,
Bruno
S.,
Tetta
C.,
Kalinina
N.,
Porta
M.,
Camussi
G.,
Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Communication and Signaling.
2014;
12
(1)
:
26
.
View Article PubMed Google Scholar -
Kim
H.S.,
Choi
D.Y.,
Yun
S.J.,
Choi
S.M.,
Kang
J.W.,
Jung
J.W.,
Proteomic analysis of microvesicles derived from human mesenchymal stem cells. Journal of Proteome Research.
2012;
11
(2)
:
839-49
.
View Article PubMed Google Scholar -
Ferguson
S. W.,
Wang
J.,
Lee
C. J.,
Liu
M.,
The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep.
2018;
8
(1)
:
1419
.
View Article PubMed Google Scholar -
Bartholomew
A.,
Sturgeon
C.,
Siatskas
M.,
Ferrer
K.,
McIntosh
K.,
Patil
S.,
Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology.
2002;
30
(1)
:
42-8
.
View Article PubMed Google Scholar -
Deng
Y.,
Zhang
Y.,
Ye
L.,
Zhang
T.,
Cheng
J.,
Chen
G.,
Umbilical Cord-derived Mesenchymal Stem Cells Instruct Monocytes Towards an IL10-producing Phenotype by Secreting IL6 and HGF. Scientific Reports.
2016;
6
(1)
:
37566
.
View Article PubMed Google Scholar -
Weiss
A.R.,
Dahlke
M.H.,
Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Frontiers in Immunology.
2019;
10
:
1191
.
View Article PubMed Google Scholar -
Zheng
G.,
Huang
R.,
Qiu
G.,
Ge
M.,
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res.
2018;
374
(1)
:
1-15
.
View Article PubMed Google Scholar -
Abumaree
M.H.,
Al Jumah
M.A.,
Kalionis
B.,
Jawdat
D.,
Al Khaldi
A.,
Abomaray
F.M.,
Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Reviews and Reports.
2013;
9
(5)
:
620-41
.
View Article PubMed Google Scholar -
Luz-Crawford
P.,
Djouad
F.,
Toupet
K.,
Bony
C.,
Franquesa
M.,
Hoogduijn
M.J.,
Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells (Dayton, Ohio).
2016;
34
(2)
:
483-92
.
View Article PubMed Google Scholar -
Raffaghello
L.,
Bianchi
G.,
Bertolotto
M.,
Montecucco
F.,
Busca
A.,
Dallegri
F.,
Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells (Dayton, Ohio).
2008;
26
(1)
:
151-62
.
View Article PubMed Google Scholar -
Hall
S.R.,
Tsoyi
K.,
Ith
B.,
Padera
R.F.,
Lederer
J.A.,
Wang
Z.,
Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells (Dayton, Ohio).
2013;
31
(2)
:
397-407
.
View Article PubMed Google Scholar -
Le Blanc
K.,
Mougiakakos
D.,
Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews. Immunology.
2012;
12
(5)
:
383-96
.
View Article PubMed Google Scholar -
Dallegri
F.,
Ottonello
L.,
Tissue injury in neutrophilic inflammation. Inflammation Research.
1997;
46
(10)
:
382-91
.
View Article PubMed Google Scholar -
Maqbool
M.,
Vidyadaran
S.,
George
E.,
Ramasamy
R.,
Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biology International.
2011;
35
(12)
:
1247-51
.
View Article PubMed Google Scholar -
Baharlou
R.,
Rashidi
N.,
Ahmadi-Vasmehjani
A.,
Khoubyari
M.,
Sheikh
M.,
Erfanian
S.,
Immunomodulatory Effects of Human Adipose Tissue-derived Mesenchymal Stem Cells on T Cell Subsets in Patients with Rheumatoid Arthritis. Iranian Journal of Allergy, Asthma, and Immunology.
2019;
18
(1)
:
114-9
.
View Article PubMed Google Scholar -
Jimenez-Puerta
G. J.,
Marchal
J. A.,
E. López-Ruiz,
P. Gálvez-Martín,
Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J Clin Med.
2020;
9
(2)
:
445
.
View Article PubMed Google Scholar -
English
K.,
Ryan
J.M.,
Tobin
L.,
Murphy
M.J.,
Barry
F.P.,
Mahon
B.P.,
Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clinical and Experimental Immunology.
2009;
156
(1)
:
149-60
.
View Article PubMed Google Scholar -
Gonzalez-Rey
E.,
Gonzalez
M.A.,
Varela
N.,
O'Valle
F.,
Hernandez-Cortes
P.,
Rico
L.,
Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Annals of the Rheumatic Diseases.
2010;
69
(1)
:
241-8
.
View Article PubMed Google Scholar -
Xu
C.,
Yu
P.,
Han
X.,
Du
L.,
Gan
J.,
Wang
Y.,
TGF-β promotes immune responses in the presence of mesenchymal stem cells. Journal of Immunology (Baltimore, Md.: 1950).
2014;
192
(1)
:
103-9
.
View Article PubMed Google Scholar -
Han
X.,
Yang
Q.,
Lin
L.,
Xu
C.,
Zheng
C.,
Chen
X.,
Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death and Differentiation.
2014;
21
(11)
:
1758-68
.
View Article PubMed Google Scholar -
Liu
H.,
Li
R.,
Liu
T.,
Yang
L.,
Yin
G.,
Xie
Q.,
Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Frontiers in Immunology.
2020;
11
:
1912
.
View Article PubMed Google Scholar -
Chatterjee
D.,
Tufa
D.M.,
Baehre
H.,
Hass
R.,
Schmidt
R.E.,
Jacobs
R.,
Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood.
2014;
123
(4)
:
594-5
.
View Article PubMed Google Scholar -
Carreras-Planella
L.,
Monguió-Tortajada
M.,
Borràs
F.E.,
Franquesa
M.,
Corrigendum: Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Frontiers in Immunology.
2019;
10
:
2413
.
View Article PubMed Google Scholar -
Chen
X.,
Cai
C.,
Xu
D.,
Liu
Q.,
Zheng
S.,
Liu
L.,
Human Mesenchymal Stem Cell-Treated Regulatory CD23+CD43+ B Cells Alleviate Intestinal Inflammation. Theranostics.
2019;
9
(16)
:
4633-47
.
View Article PubMed Google Scholar -
Rosado
M.M.,
Bernardo
M.E.,
Scarsella
M.,
Conforti
A.,
Giorda
E.,
Biagini
S.,
Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells and Development.
2015;
24
(1)
:
93-103
.
View Article PubMed Google Scholar -
Schena
F.,
Gambini
C.,
Gregorio
A.,
Mosconi
M.,
Reverberi
D.,
Gattorno
M.,
Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis and Rheumatism.
2010;
62
(9)
:
2776-86
.
View Article PubMed Google Scholar -
Corcione
A.,
Benvenuto
F.,
Ferretti
E.,
Giunti
D.,
Cappiello
V.,
Cazzanti
F.,
Human mesenchymal stem cells modulate B-cell functions. Blood.
2006;
107
(1)
:
367-72
.
View Article PubMed Google Scholar -
Ge
W.,
Jiang
J.,
Arp
J.,
Liu
W.,
Garcia
B.,
Wang
H.,
Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation.
2010;
90
(12)
:
1312-20
.
View Article PubMed Google Scholar -
Melief
S.M.,
Geutskens
S.B.,
Fibbe
W.E.,
Roelofs
H.,
Multipotent stromal cells skew monocytes towards an anti-inflammatory function: the link with key immunoregulatory molecules. Haematologica.
2013;
98
(9)
:
e121-2
.
View Article PubMed Google Scholar -
Allavena
P.,
Piemonti
L.,
Longoni
D.,
Bernasconi
S.,
Stoppacciaro
A.,
Ruco
L.,
IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. European Journal of Immunology.
1998;
28
(1)
:
359-69
.
View Article PubMed Google Scholar -
Djouad
F.,
Charbonnier
L.M.,
Bouffi
C.,
Louis-Plence
P.,
Bony
C.,
Apparailly
F.,
Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells (Dayton, Ohio).
2007;
25
(8)
:
2025-32
.
View Article PubMed Google Scholar -
Krasnodembskaya
A.,
Samarani
G.,
Song
Y.,
Zhuo
H.,
Su
X.,
Lee
J.W.,
Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. American Journal of Physiology. Lung Cellular and Molecular Physiology.
2012;
302
(10)
:
1003-13
.
View Article PubMed Google Scholar -
Jeyaraman
M.,
John
A.,
Koshy
S.,
Ranjan
R.,
Anudeep
T.C.,
Jain
R.,
Fostering mesenchymal stem cell therapy to halt cytokine storm in COVID-19. Biochimica et Biophysica Acta. Molecular Basis of Disease.
2021;
1867
(2)
:
166014
.
View Article PubMed Google Scholar -
Sotiropoulou
P.A.,
Perez
S.A.,
Gritzapis
A.D.,
Baxevanis
C.N.,
Papamichail
M.,
Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells (Dayton, Ohio).
2006;
24
(1)
:
74-85
.
View Article PubMed Google Scholar -
Chen
X.,
Shao
H.,
Zhi
Y.,
Xiao
Q.,
Su
C.,
Dong
L.,
CD73 Pathway Contributes to the Immunosuppressive Ability of Mesenchymal Stem Cells in Intraocular Autoimmune Responses. Stem Cells and Development.
2016;
25
(4)
:
337-46
.
View Article PubMed Google Scholar -
Kyurkchiev
D.,
Bochev
I.,
Ivanova-Todorova
E.,
Mourdjeva
M.,
Oreshkova
T.,
Belemezova
K.,
Secretion of immunoregulatory cytokines by mesenchymal stem cells. World Journal of Stem Cells.
2014;
6
(5)
:
552-70
.
View Article PubMed Google Scholar -
Lorenz
H.P.,
Whitby
D.J.,
Longaker
M.T.,
Adzick
N.S.,
Fetal wound healing. The ontogeny of scar formation in the non-human primate. Annals of Surgery.
1993;
217
(4)
:
391-6
.
View Article PubMed Google Scholar -
Eming
S. A.,
Wynn
T. A.,
Inflammation and metabolism in tissue repair and regeneration. Science.
2017;
356
(6342)
:
1026-1030
.
View Article PubMed Google Scholar -
Jin
L.,
Deng
Z.,
Zhang
J.,
Yang
C.,
Liu
J.,
Han
W.,
Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. Journal of Translational Medicine.
2019;
17
(1)
:
251
.
View Article PubMed Google Scholar -
Liu
Y.C.,
Zou
X.B.,
Chai
Y.F.,
Yao
Y.M.,
Macrophage polarization in inflammatory diseases. International Journal of Biological Sciences.
2014;
10
(5)
:
520-9
.
View Article PubMed Google Scholar -
Mazini
L.,
Rochette
L.,
Hamdan
Y.,
Malka
G.,
Skin Immunomodulation during Regeneration: Emerging New Targets. J Pers Med.
2021;
11
(2)
:
85
.
View Article PubMed Google Scholar -
Daltro
S.R.,
Meira
C.S.,
Santos
I.P.,
Ribeiro Dos Santos
R.,
Soares
M.B.,
Mesenchymal Stem Cells and Atopic Dermatitis: A Review. Frontiers in Cell and Developmental Biology.
2020;
8
:
326
.
View Article PubMed Google Scholar -
Blanpain
C.,
Fuchs
E.,
Epidermal homeostasis: a balancing act of stem cells in the skin. Nature Reviews. Molecular Cell Biology.
2009;
10
(3)
:
207-17
.
View Article PubMed Google Scholar -
Choi
E. W.,
Seo
M. K.,
Woo
E. Y.,
Kim
S. H.,
Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol.
2018;
27
(10)
:
1170-1172
.
View Article PubMed Google Scholar -
Ding
J.,
Wang
X.,
Chen
B.,
Zhang
J.,
Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed Res Int.
2019;
2019
:
9742765
.
PubMed Google Scholar -
Wang
L.,
Hu
L.,
Zhou
X.,
Xiong
Z.,
Zhang
C.,
Shehada
H.M.,
Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Scientific Reports.
2017;
7
(1)
:
13321
.
View Article PubMed Google Scholar -
Zhang
W.,
Bai
X.,
Zhao
B.,
Li
Y.,
Zhang
Y.,
Li
Z.,
Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Experimental Cell Research.
2018;
370
(2)
:
333-42
.
View Article PubMed Google Scholar -
Lee
S.H.,
Jin
S.Y.,
Song
J.S.,
Seo
K.K.,
Cho
K.H.,
Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Annals of Dermatology.
2012;
24
(2)
:
136-43
.
View Article PubMed Google Scholar -
Bhora
F.Y.,
Dunkin
B.J.,
Batzri
S.,
Aly
H.M.,
Bass
B.L.,
Sidawy
A.N.,
Effect of growth factors on cell proliferation and epithelialization in human skin. The Journal of Surgical Research.
1995;
59
(2)
:
236-44
.
View Article PubMed Google Scholar -
Li
J.-Y.,
Ren
K.-K.,
Zhang
W.-J.,
Xiao
L.,
Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther.
2019;
10
(1)
:
247
.
View Article PubMed Google Scholar -
Zhou
P.,
Li
X.,
Zhang
B.,
Shi
Q.,
A human umbilical cord mesenchymal stem cell-conditioned medium/chitosan/collagen/β-glycerophosphate thermosensitive hydrogel promotes burn injury healing in mice. Biomed Res Int.
2019;
2019
:
5768285
.
View Article PubMed Google Scholar -
Sun
J.,
Zhang
Y.,
Song
X.,
Zhu
J.,
The Healing Effects of Conditioned Medium Derived from Mesenchymal Stem Cells on Radiation-Induced Skin Wounds in Rats. Cell Transplant.
2019;
28
(1)
:
105-115
.
View Article PubMed Google Scholar -
Lee
Y. S.,
Sah
S. K.,
Lee
J. H.,
Seo
K.-W.,
Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochem Biophys Rep.
2017;
2016
:
281-288
.
View Article PubMed Google Scholar -
Wang
L.,
Wang
F.,
Zhao
L.,
Yang
W.,
Mesenchymal stem cells coated by the extracellular matrix promote wound healing in diabetic rats. Stem Cells Int.
2019;
2019
:
9564869
.
View Article PubMed Google Scholar -
Zheng
X.,
Ding
Z.,
Cheng
W.,
Lu
Q.,
Microskin‐Inspired Injectable MSC‐Laden Hydrogels for Scarless Wound Healing with Hair Follicles. Advanced healthcare materials.
2020;
9
(10)
:
2000041
.
-
Zhao
G.,
Liu
F.,
Liu
Z.,
Zuo
K.,
MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem cell research & therapy.
2020;
11
(1)
:
1-8
.
View Article Google Scholar -
Myung
H.,
Jang
H.,
Myung
J. K.,
Lee
C.,
Platelet‐rich plasma improves the therapeutic efficacy of mesenchymal stem cells by enhancing their secretion of angiogenic factors in a combined radiation and wound injury model. Experimental Dermatology.
2020;
29
(2)
:
158-67
.
View Article Google Scholar -
He
X.,
Dong
Z.,
Cao
Y.,
Wang
H.,
MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int.
2019;
2019
:
7132708
.
View Article PubMed Google Scholar -
Chang
Y.-W.,
Wu
Y.-C.,
Huang
S.-H.,
Wang
H.-M. D.,
Autologous and not allogeneic adipose-derived stem cells improve acute burn wound healing. PLoS One.
2018;
13
(5)
:
e0197744
.
View Article PubMed Google Scholar -
Gholipourmalekabadi
M.,
Seifalian
A. M.,
Urbanska
A. M.,
Omrani
M. D.,
3D protein-based bilayer artificial skin for the guided scarless healing of third-degree burn wounds in vivo. Biomacromolecules.
2018;
19
(7)
:
2409-2422
.
View Article PubMed Google Scholar -
Lee
B.R.,
Kim
J.H.,
Choi
E.S.,
Cho
J.H.,
Kim
E.,
Effect of young exosomes injected in aged mice. International Journal of Nanomedicine.
2018;
13
:
5335-45
.
View Article PubMed Google Scholar -
Pham
P.V.,
Stem Cell Drugs-A New Generation of Biopharmaceuticals. Springer International Publishing; 2018 Oct 25.. 2018
.
View Article Google Scholar -
Wei
W.,
Ao
Q.,
Wang
X.,
Cao
Y.,
Liu
Y.,
Zheng
S.G.,
Mesenchymal Stem Cell-Derived Exosomes: A Promising Biological Tool in Nanomedicine. Frontiers in Pharmacology.
2021;
11
:
590470
.
View Article PubMed Google Scholar -
Ahangar
P.,
Mills
S. J.,
Smith
L. E.,
Strudwick
X. L.,
Human multipotent adult progenitor cell-conditioned medium improves wound healing through modulating inflammation and angiogenesis in mice. Stem Cell Res Ther.
2020;
11
(1)
:
299
.
View Article PubMed Google Scholar -
Jung
J.W.,
Kwon
M.,
Choi
J.C.,
Shin
J.W.,
Park
I.W.,
Choi
B.W.,
Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Medical Journal.
2013;
54
(5)
:
1293-6
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 12 (2022)
Page No.: 5437-5449
Published on: 2022-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8396 times
- PDF downloaded - 1705 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress




