Abstract
Insomnia has a significant global incidence rate. Previous observational studies, general practitioner surveys, and medical trials suggest that a variety of patient and physician factors are associated with this, including low patient reporting of insomnia, limited healthcare professional training, office-based time constraints, and misconceptions about the seriousness of insomnia, treatment benefits, and the risks associated with hypnotic use. Here, we discuss the recently studied genetic aspects of insomnia pathogenesis and the orexin system and acupuncture as potential therapeutic strategies.
Introduction
The presence of polysomnographic indication of disturbed sleep is often referred to as “insomnia.” Long sleep onset times, numerous nocturnal awakenings, protracted periods of consciousness throughout the sleep phase, and several brief awakenings are all considered signs of insomnia1. Insomnia symptoms include difficulty falling asleep, frequent awakenings with difficulty falling back asleep, or non-restorative or poor-quality sleep, which is commonly accompanied by the feeling of getting little sleep overall1. Sleep-related symptoms are seen in a significant majority of epidemiological studies, with a peak incidence of 20–50%. Sleeplessness is associated with severe daytime distress, irritability, diminished focus, or weariness. Approximately 15% of adults have insomnia2. Insomnia is typically accompanied by a physical or psychological condition. Contrary to the long-held belief that sleeplessness was a marker of other ailments, the relationship between other illnesses and insomnia is complex and occasionally bidirectional2. Risk factors for sleeplessness include severe depression, mental disorders, drug and alcohol abuse, suicidality, high blood pressure, and diabetes3, 4 (Figure 1). As insomnia is associated with deficits in an individual’s quality of life and an increased risk of accidents and falls, therapy should specifically aim to resolve insomnia, including when it occurs concurrently with other medical or mental disorders4.
Although the vast majority of individuals with mental illnesses do not die by suicide, over 90% of those who do die by suicide have a mental disorder at the time of their death5. Therefore, evaluating additional risk factors besides mental illnesses may aid in identifying individuals who are most at risk of engaging in suicidal behavior5.
Several suicidal behaviors, including suicidal thoughts, suicide attempts, and suicidal behavior mortality, have been linked to insomnia symptoms in previous studies. Furthermore, several studies have reported a link between suicidal ideation and dreams6, 7, 8. However, a limited number of studies have investigated whether dreams or sleeplessness symptoms are linked to suicidal thoughts and conduct in the absence of psychiatric disease. Other symptoms include nightmares, depression, anxiety, and suicide ideation9, 10, 11. This review discusses the recently studied genetic aspects involved in insomnia pathogenesis and explores the orexin system and acupuncture as potential therapeutic strategies.
Insomnia
It is crucial to characterize the various phenotypes to identify clinically significant subtypes of insomnia, as this may lessen the heterogeneity of insomnia and make it easier to identify its causes and develop specialized treatments. However, there are few standardized tools available for diagnosing insomnia that enable phenotyping12. Although they achieve more overall hours of sleep, insomniacs have higher mortality rates13. The success of cognitive-behavioral therapy (CBT), therapy outcomes, and concomitant bipolar disorder are all influenced by the 6-hour distinction in sleep duration13. A recent study investigated different types of insomnia based on psychological stress14. Following interviews with 2,224 volunteers, five different types were identified: extremely depressed, moderately distressed but insensitive to positive reinforcement, moderately distressed but highly reactive to their environment and life circumstances, and slightly distressed but with low reactivity. Furthermore, participants were consistently placed in the same category throughout the five-year investigation15.
Prevalence
Insomnia has several potential causes, with some being directly linked to the disease. The two demographic risk factors are age and gender, with the highest incidences seen in women and older adults. Although the reason for the age risk factor is unknown, it may be related to the older population’s propensity for insomnia and the partial functional degradation of sleep regulation mechanisms associated with aging12.
The increasing prevalence of insomnia among elderly individuals is significantly influenced by the presence of concomitant medical disorders. Furthermore, menstruation and menopause onset both increase the prevalence of sleeplessness in women16. Working night or rotational shifts, having comorbid medical conditions, a chronic illness, or having psychiatric conditions are all risk factors for sleeplessness16. Approximately 75–90% of insomniacs are considered to be at an elevated risk for other medical conditions, including those that cause hypoxemia and dyspnea, gastric reflux disease, pain syndromes, and neurodegenerative diseases. It is important to note that a range of primary sleep disorders and circadian rhythm abnormalities frequently coexist with and contribute to insomnia17, 18.
Insomnia symptoms with the highest prevalence include early morning wakeups (2.2%), difficulties falling asleep (7.7%), and nonrestorative sleep (25.2%)19. The difficulty sustaining sleep symptom was the most common symptom (61%). While the incidence of insomnia among working individuals is 23.2%, women are substantially more likely to develop it19. This sex-based difference begins to appear during adolescence and peaks after menopause. In addition to women, individuals with sociological difficulties, poorer health, or lower standards of living are more prone to insomnia20. In longitudinal studies, 40–70% of patients had insomnia that persisted for up to 4 years. While some individuals’ symptoms do not disappear, others may experience a waxing and waning course of insomnia21.
To the best of our knowledge, there is no information regarding the prevalence of insomnia, the contributing variables, and the incidence of adults living in urban and semi-urban areas using sleep aids among Pakistan’s general population, nor in the specific setting of Karachi22, 23. Sleep issues were reported by one-third of our sample at the time of the survey.
This prevalence rate was comparable to those reported in other Asian nations. For instance, samples from Turkey24 and South India both demonstrated a prevalence rate of insomnia or other sleep-related problems of 20% and 34%, respectively. However, our prevalence rate was lower than that of Japan (44.8%) and Hong Kong 19.4% and 39.4%, respectively25.
Insomnia pathophysiology
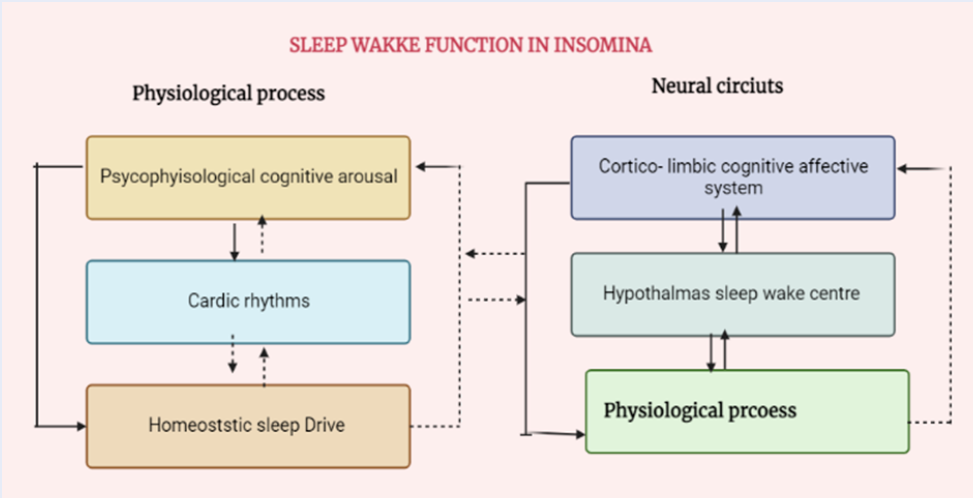
The intricate combination of altered circadian and homeostatic processes and psychological and cognitive arousal can cause insomnia26. Insomnia may also be caused by sleep-wake disorders and switch to work26, 27. Stages N1–N3 are non-REM sleep stages, during which cortical activity is minimal, as REM sleep is characterized by intense brain activity26.
The sleep-wake phase is a complex process that regulates alertness and sleep through a feedback mechanism between opposing systems26, 28.
Wakefulness is mediated by the ascending reticular activation pathway, which involves multiple brainstem and posterior hypothalamic nuclei. This process reaches deep into the cerebral brain. The brainstem and hypothalamic arousal zones receive orexin-producing neurons from the lateral hypothalamus, which functionally encourage their activity during periods of wakefulness29 (Figure 2).
Insomnia Genetics
Seugnet et al. reported 30 isolated Drosophila flies demonstrating rest-activity traits that resembled those of people with insomnia, including shorter rest duration, longer delay to enter a resting state after turning lights off, more fragmented rest intervals, and higher activity levels30, 31. Furthermore, 755 human homologous genes with different expression levels were found in ins-1 flies in comparison to wild-type flies following whole-genome transcription profiling. The study of insomnia genetics may benefit from analyzing the genes connected to metabolism, cell surface interaction, sensory perception, and brain activity in ins-1 flies32.
The classification of insomnia symptom phenotypes using self-report items produces a wide range of heritability estimates. The majority of research on human genetics has focused on a small set of genes33, 34. Family history and twin studies on insomnia that employed stricter areas to detect insomnia have produced more realistic and consistent h2 estimates ranging from 31–58% (h2 = 0–81%)35. The homozygous clock gene and the 5-HTTP short (s-) allele are two examples of gene variations that have been identified through candidate gene research and may be important in insomnia pathophysiology. In a genome-wide association study, several single-nucleotide polymorphisms (SNPs) were shown to be highly associated with insomnia symptoms36. The most significant SNPs were found in genes related to neuroplasticity, neural excitability, and mental health37. In 1972, Bootzin proposed that sleep-related factors (such as a quiet, dark bedroom) might act as selective triggers that encourage sleep, and a lack of these sleep-promoting cues might lead to insomnia. A phone call, reading, or tension are some triggers that prevent sleep. The goal of stimulus management treatment is to identify the cause of sleeplessness so that an individual can sleep separately from these stimuli38.
Recent therapies for insomnia
Despite various criteria employed across research, a considerable amount of identical twin and family literature demonstrates that sleeping problems are somewhat heritable39. Many studies have focused on insomnia features rather than strict insomnia disease characteristics39. Overall, these studies are consistent with the idea that insomnia runs in a family’s genes; however, they have not distinguished between shared genetic and environmental factors40, 41. Twin studies allow for the simultaneous analysis of both genes and shared environments, as twins are presumed to share similar environments. There is a substantially greater body of literature on twins, with over 20 twin studies to date involving insomnia phenotypes. Estimates of insomnia heritability range from 22–59% in adults and 14–71% in children39.
Insomnia-related genes
Laposky et al. investigated mice lacking the essential circadian clock gene, BMAL1/Mop3, which is also known as mop342, 43. These mice displayed altered sleep-wake patterns, including more fragmented sleep, fewer sleep episodes, and changes in overall sleep duration. In a human study, Viola et al. studied the PER3 gene, comparing individuals homozygous for either the short (PER34/4) or long (PER35/5) alleles. Individuals with the long alleles had reduced sleep latency and spent more of the night in slow-wave sleep compared to individuals with the short allele43, 44.
Numerous studies have investigated the links between circadian genes and the sleep-wake cycles of individuals with mood disorders. For example, Serretti et al. discovered a link between sleeplessness symptoms in individuals with severe depression and 3111T/C CLOCK gene allele frequencies. Similarly, Urge et al. studied the correlations between 113 unique SNPs in clock genes and sleep disturbance in patients with depression and control patients in a large cohort study in Finland45, 46. They observed that the classic gene was associated with early morning awakenings in the depressed group; however, its effect varied between men and women46, 47.
The role of serotonin
Serotonin is a well-known neurotransmitter that has a significant impact on the immune system and peripheral tissues. An increasing body of research suggests that several immune cell types, such as T cells, macrophages, mast cells, dendritic cells, and platelets, express the machinery needed to produce, store, react to, and/or transport serotonin48. Brummett et al. investigated the link between sleep patterns and the serotonin gene in dementia caregivers. They discovered a strong gene X environment correlation with caregivers, whereby caregivers with the short allele were more to report poor sleep quality. However, there was no link between caregivers who did not show concern for others49. Monoamine oxidase A (MAO-A) influences serotonin availability in the brain. Two studies have revealed links between MAO-A polymorphisms and insomnia characteristics50.
The role of adenosine
Adenosine receptors have been linked to a variety of diseases, including cancer, immunological and inflammatory disorders, cerebral and heart ischemia, and immune and inflammatory disorders. They minimize overexcitation that could harm the brain and control sleep- and psychiatric-disorder-related systems. Adenosine is assumed to play a role in sleep regulation, and genes that affect adenosine activity may influence sleep/wake phase patterns and, therefore, sleeplessness51. Adenosine deaminase (ADA) gene G/A allele carriers spend longer in slow-wave sleep, wake up less at night, and demonstrate higher strength than G/G variation carriers. Gass et al. examined 117 SNPs from 13 genes associated with adenosine transporters, receptors, and metabolic enzymes in depressed people and a control group52. Polymorphisms in the SLC29A3 gene, which is associated with adenosine metabolism, were only seen in women52.
Recent Therapies for Insomnia
The present guidelines for insomnia treatment include both pharmacological and non-pharmacological therapies53, 54. Numerous approaches are used for non-pharmacological therapy, including CBT, managing external disturbances, and relaxation training, representing both psychological and behavioral treatments for insomnia. Typically, therapy for good sleep hygiene is combined with these therapeutic techniques54.
Benzodiazepines, non-benzodiazepines GABAA receptor stimulators (Z-drugs), melatonin receptor activators, ORAs (suvorexant and Lemborexant), and antidepressants are examples of sleep aids (hypnotics) prescribed to treat insomnia (low-dose doxepin). Benzodiazepines belong to a family of drugs that target several GABAA receptor subtypes54, 55. In the past, prescriptions for benzodiazepines, such as flurazepam, brotizolam, temazepam, triazolam, estazolam, and quazepam, were often given for insomnia54. Although the effectiveness of these drugs has been well established, their usefulness is constrained by side effects, like daytime sedation (such as hangover in the morning or the following day), cognitive impairment (including anterograde amnesia), motor incoordination, abuse potential, and dependence56.
The orexin system as a potential therapeutic target
The neuropeptides orexin A and orexin B, which are essential for the regulation of the brain’s reward and aversion systems, energy balance, and wakefulness, are produced by a limited number of neurons in the lateral hypothalamic area (LHA)57. Prepro-orexin, a common precursor polypeptide, is the source of orexin A and B. Prohormone convertases are thought to be responsible for the proteolytic processing of prepro-orexin58. In projection neurons expressing orexin receptors, endogenous orexins activate the orexin-1 (OX1R) and orexin-2 (OX2R) receptors, two closely linked G protein-coupled receptors (GPCRs). While both OX1R and OX2R bind orexin A and B, OX2R binds with less selective affinity57.
The neurostructural distribution of OX1R and OXR2 supports their crucial functions of boosting alertness and sustaining wakefulness in conditions of high motivational importance, such as physiological stress59. The orexin peptides and their receptors are largely conserved throughout vertebrate species. States of general need, such as hunger, reward possibility, and threat exposure. Regions important for emotion regulation, circadian rhythm, and numerous afferent inputs are all received by orexin neurons in the LHA59, 60 autonomic tone, rhythms, and hunger.
The basal forebrain, corticolimbic structures, and brainstem receive extensive and dense projections of nerve fibers from orexin immunoreactive neurons of the LHA, particularly in areas involved in wake and sleep control61. Orexin-producing neurons are active during wakefulness and dormant during sleep60. Orexin A levels vary according to circadian cycles in the cerebrospinal fluid of several animals, peaking during active waking periods. An imbalance in sleep-wake regulation, consisting of either overactivity of the arousal systems or hypoactivity of the sleep-inducing systems, is thought to be the last common route of insomnia pathophysiology62.
Chinese hemopathy acupuncture as a potential treatment
Traditional Chinese treatments, modern medicine therapies, and other approaches have demonstrated success in insomnia treatment63. The primary medications prescribed for insomnia treatment are diazepam, estazolam, and alprazolam64. Although pharmaceutical treatments for insomnia are effective, their use has been reduced due to concerns about misuse, addiction, dependency, breathing restrictions, and unpleasant reactions. Consequently, many patients try complementary and alternative therapies to treat their insomnia64. Using pins is one of the most successful insomnia therapies64. The ancient Chinese therapy known as acupuncture involves inserting small, solid, metal needles into the skin either manually or using electrical stimulation65. The China Sleep Research Association’s most recent recommendations for the identification and treatment of insomnia in China now include acupuncture as a secure and reliable natural therapy65.
Conclusion
This review highlights the complex nature of insomnia and the need for further research on various aspects of this condition. The findings suggest that the pathophysiology of insomnia involves both environmental and genetic factors. Human genetic studies using better sleep metrics could provide a deeper understanding of the genetic aspect of insomnia pathophysiology. Furthermore, research investigating the relationship between nightmares and suicidal ideation is needed, particularly among different age groups. Prospective studies could be useful for predicting changes in suicidal ideation based on changes in insomnia symptoms or dreams. Diagnoses should be included in future research, as evaluating symptoms alone may not provide a complete picture of insomnia. Future studies could explore the use of emerging techniques, such as diaries, actigraphy, and PSG, to investigate the circuit and local level thalamic dysregulation that contributes to insomnia. Such studies may also help to determine the efficacy of potential therapeutic strategies, such as targeting the orexin system and using acupuncture.
Abbreviations
GABBA: Gamma aminobutyric acid, LHA: lateral hypothalamic areas, MAO A: Monoamine oxidase, OXIR: orexins activate the orexin- 1 receptor, REM: Rapid eye movement
Acknowledgments
Authors are grateful for vice chancellor of University of Narowal for providing support for this study.
Author’s contributions
All authors significantly contributed this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Van Someren
E.J.,
Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiological Reviews.
2021;
101
(3)
:
995-1046
.
View Article PubMed Google Scholar -
Paul
R.H.,
Salminen
L.E.,
Heaps
J.,
Cohen
L.M.,
The Wiley Encyclopedia of Health Psychology. 2021
.
-
Morin
C.M.,
Bootzin
R.R.,
Buysse
D.J.,
Edinger
J.D.,
Espie
C.A.,
Lichstein
K.L.,
Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). Sleep.
2006;
29
(11)
:
1398-414
.
View Article PubMed Google Scholar -
Krystal
A.D.,
Prather
A.A.,
Ashbrook
L.H.,
The assessment and management of insomnia: an update. World Psychiatry ; Official Journal of the World Psychiatric Association (WPA).
2019;
18
(3)
:
337-52
.
View Article PubMed Google Scholar -
Riemann
D.,
Krone
L.B.,
Wulff
K.,
Nissen
C.,
Sleep, insomnia, and depression. Neuropsychopharmacology.
2020;
45
(1)
:
74-89
.
View Article PubMed Google Scholar -
McGirr
A.,
Renaud
J.,
Seguin
M.,
Alda
M.,
Benkelfat
C.,
Lesage
A.,
An examination of DSM-IV depressive symptoms and risk for suicide completion in major depressive disorder: a psychological autopsy study. Journal of Affective Disorders.
2007;
97
(1-3)
:
203-9
.
View Article PubMed Google Scholar -
Woznica
A.A.,
Carney
C.E.,
Kuo
J.R.,
Moss
T.G.,
The insomnia and suicide link: toward an enhanced understanding of this relationship. Sleep Medicine Reviews.
2015;
22
:
37-46
.
View Article PubMed Google Scholar -
McCall
W.V.,
Black
C.G.,
The link between suicide and insomnia: theoretical mechanisms. Current Psychiatry Reports.
2013;
15
(9)
:
389
.
View Article PubMed Google Scholar -
Bos
S.C.,
Macedo
A.F.,
Literature review on Insomnia (2010-2016). Biological Rhythm Research.
2019;
50
(1)
:
94-163
.
View Article Google Scholar -
Chu
C.,
Hom
M.A.,
Rogers
M.L.,
Stanley
I.H.,
Ringer-Moberg
F.B.,
Podlogar
M.C.,
Insomnia and suicide-related behaviors: A multi-study investigation of thwarted belongingness as a distinct explanatory factor. Journal of Affective Disorders.
2017;
208
:
153-62
.
View Article PubMed Google Scholar -
Miller
B.J.,
Parker
C.B.,
Rapaport
M.H.,
Buckley
P.F.,
McCall
W.V.,
Insomnia and suicidal ideation in nonaffective psychosis. Sleep.
2019;
42
(2)
.
View Article PubMed Google Scholar -
Sosso
F.,
Kuss
D.,
Vandelanotte
C.,
Jasso-Medrano
J.,
Husain
M.,
Curcio
G.,
RETRACTED ARTICLE: Insomnia, sleepiness, anxiety and depression among different types of gamers in African countries. Scientific Reports.
2020;
10
(1)
:
1-12
.
View Article PubMed Google Scholar -
Wallace
M.L.,
Lee
S.,
Hall
M.H.,
Stone
K.L.,
Langsetmo
L.,
Redline
S.,
MrOS
Research Groups
SOF,
Heightened sleep propensity: a novel and high-risk sleep health phenotype in older adults. Sleep Health.
2019;
5
(6)
:
630-8
.
View Article PubMed Google Scholar -
Blanken
T.F.,
Benjamins
J.S.,
Borsboom
D.,
Vermunt
J.K.,
Paquola
C.,
Ramautar
J.,
Insomnia disorder subtypes derived from life history and traits of affect and personality. The Lancet. Psychiatry.
2019;
6
(2)
:
151-63
.
View Article PubMed Google Scholar -
Bondar
J.,
Caye
A.,
Chekroud
A.M.,
Kieling
C.,
Symptom clusters in adolescent depression and differential response to treatment: a secondary analysis of the Treatment for Adolescents with Depression Study randomised trial. The Lancet. Psychiatry.
2020;
7
(4)
:
337-43
.
View Article PubMed Google Scholar -
Zeng
L.N.,
Zong
Q.Q.,
Yang
Y.,
Zhang
L.,
Xiang
Y.F.,
Ng
C.H.,
Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Frontiers in Psychiatry.
2020;
11
:
577429
.
View Article PubMed Google Scholar -
Katz
D.A.,
McHorney
C.A.,
Clinical correlates of insomnia in patients with chronic illness. Archives of Internal Medicine.
1998;
158
(10)
:
1099-107
.
View Article PubMed Google Scholar -
Phillips
B.,
Hening
W.,
Britz
P.,
Mannino
D.,
Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest.
2006;
129
(1)
:
76-80
.
View Article PubMed Google Scholar -
Walsh
J.K.,
Coulouvrat
C.,
Hajak
G.,
Lakoma
M.D.,
Petukhova
M.,
Roth
T.,
Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep.
2011;
34
(8)
:
997-1011
.
View Article PubMed Google Scholar -
Salari
N.,
Darvishi
N.,
Khaledi-Paveh
B.,
Vaisi-Raygani
A.,
Jalali
R.,
Daneshkhah
A.,
A systematic review and meta-analysis of prevalence of insomnia in the third trimester of pregnancy. BMC Pregnancy and Childbirth.
2021;
21
(1)
:
284
.
View Article PubMed Google Scholar -
Ellis
J.G.,
Perlis
M.L.,
Neale
L.F.,
Espie
C.A.,
Bastien
C.H.,
The natural history of insomnia: focus on prevalence and incidence of acute insomnia. Journal of Psychiatric Research.
2012;
46
(10)
:
1278-85
.
View Article PubMed Google Scholar -
Kidwai
R.,
Ahmed
S.H.,
Prevalence of insomnia and use of sleep medicines in urban communities of Karachi, Pakistan. JPMA. The Journal of the Pakistan Medical Association.
2013;
63
(11)
:
1358-63
.
PubMed Google Scholar -
Chowdhury
A.I.,
Ghosh
S.,
Hasan
M.F.,
Khandakar
K.A.,
Azad
F.,
Prevalence of insomnia among university students in South Asian Region: a systematic review of studies. Journal of Preventive Medicine and Hygiene.
2021;
61
(4)
:
525-9
.
PubMed Google Scholar -
Aslan
S.,
Gulcat
Z.,
Selda Albayrak
F.,
Maral
I.,
Yetkin
S.,
Sutcigil
L.,
Prevalence of insomnia symptoms: results from an urban district in Ankara, Turkey 1. International Journal of Psychiatry in Clinical Practice.
2006;
10
(1)
:
52-8
.
View Article PubMed Google Scholar -
Asai
T.,
Kaneita
Y.,
Uchiyama
M.,
Takemura
S.,
Asai
S.,
Yokoyama
E.,
Epidemiological study of the relationship between sleep disturbances and somatic and psychological complaints among the Japanese general population. Sleep and Biological Rhythms.
2006;
4
(1)
:
55-62
.
View Article Google Scholar -
Fuller
P.M.,
Gooley
J.J.,
Saper
C.B.,
Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. Journal of Biological Rhythms.
2006;
21
(6)
:
482-93
.
View Article PubMed Google Scholar -
Dopheide
J.A.,
Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. The American Journal of Managed Care.
2020;
26
(4)
:
76-84
.
PubMed Google Scholar -
Jhawar
S.,
Krishna
G.G.,
Chikkanna
U.,
Understanding the pathophysiology of insomnia (Anidra) with special reference to primary insomnia using neurotransmitter sleep theories: a narrative review. Journal of Indian System of Medicine.
2022;
10
(1)
:
27
.
View Article Google Scholar -
Saper
C.B.,
Fuller
P.M.,
Pedersen
N.P.,
Lu
J.,
Scammell
T.E.,
Sleep state switching. Neuron.
2010;
68
(6)
:
1023-42
.
View Article PubMed Google Scholar -
Kelly
J.M.,
Bianchi
M.T.,
Mammalian sleep genetics. Neurogenetics.
2012;
13
(4)
:
287-326
.
View Article Google Scholar -
Lane
J.M.,
Jones
S.E.,
Dashti
H.S.,
Wood
A.R.,
Aragam
K.G.,
van Hees
V.T.,
All In Sleep
HUNT,
Biological and clinical insights from genetics of insomnia symptoms. Nature Genetics.
2019;
51
(3)
:
387-93
.
View Article PubMed Google Scholar -
Seugnet
L.,
Suzuki
Y.,
Thimgan
M.,
Donlea
J.,
Gimbel
S.I.,
Gottschalk
L.,
Identifying sleep regulatory genes using a Drosophila model of insomnia. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience.
2009;
29
(22)
:
7148-57
.
View Article PubMed Google Scholar -
Gehrman
P.R.,
Meltzer
L.J.,
Moore
M.,
Pack
A.I.,
Perlis
M.L.,
Eaves
L.J.,
Heritability of insomnia symptoms in youth and their relationship to depression and anxiety. Sleep.
2011;
34
(12)
:
1641-6
.
View Article PubMed Google Scholar -
Baranova
A.,
Cao
H.,
Zhang
F.,
Shared genetic liability and causal effects between major depressive disorder and insomnia. Human Molecular Genetics.
2022;
31
(8)
:
1336-45
.
View Article PubMed Google Scholar -
Wing
Y.K.,
Zhang
J.,
Lam
S.P.,
Li
S.X.,
Tang
N.L.,
Lai
K.Y.,
Familial aggregation and heritability of insomnia in a community-based study. Sleep Medicine.
2012;
13
(8)
:
985-90
.
View Article PubMed Google Scholar -
Deuschle
M.,
Schredl
M.,
Schilling
C.,
Wüst
S.,
Frank
J.,
Witt
S.H.,
Association between a serotonin transporter length polymorphism and primary insomnia. Sleep.
2010;
33
(3)
:
343-7
.
View Article PubMed Google Scholar -
Ban
H.J.,
Kim
S.C.,
Seo
J.,
Kang
H.B.,
Choi
J.K.,
Genetic and metabolic characterization of insomnia. PLoS One.
2011;
6
(4)
:
e18455
.
View Article PubMed Google Scholar -
Traupman
E.K.,
Dixon
M.A.,
Cognitive-behavioral therapy for insomnia for primary care: review of components and application for residents in primary care. International Journal of Psychiatry in Medicine.
2022;
57
(5)
:
423-33
.
View Article PubMed Google Scholar -
Gehrman
P.R.,
Byrne
E.,
Gillespie
N.,
Martin
N.G.,
Genetics of insomnia. Sleep Medicine Clinics.
2011;
6
(2)
:
191-202
.
View Article Google Scholar -
Barclay
N.L.,
Gregory
A.M.,
Quantitative genetic research on sleep: a review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Medicine Reviews.
2013;
17
(1)
:
29-40
.
View Article PubMed Google Scholar -
Abe
K.,
Shimakawa
M.,
Genetic-constitutional factor and childhood insomnia. Psychiatria et Neurologia.
1966;
152
(6)
:
363-9
.
View Article PubMed Google Scholar -
Laposky
A.,
Easton
A.,
Dugovic
C.,
Walisser
J.,
Bradfield
C.,
Turek
F.,
Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep.
2005;
28
(4)
:
395-409
.
View Article PubMed Google Scholar -
Li
T.,
Liu
Z.,
Wang
Y.,
Zuo
D.,
Wang
S.,
Ju
H.,
Multiplexed Visualization Method to Explore Complete Targeting Regulatory Relationships Among Circadian Genes for Insomnia Treatment. Frontiers in Neuroscience.
2022;
16
:
877802
.
View Article PubMed Google Scholar -
Viola
A.U.,
Archer
S.N.,
James
L.M.,
Groeger
J.A.,
Lo
J.C.,
Skene
D.J.,
PER3 polymorphism predicts sleep structure and waking performance. Current Biology.
2007;
17
(7)
:
613-8
.
View Article PubMed Google Scholar -
Serretti
A.,
Benedetti
F.,
Mandelli
L.,
Lorenzi
C.,
Pirovano
A.,
Colombo
C.,
Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics.
2003;
121B
(1)
:
35-8
.
View Article PubMed Google Scholar -
Utge
S.J.,
Soronen
P.,
Loukola
A.,
Kronholm
E.,
Ollila
H.M.,
Pirkola
S.,
Systematic analysis of circadian genes in a population-based sample reveals association of TIMELESS with depression and sleep disturbance. PLoS One.
2010;
5
(2)
:
e9259
.
View Article PubMed Google Scholar -
Bin Heyat
M.B.,
Akhtar
F.,
Ansari
M.A.,
Khan
A.,
Alkahtani
F.,
Khan
H.,
Progress in detection of insomnia sleep disorder: a comprehensive review. Current Drug Targets.
2021;
22
(6)
:
672-84
.
View Article PubMed Google Scholar -
Wu
H.,
Denna
T.H.,
Storkersen
J.N.,
Gerriets
V.A.,
Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacological Research.
2019;
140
:
100-14
.
View Article PubMed Google Scholar -
Brummett
B.H.,
Krystal
A.D.,
Ashley-Koch
A.,
Kuhn
C.M.,
Züchner
S.,
Siegler
I.C.,
Sleep quality varies as a function of 5-HTTLPR genotype and stress. Psychosomatic Medicine.
2007;
69
(7)
:
621-4
.
View Article PubMed Google Scholar -
Craig
D.,
Hart
D.J.,
Passmore
A.P.,
Genetically increased risk of sleep disruption in Alzheimer's disease. Sleep.
2006;
29
(8)
:
1003-7
.
View Article PubMed Google Scholar -
van Calker
D.,
Biber
K.,
Domschke
K.,
Serchov
T.,
The role of adenosine receptors in mood and anxiety disorders. Journal of Neurochemistry.
2019;
151
(1)
:
11-27
.
View Article PubMed Google Scholar -
Rétey
J.V.,
Adam
M.,
Honegger
E.,
Khatami
R.,
Luhmann
U.F.,
Jung
H.H.,
A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proceedings of the National Academy of Sciences of the United States of America.
2005;
102
(43)
:
15676-81
.
View Article PubMed Google Scholar -
Chung
K.F.,
Lee
C.T.,
Yeung
W.F.,
Chan
M.S.,
Chung
E.W.,
Lin
W.L.,
Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Family Practice.
2018;
35
(4)
:
365-75
.
View Article PubMed Google Scholar -
Muehlan
C.,
Vaillant
C.,
Zenklusen
I.,
Kraehenbuehl
S.,
Dingemanse
J.,
Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opinion on Drug Metabolism & Toxicology.
2020;
16
(11)
:
1063-78
.
View Article PubMed Google Scholar -
Pollack
M.H.,
Hoge
E.A.,
Worthington
J.J.,
Moshier
S.J.,
Wechsler
R.S.,
Brandes
M.,
Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: a randomized, double-blind, placebo-controlled trial. The Journal of Clinical Psychiatry.
2011;
72
(7)
:
892-7
.
View Article PubMed Google Scholar -
Buscemi
N.,
Vandermeer
B.,
Friesen
C.,
Bialy
L.,
Tubman
M.,
Ospina
M.,
The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. Journal of General Internal Medicine.
2007;
22
(9)
:
1335-50
.
View Article PubMed Google Scholar -
Gunn
H.E.,
Tutek
J.,
Buysse
D.J.,
Brief behavioral treatment of insomnia. Sleep Medicine Clinics.
2019;
14
(2)
:
235-43
.
View Article PubMed Google Scholar -
Kilduff
T.S.,
Peyron
C.,
The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends in Neurosciences.
2000;
23
(8)
:
359-65
.
View Article PubMed Google Scholar -
Venner
A.,
Karnani
M.M.,
Gonzalez
J.A.,
Jensen
L.T.,
Fugger
L.,
Burdakov
D.,
Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. The Journal of Physiology.
2011;
589
(Pt 23)
:
5701-8
.
View Article PubMed Google Scholar -
Taddei-Allen
P.,
Economic burden and managed care considerations for the treatment of insomnia. The American Journal of Managed Care.
2020;
26
(4)
:
91-6
.
PubMed Google Scholar -
Riemann
D.,
Spiegelhalder
K.,
Feige
B.,
Voderholzer
U.,
Berger
M.,
Perlis
M.,
The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Medicine Reviews.
2010;
14
(1)
:
19-31
.
View Article PubMed Google Scholar -
Levenson
J.C.,
Kay
D.B.,
Buysse
D.J.,
The pathophysiology of insomnia. Chest.
2015;
147
(4)
:
1179-92
.
View Article PubMed Google Scholar -
Kalavapalli
R.,
Singareddy
R.,
Role of acupuncture in the treatment of insomnia: a comprehensive review. Complementary Therapies in Clinical Practice.
2007;
13
(3)
:
184-93
.
View Article PubMed Google Scholar -
He
W.,
Li
M.,
Zuo
L.,
Wang
M.,
Jiang
L.,
Shan
H.,
Acupuncture for treatment of insomnia: an overview of systematic reviews. Complementary Therapies in Medicine.
2019;
42
:
407-16
.
View Article PubMed Google Scholar -
J. LIN,
L.H. Pao,
S.Y. Su,
S.S. Huang,
J.G. Lin,
Y.C. Cheng,
J. Chao,
Traditional Chinese Medicine Formulas against Insomnia: A Systematic Review and Meta-analysis. Researchsquare.
2022;
:
Epub
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 4 (2023)
Page No.: 5630-5637
Published on: 2023-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 5518 times
- PDF downloaded - 1412 times
- XML downloaded - 124 times
 Biomedpress
Biomedpress




