Abstract
Background: Glioblastoma is a grade IV glioma tumor, and is the most frequent neoplasia in the central nervous system. Patients with glioblastoma have a very low overall survival rate and poor prognosis. Their major therapeutic challenges are the limited penetration of therapeutic agents through the blood-brain barrier, increased treatment resistance, and tumor heterogeneity.
Case presentation: Anti-programmed cell death 1 (PD-1) immune checkpoint blockade-based therapy using pembrolizumab achieved good stability and non-recurrence in a Kurdish patient with highgrade glioblastoma who was refractory to first-line therapy.
Conclusion: It appears that after the failure of routine and standard treatments in patients with glioblastoma, an immunotherapy-based therapeutic strategy is suitable for improving their clinical outcomes and creating antitumor effects.
INTRODUCTION
Glioma is a general term used to describe tumors originating from glial cells in the supporting tissue of the brain or primary brain tumors1. Glioblastoma multiform (GBM) is the most common malignant type of central nervous system (CNS) tumor, with about 48.6% of malignant tumors arising in this region2. These tumors are frequently formed in the brain hemispheres, and less often in the cerebellum, brain stem, and spinal cord3. The World Health Organization (WHO) grading system categorizes gliomas as grades I to IV based on histopathological criteria reflecting their malignancy4. Glioblastomas are graded based on their response to treatment, malignancy type, and potential for proliferation as (I) the lowest proliferative power and response to surgical treatment, (II) diffuse astrocytoma, (III) anaplastic astrocytoma, (IV) GBM5.
Regarding epidemiology, GBM has a low prognosis and can occur at any age. However, it occurs often in those aged 55–60 years and is more frequent in men. In addition, the post-diagnosis survival rate is 14–15 months6. Moreover, complete and accurate information about its causes remains unavailable, and its specific carcinogens have not been identified. The only known risk factor is exposure to high doses of ionizing radiation7. However, genetic predisposition has been reported in 5%–10% of all cases8.
The treatment response will differ based on the glioma grade. However, GBMs do not respond well to standard treatments such as surgery and chemotherapy due to the limited penetration of therapeutic agents through the blood-brain barrier and increased drug resistance. Previous studies have shown that immunotherapy targeting the pathway involving programmed cell death 1 (PD-1) and its ligand (PD-L1) can be used to treat GBM9. In this case report, we present a patient with grade IV glioblastoma who was unresponsive to routine treatments but showed promising results with anti-PD-1 immune checkpoint blockade-based immunotherapy.
CASE PRESENTATION
In July 2022, a 67-year-old Kurdish woman without a medical history was referred to Kermanshah Hematology and Oncology Clinic for headache, confusion, and numbness. The patient had no relevant clinical history. Following a physical examination, the physician requested a magnetic resonance imaging scan of her head. It showed a right parietal brain lesion, 45 × 40 mm in size. Since these findings indicated a glioma tumor, we performed an open biopsy for a definitive diagnosis. Microscopic evaluation of the biopsy sample revealed an infiltrative, diffuse growth pattern of glioma cells, and the immunohistochemistry (IHC) findings for glial fibrillary acidic protein (GFAP) were positive. Based on these results, the patient was diagnosed with grade IV GBM. Therefore, the patient was concomitantly treated with radiation therapy (30 sessions) and chemotherapy with temozolomide (250 mg). Then, a surgical procedure was performed in the parietal brain region to remove the lesion.
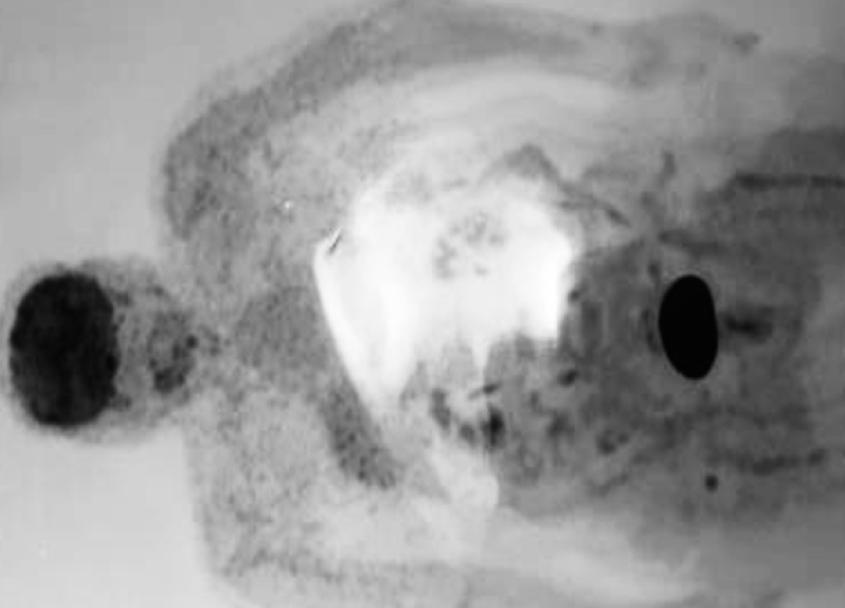
A fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan performed four months after surgery showed disease progression and worsened symptoms (Figure 1). Tumor regrowth was not inhibited, and a second open biopsy was performed. The IHC analysis of PD-L1 was positive, indicating an ideal candidate for immunotherapy (Figure 2). Therefore, the patient underwent immunotherapy with pembrolizumab (200 mg) and a combination of bevacizumab (400 mg) and irinotecan (300 mg) at three-week intervals. After the third immunotherapy cycle, the patient was stable and is being monitored regularly.
DISCUSSION
GBM is an aggressive tumor and the most common cancer of the CNS. It is thought to be caused by genetic abnormalities that affect neuroglial stem or progenitor cells10. Grade IV is the aggressive GBM stage, characterized by microvascular proliferation, necrosis, or both11. Therapeutic options for these patients include surgery, combination chemotherapy, and radiotherapy. However, their effectiveness is decreased due to the tumor’s metastatic and aggressive nature12. Nevertheless, immunotherapy using immune checkpoint inhibitors has opened a new window for treating these patients. As modulators of the immune response, immune checkpoints control immune system function by balancing inhibitory and stimulatory signals. In addition to preserving immune homeostasis, they also prevent autoimmunity13. Moreover, checkpoint inhibitors have been successfully approved to treat 12 types of cancerous tumors14.
Two critical immune checkpoints identified are cytotoxic T lymphocyte antigen-4 (CTLA-4) and PD-1/PD-L1. PD-L1 is encoded by the PDCDL1 gene located at p24.1 on chromosome 9. PD-L1 is a type I membrane protein activated by binding to the PD-1 receptor10. They play an essential role in tumor cells escaping the immune system15 because PD-1 is expressed as a co-inhibitory receptor on the surface of activated CD4+ or CD8+ T cells, and PD-L1 is expressed by various cells, including tumors. Therefore, their interaction allows tumor cells to survive in the natural physiological environment of the body16. Studies have shown that PD-L1 expression in glioma cells correlates with glioblastoma grading under the WHO criteria and can be considered a diagnostic biomarker for glioma cells17. It can also be very useful in targeted treatments and disease management.
Anti-PD-1 immune checkpoint inhibitors enhance the antitumor immune response in patients18. This new immunotherapy-based therapeutic strategy has been investigated in various preclinical- or clinical-stage cancers, such as melanoma, Hodgkin’s lymphoma, breast cancer, and non-small cell lung cancer (NSCLC)19, 20, 21. Therapy resistance and tumor heterogeneity are considered key challenges for the clinical application of this strategy since previous studies showed that only about 50% of patients with PD-L1 overexpression might respond adequately to it22. However, monoclonal antibodies against PD-1 (nivolumab, pembrolizumab, and cemiplimab) or PD-L1 (atezolizumab, durvalumab, and avelumab) have been approved by the US Food and Drug Administration (FDA) to treat different cancers23. The goal of using PD-1/PD-L1 inhibitors is to disrupt the interaction between PD-1 and PD-L1, preventing immune system escape and normalizing the induction of the apoptotic pathway in tumor cells (Figure 3).
Our patient was diagnosed with high-grade glioblastoma. After the failure of the first-line treatment (surgery combined with temozolomide and bevacizumab chemotherapy), IHC analysis showed PD-L1 positivity, making her a potential candidate for immunotherapy. Therefore, she underwent anti-PD-1 immunotherapy using pembrolizumab (Keytruda), which showed very promising results. Pembrolizumab is a humanized anti-PD-1 monoclonal antibody that has shown favorable anti-cancer activity. The FDA has approved it to treat resistant and metastatic tumors and those with microsatellite instability24. In addition, it has shown a long-term response advantage and a high overall survival rate during five-year follow-up compared to chemotherapy for some cancers, such as PD-L1 positive metastatic NSCLC25. Furthermore, studies have shown that patients with glioblastoma who received neoadjuvant pembrolizumab after surgery had increased OS, overexpressed interferon-γ, and induced PD-L1 in the tumor microenvironment26.
It is crucial to note that our patient had the following distinctive characteristics:
1- High-grade glioblastoma (IV)
2- Older age (67 years)
3- Kurdish ethnicity
4- A positive response to treatment with an appropriate pembrolizumab dose (200 mg)
5- High PD-L1 expression after the failure of first-line therapy with surgery and chemotherapy
CONCLUSIONS
Our study provides important evidence of pembrolizumab’s efficacy in patients with high-grade glioblastoma overexpressing PD-L1. After the failure of first-line treatment or resistance to therapy in these patients, pembrolizumab appears useful for improving clinical outcomes and creating relatively long-lasting antitumor effects. Therefore, our results highlight the importance of further prospective studies with larger volumes.
Abbreviations
CNS: Central nervous system, CTLA-4: Cytotoxic T lymphocyte antigen-4, FDA: Food and drug administration, FDG: Fluorodeoxyglucose, GBM: Glioblastoma, GFAP: Glial fibrillary acidic protein, IHC: Immunohistochemistry, MSI: Microsatellite instability, NSCLC: Non-small cell lung cancer, OS: Overall survival, PD-1: Programmed cell death protein-1, PD-L1: Programmed cell death-ligand1, WHO: World health organization.
Acknowledgments
The authors are grateful to all colleagues in the Clinic of Hematology and Oncology.
Author’s contributions
Mehrdad Payandeh & Noorodin Karami: Literature search, Clinical studies, Data acquisition, Data analysis, Guarantor; Noorodin Karami, Mahboobeh Jafari: Manuscript review, Figure design; Afshin Karami: Concepts, Design, Definition of intellectual content, Manuscript preparation; Samira Asadollahi & Sareh Bakhshandeh Bavarsad: Manuscript editing, Referencing by EndNote, Manuscript preparation, Literature search. All authors read and confirmed the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
The procedures have been reviewed and approved by the ethics committee. All procedures were in accordance with the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Agnihotri
S.,
Burrell
K.E.,
Wolf
A.,
Jalali
S.,
Hawkins
C.,
Rutka
J.T.,
Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Archivum Immunologiae et Therapiae Experimentalis.
2013;
61
(1)
:
25-41
.
View Article PubMed Google Scholar -
Baskin
D.S.,
Sharpe
M.A.,
Nguyen
L.,
Helekar
S.A.,
Case Report: End-Stage Recurrent Glioblastoma Treated With a New Noninvasive Non-Contact Oncomagnetic Device. Frontiers in Oncology.
2021;
11
:
708017
.
View Article PubMed Google Scholar -
Esfahani
H.,
Dehghan
A.,
Sami
G.,
Eskandari
N.,
Glioblastoma Multiforme in a nine-year-old girl: a case report. Iranian Journal of Pediatric Hematology and Oncology.
2019;
9
(2)
:
131-4
.
View Article Google Scholar -
Louis
D.N.,
Ohgaki
H.,
Wiestler
O.D.,
Cavenee
W.K.,
Burger
P.C.,
Jouvet
A.,
The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica.
2007;
114
(2)
:
97-109
.
View Article PubMed Google Scholar -
Jov\vcevska
I.,
Ko\vcevar
N.,
Komel
R.,
Glioma and glioblastoma - how much do we (not) know?. Molecular and Clinical Oncology.
2013;
1
(6)
:
935-41
.
View Article PubMed Google Scholar -
Grochans
S.,
Cybulska
A.M.,
Simińska
D.,
Korbecki
J.,
Kojder
K.,
Chlubek
D.,
Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers (Basel).
2022;
14
(10)
:
2412
.
View Article PubMed Google Scholar -
Bondy
M.L.,
Scheurer
M.E.,
Malmer
B.,
Barnholtz-Sloan
J.S.,
Davis
F.G.,
Il'yasova
D.,
Brain Tumor Epidemiology Consortium
Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer.
2008;
113
(7)
:
1953-68
.
View Article PubMed Google Scholar -
Roviello
G.,
Petrioli
R.,
Cerase
A.,
Marsili
S.,
Miracco
C.,
Rubino
G.,
A husband and a wife with simultaneous presentation of glioblastoma multiforme: a case report. Case Reports in Oncology.
2013;
6
(3)
:
538-43
.
View Article PubMed Google Scholar -
Lee
G.A.,
Lin
W.L.,
Kuo
D.P.,
Li
Y.T.,
Chang
Y.W.,
Chen
Y.C.,
Detection of pd-l1 expression in temozolomide-resistant glioblastoma by using pd-l1 antibodies conjugated with lipid-coated superparamagnetic iron oxide. International Journal of Nanomedicine.
2021;
16
(July)
:
5233-46
.
View Article PubMed Google Scholar -
Wesseling
P.,
Capper
D.,
WHO 2016 Classification of gliomas. Neuropathology and Applied Neurobiology.
2018;
44
(2)
:
139-50
.
View Article PubMed Google Scholar -
Batash
R.,
Asna
N.,
Schaffer
P.,
Francis
N.,
Schaffer
M.,
Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Current Medicinal Chemistry.
2017;
24
(27)
:
3002-9
.
View Article PubMed Google Scholar -
Karami
A.,
Hossienpour
M.,
Mohammadi Noori
E.,
Rahpyma
M.,
Najafi
K.,
Kiani
A.,
Synergistic Effect of Gefitinib and Temozolomide on U87MG Glioblastoma Angiogenesis. Nutrition and Cancer.
2022;
74
(4)
:
1299-307
.
View Article PubMed Google Scholar -
Marin-Acevedo
J.A.,
Dholaria
B.,
Soyano
A.E.,
Knutson
K.L.,
Chumsri
S.,
Lou
Y.,
Next generation of immune checkpoint therapy in cancer: new developments and challenges. Journal of Hematology {&}amp; Oncology.
2018;
11
(1)
:
39
.
View Article PubMed Google Scholar -
La-Beck
N.M.,
Jean
G.W.,
Huynh
C.,
Alzghari
S.K.,
Lowe
D.B.,
Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy.
2015;
35
(10)
:
963-76
.
View Article PubMed Google Scholar -
Blank
C.,
Gajewski
T.F.,
Mackensen
A.,
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunology, Immunotherapy.
2005;
54
(4)
:
307-14
.
View Article PubMed Google Scholar -
Yu
W.,
Shao
A.,
Ren
X.,
Chen
Z.,
Xu
J.,
Wei
Q.,
Comparison of Immune Checkpoint Molecules PD-1 and PD-L1 in Paired Primary and Recurrent Glioma: Increasing Trend When Recurrence. Brain Sciences.
2022;
12
(2)
:
266
.
View Article PubMed Google Scholar -
Wilmotte
R.,
Burkhardt
K.,
Kindler
V.,
Belkouch
M.C.,
Dussex
G.,
Tribolet
N.,
B7-homolog 1 expression by human glioma: a new mechanism of immune evasion. Neuroreport.
2005;
16
(10)
:
1081-5
.
View Article PubMed Google Scholar -
Restrepo
P.,
Yong
R.,
Laface
I.,
Tsankova
N.,
Nael
K.,
Akturk
G.,
Tumoral and immune heterogeneity in an anti-PD-1-responsive glioblastoma: a case study. Cold Spring Harbor Molecular Case Studies.
2020;
6
(2)
:
a004762
.
View Article PubMed Google Scholar -
Sun
J.,
Zhang
D.,
Wu
S.,
Xu
M.,
Zhou
X.,
Lu
X.,
Resistance to PD-1 / PD-L1 blockade cancer immunotherapy : mechanisms , predictive factors , and future perspectives. Frontiers in Pharmacology.
2020;
2020
(11)
:
441
.
View Article Google Scholar -
Betof Warner
A.,
Palmer
J.S.,
Shoushtari
A.N.,
Goldman
D.A.,
Panageas
K.S.,
Hayes
S.A.,
Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade. Journal of Clinical Oncology.
2020;
38
(15)
:
1655-63
.
View Article PubMed Google Scholar -
Garon
E.B.,
Hellmann
M.D.,
Rizvi
N.A.,
Carcereny
E.,
Leighl
N.B.,
Ahn
M.J.,
Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. Journal of Clinical Oncology.
2019;
37
(28)
:
2518-27
.
View Article PubMed Google Scholar -
M. Reck,
D. Rodríguez-Abreu,
A.G. Robinson,
R. Hui,
T. Csőszi,
A. Fülöp,
M. Gottfried,
N. Peled,
A. Tafreshi,
S. Cuffe,
M. O’Brien,
Pembrolizumab versus chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England Journal of Medicine.
2016;
2016
(375)
:
1823-33
.
View Article Google Scholar -
Chen
S.,
Zhang
Z.,
Zheng
X.,
Tao
H.,
Zhang
S.,
Ma
J.,
Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Frontiers in Oncology.
2021;
11
(April)
:
562315
.
View Article PubMed Google Scholar -
Reardon
D.A.,
Kim
T.M.,
Frenel
J.S.,
Simonelli
M.,
Lopez
J.,
Subramaniam
D.S.,
Treatment with pembrolizumab in programmed death ligand 1-positive recurrent glioblastoma: results from the multicohort phase 1 KEYNOTE-028 trial. Cancer.
2021;
127
(10)
:
1620-9
.
View Article PubMed Google Scholar -
Reck
M.,
Rodriguez-Abreu
D.,
Robinson
A.G.,
Hui
R.,
Csoszi
T.,
Fulop
A.,
Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score > 50%. Journal of Clinical Oncology.
2021;
39
(21)
:
2339-49
.
View Article PubMed Google Scholar -
Cloughesy
T.F.,
Mochizuki
A.Y.,
Orpilla
J.R.,
Hugo
W.,
Lee
A.H.,
Davidson
T.B.,
Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nature Medicine.
2019;
25
(3)
:
477-86
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 5 (2023)
Page No.: 5680-5685
Published on: 2023-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3081 times
- PDF downloaded - 1160 times
- XML downloaded - 96 times
 Biomedpress
Biomedpress




