Abstract
Introduction: After nearly two years, there is still no proven treatment for infection with severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)?the virus that causes Covid-19. Currently, the two most widely known drugs for treating Covid-19 are remdesivir and favipiravir. Therefore, this study aimed to evaluate the effects of remdesivir and favipiravir on Covid-19 clinical outcomes.
Methods: A systematic review of the literature on the PubMed and Scopus databases was undertaken to identify studies that have examined the effects of remdesivir and favipiravir on Covid-19 outcomes. To weighted group mean differences for within- and between-group comparisons, odds ratio effect sizes, and random-effects models were used. Subgroup analyses were also conducted to determine the effects of potential sources of heterogeneity, which was assessed using the I-squared (I2) test.
Results: Twenty-eight studies with a total of 10,871 adult participants were included in the analysis. According to pooled analysis results, there was no statistically significant difference between the remdesivir/favipiravir and control groups in terms of mortality, intensive care unit admissions, or adverse effects (p > 0.05). Mean hospitalization duration was significantly different for those receiving remdesivir (0.1-day increase) and favipiravir (0.06-day decrease), but these findings included significant levels of publication bias. Treatment duration was found to be a significant source of heterogeneity in the mortality results.
Conclusion: Remdesivir and favipiravir have no effect on mortality, intensive care unit admissions, or duration of hospitalization for Covid-19 patients.
Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) was first reported in late December 2019 in Wuhan, China, and has since spread globally1. By October 2021, more than 237 million people had been infected by the virus that causes Covid-19 and approximately 4.5 million people had died from their infections2. The Covid-19 pandemic is an ongoing global health crisis that requires immediate attention to quickly find an appropriate treatment to reduce global mortality and morbidity associated with the disease. Currently, drugs including arbidol3, ribavirin4, chloroquine or hydroxychloroquine, lopinavir/ritonavir, remdesivir, and favipiravir are among those used to treat the infection experimentally. There is no known cure for SARS-CoV-2 infection5, although there are some effective treatments. Specifically, convalescent plasma6, interleukin (IL)-1 or IL-6 inhibitors7, and interferons3 have been used as supportive therapy. Medications given to COVID-19 patients include antimalarial drugs such as chloroquine and hydroxychloroquine, which are also used to treat autoimmune diseases8, while lopinavir/ritonavir is an FDA-approved HIV treatment drug9. Gilead Science collaborated with the US Centers for Disease Control and Prevention (CDC) and the US Army Medical Research Institute of Infectious Diseases to develop remdesivir, an intravenous adenosine nucleotide analog prodrug with activity against several RNA viruses10, 11. Similarly, favipiravir is an antiviral that works against viruses containing RNA. Toyama Chemical Company was the first to approve this drug, which was used to treat influenza in Japan and China3, 12, 13, 14, 15.
The Solidarity World Health Organization International Trial was a collaborative effort to find potential treatments for Covid-19 that involved 52 countries. Drugs that were investigated included remdesivir, hydroxychloroquine, lopinavir, and interferon, of which remdesivir, hydroxychloroquine, lopinavir, and interferon were found to be ineffective or have little effect for the treatment of Covid-19 hospitalized patients16. In contrast, according to the findings of another review study, there was a higher rate of improvement in patients who received remdesivir than in those who received a placebo; however, there was no difference in the 14-day mortality rate17. Another review found that remdesivir significantly reduced recovery time and the occurrence of side effects, but was ineffective in treating the disease if used alone. Hence, there was improved performance when remdesivir was combined with other antiviral drugs18. Favipiravir was found to be effective in treating patients with mild to moderate disease only19.
Covid-19 is treated with antiviral drugs and supportive therapies, and numerous studies and clinical trials have been carried out to confirm the effectiveness of the drugs in combating infection. Therefore, this study aims to support the development and implementation of effective treatments for Covid-19 and analyze the results of published studies investigating the use of either remdesivir or favipiravir in COVID-19 patients to clarify their efficacy in relation to different patient outcomes.
Methods
Research Design
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines20, 21. A quality check was conducted using the Critical Appraisal Skills Program checklist for randomized control trials (RCTs) and cohort studies22, 23.
Search Strategy
Three authors independently searched the MEDLINE (PubMed) and Scopus databases for published articles. The search strategy was guided by the keywords "COVID-19," "remdesivir," and "favipiravir". A complete list of the keywords used for the search is presented in the appendix. Case-control, cohort, and RTCs were included in the searches. All of the articles were examined and there was no limitation according to study time or location. The population, intervention type, and study comparison criteria were adjusted to determine study inclusion and exclusion criteria.
Inclusion and Exclusion Criteria
The inclusion criteria were:
1) Studies using case-control, cohort, or RCT designs;
2) COVID-19 patients with positive laboratory tests;
3) Remdesivir and/or favipiravir having been administered to the treatment/intervention group;
4) Any medicines other than Remdesivir and Favipiravir in the control group; and
5) Disease and treatment-related outcomes were measured.
Case reports, reviews, animal research, in silico and in vitro studies, as well as articles with full texts that were unavailable (after contacting the authors), were excluded from the study.
Data Extraction and Quality Control
Two authors independently extracted data from the selected articles using a checklist. First, the titles and abstracts of identified articles were examined, and articles that were unrelated to the meta-analysis were excluded. The full texts of the remaining articles were then reviewed and included in the analysis based on the inclusion criteria. Data on the first author, year of publication, location, type of study, blinding, randomization, disease severity, sample size, type and dose of the treatment drug, population type, other treatments used, duration of treatment, age, gender, length of the follow-up period, length of the hospitalization period, recovery ratio, recovery time, mortality rate, days to first improvement, mechanical ventilation, intensive care unit (ICU) admission, ICU length of stay, acute respiratory distress syndrome, intubation, and any adverse effects in treatment and control groups were collected.
Statistical Analysis
We used a proposed estimation model24 to justify the scale and outcome indicators (median, interquartile range (IQR), mean and standard deviation (SD)). We anticipated significant heterogeneity among the studies and, therefore, used a random effects model. To examine the heterogeneity of the effect-size estimates among the studies, the Q-statistic, its p-value, a forest plot, and I2 were used. The Q-statistic was used to compare the observed and expected effect size dispersions across the studies, and the p-values for statistical significance are provided. The I2 value is the ratio of real to observed heterogeneity. I2 values between 0% and 50% were considered to be acceptable heterogeneity, while values greater than 50% were considered to indicate significant heterogeneity25. Subgroup analysis and meta-regression were used to determine the sources of heterogeneity when it was significant26. A funnel plot and Egger's regression test were used to evaluate publication bias (given the low power of the test, a = 0.1 was used)27. Stata Statistical Software Version 15.1. (StataCorp LP., College Station, TX, USA) was used for all analyses.
| No | First author | Country | Study Design | Blinding type | Randomization | Covid status | Sample Size | Population type | Treatment protocol (days and dose) | Mean Age | Follow-up Duration (days) | Control group | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alessandro Russo 28 | Italy | Observentional-Cohort | No data | No data | Hospitalization | 294 | Normal | Remidisivir | 63.20 | 30 | Non | -Hospitalization Days -Mortality |
| 2 | Andreas Barratt-Due 29 | Norway | Interventional | Triple | Yes | Hospitalization | 42 | Normal | Remidisivir 100 mg per day | 59.70 | 90 | Routine cares | -Mortality |
| 3 | Anıl Uc 30 | Turkey | Observentional-Cohort | No data | Yes | Hospitalization | 48 | Normal | Favipiravir + Hydro 1200 mg per day | 58.50 | 14 | Hydrox | -Mortality -ICU admition |
| 4 | Areej A Malhani 31 | Saudi Arabia | Observentional-Cohort | No | No | Hospitalization | 154 | Normal | Favipiravir 1600 mg per day | 55 | 28 | IFN | -Hospitalization Days -Mortality -ICU admition |
| 5 | Carlos K H Wong 32 | Hong Kong | Observentional-Cohort | No | No | Hospitalization | 466 | Normal | Remid+Dexametasone | 64.80 | 11 | Dexa | -Mortality |
| 6 | Christoph D. Spinner 33 | United States, Europe, and Asia | Interventional | No | Yes | Hospitalization | 193 | Normal | Remidisivir 100 mg per day | 55.66 | 11 | Routine cares | -Mortality -Adverse effect |
| 7 | Eun-Jeong Joo 34 | S. Korea | Observentional-Cohort | No | No | Hospitalization | 48 | Normal | Remidisivir 100 mg per day | 69.02 | 30 | Routine cares | -Hospitalization Days -Time to recovery -Mortality |
| 8 | Faryal Khamis 35 | Oman | Interventional | No | Yes | Hospitalization | 44 | Normal | Favipiravir 1600 mg per day | 54 | 14 | Routine cares | -Hospitalization Days -Mortality -ICU admition |
| 9 | George A Diaz 36 | USA | Observentional-Cohort | No | No | Hospitalization | 286 | Normal | Remidisivir 100 mg per day | 61.40 | 30 | Routine cares | -Mortality |
| 10 | Halit ÇINARKA 37 | Turkey | Observentional-Cohort | No data | No data | Hospitalization | 131 | Normal | Favipiravir | 55.97 | 14 | Iopinavir | -Hospitalization Days -Mortality -ICU admition |
| 11 | Hany M Dabbous 38 | Egypt | Interventional | No data | Yes | Hospitalization | 44 | Normal | Favipiravir 1200 mg per day | 34.86 | 10 | Chloroquine | -Hospitalization Days -Mortality |
| 12 | Havva Kocayiğit 39 | Turkey | Observentional-Cohort | No | No data | ICU | 65 | Normal | Favipiravir | 69.80 | 70 | lopinavir | -Mortality -ICU stay |
| 13 | J.H. Beigel 40 | United States (45 sites), Denmark (8), the United Kingdom (5), Greece (4), Germany (3), Korea (2), Mexico (2), Spain (2), Japan (1), and Singapore (1) | Interventional | Double | Yes | Hospitalization | 541 | Normal | Remidisivir 100 mg per day | 58.60 | 10 | Placebo | -Time to recovery -Mortality -Adverse effect |
| 14 | Lakshmi Mahajan 41 | India | Interventional | No | Yes | Hospitalization | 34 | Normal | Remidisivir 100 mg per day | 58.08 | 12 | Routine cares | -Mortality |
| 15 | Markos Kalligeros 42 | USA | Observentional-Cohort | No | No | Hospitalization | 99 | Normal | Remidisivir 100 mg per day | 58.66 | 28 | Routine cares | -Mortality |
| 16 | Masaharu Shinkai 43 | Japan | Interventional | Single | Yes | No data | 107 | Normal | Favipiravir 1600 mg per day | 43.80 | 28 | Placebo | -Mortality -Adverse effect |
| 17 | Masoud Solaymani-Dodaran 44 | Iran | Interventional | Single | Yes | Hospitalization | 190 | Normal | Favipiravir 1800 mg per day | 58.60 | 10 | lopinavir | -Mortality -ICU admition |
| 18 | Michael E Ohl 45 | USA | Observentional-Cohort | No data | No data | Hospitalization | 1172 | Normal | Remidisivir | 66.60 | 30 | Routine cares | -Mortality -ICU admition |
| 19 | Nouf K Almaghlouth 46 | USA | Observentional-Cohort | No | No | Hospitalization | 33 | Normal | Remid+Tocilizumab 100 mg per day | 7 | Tocli | -Mortality | |
| 20 | Regine Padilla 47 | USA | Observentional-Cohort | No | No | Hospitalization | 11 | Normal | Remidisivir 100 mg per day | 7 | Convalescent plasma | -Mortality | |
| 21 | Robert Flisiak 48 | Poland | Observentional-Cohort | No | No | Hospitalization | 122 | Normal | Remidisivir 100 mg per day | 58.70 | 28 | lopinavir | -Hospitalization Days -Mortality -Adverse effect |
| 22 | Susan A Olender 49 | USA | Observentional-Cohort | No | No | Hospitalization | 298 | Normal | Remidisivir 100 mg per day | 14 | Routine cares | -Time to recovery -Mortality | |
| 23 | Toshiki Kuno 50 | Japan, USA | Observentional-Cohort | No | No | Hospitalization | 1336 | Normal | Remidisivir 100 mg per day | 65.70 | 14 | Steroids | -Mortality -ICU admition |
| 24 | Vishal Gupta 51 | India | Observentional-Cohort | No data | No | Hospitalization | 414 | Normal | Remidisivir | 57 | 14 | Tocli | -Hospitalization Days -Mortality |
| 25 | WHO Solidarity Trial Consortium; Hongchao Pan 52 | WHO | Interventional | Double | Yes | Hospitalization | 2743 | Normal | Remidisivir 100 mg per day | 30 | Placebo | -Mortality | |
| 26 | Yeming Wang 53 | Italy | Interventional | Double | Yes | Hospitalization | 158 | Normal | Remidisivir 200 mg per day | 64 | 28 | Placebo | -Hospitalization Days -Time to recovery -Mortality |
| 27 | Zainab Almoosa 54 | Saudi Arabia | Observentional-Cohort | No data | No data | Hospitalization | 110 | Normal | Favipiravir 1400 mg per day | 56.80 | 14 | Routine cares | -Time to recovery -Mortality -ICU admition |
| 28 | Zeno Pasquini 55 | Italy | Observentional-Cohort | No data | No data | ICU | 25 | Normal | Remidisivir 100 mg per day | 64 | 10 | Ventilation | -Mortality |
| Outcome | N studies | OR (95% CI) | Heterogeneity %I 2 (p-value) | Egger’s test p-value |
|---|---|---|---|---|
| Mortality 1 | ||||
| Remdesivir | 17 | 0.893 (0.676-1.180) | 78.47 (0.003) | 0.654 |
| Favipiravir | 8 | 0.984 (0.540-1.793) | 54.65 (0.038) | |
| ICU admission | ||||
| Remdesivir | 3 | 0.74 (0.26-1.86) | 97.09 (0.001) | 0.772 |
| Favipiravir | 4 | 0.49 (0.11-2.09) | 91.44 (0.001) | |
| Any adverse effects | ||||
| Remdesivir | 4 | 0.86 (0.46-1.58) | 90.06 (0.002) | 0.583 |
| Outcome | N studies | Mean difference p-value | Heterogeneity %I 2 (p-value) | Egger’s test p-value |
| Hospitalization Duration, days | ||||
| Remdesivir | 3 | 0.000 | 96.15 (0.000) | 0.017 |
| Favipiravir | 3 | 0.019 | 77.54 (0.030) | |
| Outcome | Remdesivir effect p-value/%I 2 | Favipiravir effect p-value/%I 2 | N studies |
|---|---|---|---|
| Mortality/Study design | |||
| Interventional | 0.418 / 18.90 | 0.632 / 0.00 | 11 |
| Observational | 0.325 / 28.24 | 0.125 / 79.20 | 14 |
| Treatment Duration | |||
| <7 | 0.652 / 0.00 | 0.238 / 28.15 | 10 |
| >7 | 0.896 / 0.00 | 0.623 / 0.00 | 10 |
| Mean Age | |||
| <59 | 0.119 / 62.82 | 0.156 / 42.12 | 13 |
| >59 | 0.965 / 64.23 | 0.178 / 44.27 | 10 |
| Outcome | Remdesivir p-value (%I 2 ) | Favipiravir p-value (%I 2 ) |
|---|---|---|
| Mortality | ||
| Study design | 0.835 (63.5) | 0.089 (19.2) |
| Hospitalization section | 0.864 (64.24) | - |
| Treatment Duration | 0.049 (0.00) | 0.010 (0.00) |
| Mean Age | 0.510 (62.82) | 0.473 (41.17) |
| Country (continent) | 0.711 (63.11) | 0.654 (33.12) |
Results
Systematic Search and Characteristics of the Included Studies
The initial search uncovered 8,329 relevant records of which 3,561 duplicates were removed. After screening the titles and abstracts, 633 studies were considered eligible for further screening. Next, the full texts of the studies were assessed and 28 studies with a total of 10,871 adult participants were found to be eligible for inclusion in the meta-analysis (Figure 1).
Pooled Analysis of Covid-19 Outcomes After Receiving Remdesivir or Favipiravir
As the included studies reported their outcomes differently, event counts rather than percentages and proportions were used. For mortality rates, some studies used counts while others used ORs. Therefore, an analysis was conducted for both indicators after converting the counts into ORs and 95% CIs. Hospitalization duration was also measured using the mean indicator and is presented as mean differences.
Mortality Rate
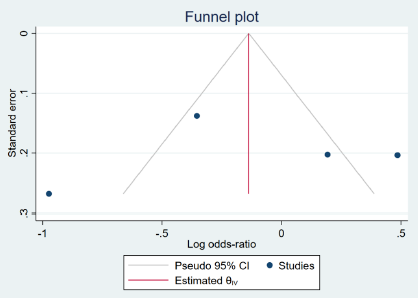
According to the results of a pooled analysis of 17 studies, there was no statistically significant difference in the mortality rate between the remdesivir and control groups (p: 0.493). Similarly, based on the results of a pooled analysis of 8 studies, there was no significant difference in the mortality rate between the favipiravir and control groups (p: 0.774). Heterogeneity was high (> 50%) for all of the studies; although, no publication bias was observed (Egger’s test p-value > 0.20) (Table 2, Figure 2, Figure 3).
Admission to the ICU
Results from a pooled analysis of 3 studies using remdesivir and 4 studies using favipiravir30, 31, 37, 44, 45, 47, 50, 54 found no statistically significant differences in ICU admission outcomes between the intervention and control groups (p-value for remdesivir: 0.785, p-value for favipiravir: 0.483). The heterogeneity was high (> 50%) and significant, but no publication bias was found (Table 2, Figure 4, Figure 5).
Adverse Effects
A pooled analysis of 4 studies33, 40, 48 indicated that patients receiving remdesivir had no significantly higher adverse effects compared to control groups (p: 0.732). While the heterogeneity was both high (> 50%) and significant, no publication bias was found. This analysis was not possible for favipiravir due to the low number of available, published studies (< 3) (Table 2,Figure 5, Figure 6).
Hospitalization Duration
The pooled analysis for hospitalization duration consisted of 3 studies34, 35, 48, which showed that the use of remdesivir significantly increased hospitalization duration in the intervention groups by 0.1 days (p: 0.000). In contrast, the results of an analysis of 3 studies31, 37, 38 that used favipiravir showed significantly reduced hospitalization duration (by 0.06 days compared to the control groups (p: 0.019)). However, high heterogeneity (> 50%) and publication bias were observed (Table 2, Figure 8, Figure 9).
The mortality rate in different subgroups was not significantly different between the intervention and control groups (Table 3). The subgroups analyzed in this meta-analysis included study design, treatment duration (median: 7 days), and age (median: 59 years). Analyzing other outcomes was not possible due to the lack of studies reporting on each possible subgroup variable.
Table 4 presents the possible sources of the high heterogeneity observed in the analysis. The only variable that significantly effected heterogeneity was treatment duration, which was significant for both remdesivir and favipiravir. Further analysis was not possible for the other outcomes due to the lack of published studies reporting on the different subgroup variables.
Discussion
This study aimed to evaluate the effectiveness of two well-known drugs, remdesivir and favipiravir, for treating Covid-19 infection. Remdesivir was introduced as an effective drug for the treatment of Covid-19 after obtaining its first emergency use authorization in May 2020 in the United States and then later in Japan. However, its use has had many critics56. Unfortunately, despite both the passage of time and an increase in the number of observational studies and RCTs, questions regarding the efficacy of these drugs remain unanswered primarily because the results have been controversial and heterogeneous between the various investigations. One way to address this issue is to conduct systematic reviews and meta-analysis studies.
As a ribonucleotide analog and selective inhibitor of the viral RNA polymerase enzyme, favipiravir performs a wide range of antiviral activities against RNA-carrying viruses, which includes blocking viral genome replication and transcription. In Japan and China, favipiravir is licensed for the treatment of novel influenza viruses. It is also effective against Ebola and other RNA-based viruses that cause hemorrhagic fevers35. However, according to the findings of the current meta-analysis, favipiravir had no significant effect on reducing the mortality rate or ICU admissions in Covid-19 patients. Other meta-analyses have also found that the drug does not decrease many of the indicators associated with Covid-19, including death, hospitalization duration, transfer to the ICU, etc.57, 58. However, in this study, favipiravir was found to reduce hospitalization duration by 0.06 days. Although this reduction was statistically significant, it is clinically equivalent to approximately 86 minutes, which would not be considered important from a patient perspective. We also found that favipiravir administration did not induce more side effects compared with controls. This result is consistent with those from another meta-analysis59. However, few interventional and secondary studies using favipiravir have been conducted, and finding high-quality interventional studies with large sample sizes is challenging. Furthermore, the high heterogeneity in the results suggests substantial variation in the target parameters of these studies.
Remdesivir is an adenosine nucleotide analogue prodrug that inhibits viral replication by inducing chain termination in the RNA-dependent RNA polymerase enzyme of SARS-CoV-260. However, there are debates concerning the effectiveness of remdesivir, and studies, including meta-analyses, have not yet reached a consensus regarding its efficacy. According to some of the articles used in the current study, the use of remdesivir did not effect the mortality or ICU admission rates in Covid-19 patients. In contrast, others have found that the use of the drug reduced the mortality rate by 34% (OR: 0.66). These inconsistent results have been found in other meta-analyses as well61, 62, 63. We also found that taking remdesivir increased treatment duration by 0.1 days (144 minutes). While this finding is not consistent with other, similar studies that have found remdesivir neither changes hospitalization duration61 nor reduces it62, 63, it should be noted that there was both high heterogeneity and publication bias, both of which could have affected our findings. The effect of the treatment duration variable in heterogeneity should be also taken into account. Specifically, if only this variable is considered, it is possible to assume that treatment duration changes remdesivir’s efficacy.
One limitation of this study (and similar meta-analyses) is the severe lack of high-quality, interventional studies with appropriate sample sizes, sufficient follow-up periods, and similar treatment protocols needed to reduce heterogeneity. One of the strengths of this study was the simultaneous review of observational and interventional studies as well as sub-group analyses of different outcome variables.
Conclusion
Based on the results of this meta-analysis, both remdesivir and favipiravir have very slight or no effect on mortality rates, ICU admissions, or hospitalization duration in Covid-19 patients. However, more vigorous interventional studies are needed before coming to firm conclusions about the effects of these drugs on covid-19 patient outcomes.
Abbreviations
FDA: United States of America Food and Drug Administration; PRISMA: The Preferred Reporting Items for Systematic Reviews and Meta-Analysis; ICUs: Intensive care units; ARDS: Acute respiratory distress syndrome; IQR: Interquartile range; SD: Standard Deviation
Acknowledgments
None.
Author’s contributions
Y.KH: Contribution to study concept and design, acquisition, analysis and interpretation of data, drafting of the manuscript. M.M: Contribution to study concept and design, drafting of the manuscript. R.K: Contribution to study concept and design, drafting of the manuscript SS. HN: Contribution to study concept and design, acquisition, analysis and interpretation of data, drafting of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Zhang
T.,
Wu
Q.,
Zhang
Z.,
Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology.
2020;
30
(7)
.
View Article PubMed Google Scholar -
worldometers 2021 [cited 2021 October 07, 2021]. Available from: https://www.worldometers.info/coronavirus/. 2021
.
-
Sanders
J.M.,
Monogue
M.L.,
Jodlowski
T.Z.,
Cutrell
J.B.,
Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Journal of the American Medical Association.
2020;
323
(18)
:
1824-36
.
View Article PubMed Google Scholar -
Khalili
J.S.,
Zhu
H.,
Mak
N.S.,
Yan
Y.,
Zhu
Y.,
Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. Journal of Medical Virology.
2020;
92
(7)
:
740-6
.
View Article PubMed Google Scholar -
Kotwani
A.,
Gandra
S.,
Potential pharmacological agents for COVID-19. Indian Journal of Public Health.
2020;
64
(6)
:
112-6
.
View Article PubMed Google Scholar -
Mair-Jenkins
J.,
Saavedra-Campos
M.,
Baillie
J.K.,
Cleary
P.,
Khaw
F.M.,
Lim
W.S.,
Convalescent Plasma Study Group
The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. The Journal of Infectious Diseases.
2015;
211
(1)
:
80-90
.
View Article PubMed Google Scholar -
Mehta
P.,
McAuley
D.F.,
Brown
M.,
Sanchez
E.,
Tattersall
R.S.,
Manson
J.J.,
HLH Across Speciality Collaboration
UK,
COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet.
2020;
395
(10229)
:
1033-4
.
View Article PubMed Google Scholar -
Wang
M.,
Cao
R.,
Zhang
L.,
Yang
X.,
Liu
J.,
Xu
M.,
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research.
2020;
30
(3)
:
269-71
.
View Article PubMed Google Scholar -
Choy
K.T.,
Wong
A.Y.,
Kaewpreedee
P.,
Sia
S.F.,
Chen
D.,
Hui
K.P.,
Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Research.
2020;
178
:
104786
.
View Article PubMed Google Scholar -
Ko
W.C.,
Rolain
J.M.,
Lee
N.Y.,
Chen
P.L.,
Huang
C.T.,
Lee
P.I.,
Arguments in favour of remdesivir for treating SARS-CoV-2 infections. International Journal of Antimicrobial Agents.
2020;
55
(4)
:
105933
.
View Article PubMed Google Scholar -
Reddy
O.S.,
Lai
W.F.,
Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options. ChemBioChem.
2020;
22
(6)
:
939-48
.
View Article PubMed Google Scholar -
Dong
L.,
Hu
S.,
Gao
J.,
Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discoveries & Therapeutics.
2020;
14
(1)
:
58-60
.
View Article PubMed Google Scholar -
Furuta
Y.,
Gowen
B.B.,
Takahashi
K.,
Shiraki
K.,
Smee
D.F.,
Barnard
D.L.,
Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Research.
2013;
100
(2)
:
446-54
.
View Article PubMed Google Scholar -
Shiraki
K.,
Daikoku
T.,
Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacology & Therapeutics.
2020;
209
:
107512
.
View Article PubMed Google Scholar -
Furuta
Y.,
Takahashi
K.,
Kuno-Maekawa
M.,
Sangawa
H.,
Uehara
S.,
Kozaki
K.,
Mechanism of action of T-705 against influenza virus. Antimicrobial Agents and Chemotherapy.
2005;
49
(3)
:
981-6
.
View Article PubMed Google Scholar -
Pan
H.,
Peto
R.,
Henao-Restrepo
A.M.,
Preziosi
M.P.,
Sathiyamoorthy
V.,
Karim
Q. Abdool,
Consortium
WHO Solidarity Trial,
Repurposed antiviral drugs for COVID-19- interim WHO SOLIDARITY trial results. The New England Journal of Medicine.
2021;
384
(6)
:
497-511
.
View Article PubMed Google Scholar -
Alexander
S.P.,
Armstrong
J.F.,
Davenport
A.P.,
Davies
J.A.,
Faccenda
E.,
Harding
S.D.,
A rational roadmap for SARS-CoV-2/COVID-19 pharmacotherapeutic research and development: IUPHAR Review 29. British Journal of Pharmacology.
2020;
177
(21)
:
4942-66
.
View Article PubMed Google Scholar -
Alexander
P.E.,
Piticaru
J.,
Lewis
K.,
Aryal
K.,
Thomas
P.,
Szczeklik
W.,
Remdesivir use in patients with coronavirus COVID-19 disease: a systematic review and meta-analysis. MedRXiv.
2020;
2020
(26)
:
2020-05
.
View Article Google Scholar -
Manabe
T.,
Kambayashi
D.,
Akatsu
H.,
Kudo
K.,
Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infectious Diseases.
2021;
21
(1)
:
489
.
View Article PubMed Google Scholar -
Liberati
A.,
Altman
D.G.,
Tetzlaff
J.,
Mulrow
C.,
G∅tzsche
P.C.,
Ioannidis
J.P.,
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine.
2009;
6
(7)
:
e1000100
.
View Article PubMed Google Scholar -
Moher
D.,
Liberati
A.,
Tetzlaff
J.,
Altman
D.G.,
Group
PRISMA,
Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research Ed.).
2009;
339
:
b2535
.
View Article PubMed Google Scholar -
Critical Appraisal Skills Programme (2020). CASP (Randomised Controlled Trial) Checklist.
.
-
Critical Appraisal Skills Programme (2018). CASP (Cohort Study) Checklist.
.
-
Wan
X.,
Wang
W.,
Liu
J.,
Tong
T.,
Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology.
2014;
14
(1)
:
135
.
View Article PubMed Google Scholar -
Higgins
J.P.,
Thompson
S.G.,
Deeks
J.J.,
Altman
D.G.,
Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.).
2003;
327
(7414)
:
557-60
.
View Article PubMed Google Scholar -
DerSimonian
R.,
Laird
N.,
Meta-analysis in clinical trials. Controlled Clinical Trials.
1986;
7
(3)
:
177-88
.
View Article PubMed Google Scholar -
Viechtbauer
W.,
Publication bias in meta-analysis: Prevention, assessment and adjustments. Psychometrika.
2007;
72
(2)
:
269-71
.
View Article Google Scholar -
Russo
A.,
Binetti
E.,
Borrazzo
C.,
Cacciola
E.G.,
Battistini
L.,
Ceccarelli
G.,
Efficacy of Remdesivir-Containing Therapy in Hospitalized COVID-19 Patients: A Prospective Clinical Experience. Journal of Clinical Medicine.
2021;
10
(17)
:
3784
.
View Article PubMed Google Scholar -
Ucan
A.,
Cerci
P.,
Efe
S.,
Akgun
H.,
Ozmen
A.,
Yagmuroglu
A.,
Benefits of treatment with favipiravir in hospitalized patients for COVID-19: a retrospective observational case-control study. Virology Journal.
2021;
18
(1)
:
102
.
View Article PubMed Google Scholar -
A
A. Malhani,
M
A. Enani,
F
Saheb Sharif-Askari,
M
R. Alghareeb,
R
T. Bin-Brikan,
S
A. AlShahrani,
R
Halwani,
IM
Tleyjeh,
Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: A cohort study. PLoS One.
2021;
16
(6)
:
e0252984
.
View Article Google Scholar -
Wong
C.K.,
Lau
K.T.,
Au
I.C.,
Xiong
X.,
Chung
M.S.,
Lau
E.H.,
Optimal timing of remdesivir initiation in hospitalized COVID-19 patients administered with dexamethasone. Clinical Infectious Diseases.
2021
.
-
Spinner
C.D.,
Gottlieb
R.L.,
Criner
G.J.,
Arribas López
J.R.,
Cattelan
A.M.,
Soriano Viladomiu
A.,
Investigators
GS-US-540-5774,
Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. Journal of the American Medical Association.
2020;
324
(11)
:
1048-57
.
View Article PubMed Google Scholar -
Joo
E.J.,
Ko
J.H.,
Kim
S.E.,
Kang
S.J.,
Baek
J.H.,
Heo
E.Y.,
Clinical and Virologic Effectiveness of Remdesivir Treatment for Severe Coronavirus Disease 2019 (COVID-19) in Korea: a Nationwide Multicenter Retrospective Cohort Study. Journal of Korean Medical Science.
2021;
36
(11)
:
e83
.
View Article PubMed Google Scholar -
Khamis
F.,
Naabi
H. Al,
Lawati
A. Al,
Ambusaidi
Z.,
Sharji
M. Al,
Barwani
U. Al,
Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. International Journal of Infectious Diseases.
2021;
102
:
538-43
.
View Article PubMed Google Scholar -
Diaz
G.A.,
Christensen
A.B.,
Pusch
T.,
Goulet
D.,
Chang
S.C.,
Grunkemeier
G.L.,
Remdesivir and Mortality in Patients with COVID-19. Clinical Infectious Diseases.
2021;
2021
:
ciab698
.
-
Çinarka
H.,
Günlüoğlu
G.,
Çörtük
M.,
Yurt
S.,
Kiyik
M.,
KOŞAR
F.,
The comparison of favipiravir and lopinavir/ritonavir combination in COVID-19 treatment. Turkish Journal of Medical Sciences.
2021;
51
(4)
:
1624-30
.
View Article PubMed Google Scholar -
Dabbous
H.M.,
Abd-Elsalam
S.,
El-Sayed
M.H.,
Sherief
A.F.,
Ebeid
F.F.,
El Ghafar
M.S.,
Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Archives of Virology.
2021;
166
(3)
:
949-54
.
View Article PubMed Google Scholar -
Kocayiğit
H.,
Özmen Süner
K.,
Tomak
Y.,
Demir
G.,
Yaylac\i
S.,
Dheir
H.,
Observational study of the effects of Favipiravir vs Lopinavir/Ritonavir on clinical outcomes in critically Ill patients with COVID-19. Journal of Clinical Pharmacy and Therapeutics.
2021;
46
(2)
:
454-9
.
View Article PubMed Google Scholar -
Barratt-Due
A.,
Olsen
I.C.,
Nezvalova-Henriksen
K.,
K\aasine
T.,
Lund-Johansen
F.,
Hoel
H.,
NOR-Solidarity trial
Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 : A Randomized Trial. Annals of Internal Medicine.
2021;
174
(9)
:
1261-9
.
View Article PubMed Google Scholar -
Beigel
J.H.,
Tomashek
K.M.,
Dodd
L.E.,
Mehta
A.K.,
Zingman
B.S.,
Kalil
A.C.,
Study Group Members
ACTT-1,
Remdesivir for the Treatment of Covid-19 - Final Report. The New England Journal of Medicine.
2020;
383
(19)
:
1813-26
.
View Article PubMed Google Scholar -
Mahajan
L.,
Singh
A.P.,
Gifty
Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: A prospective randomised study. Indian Journal of Anaesthesia.
2021;
65
(13)
:
41-6
.
View Article PubMed Google Scholar -
Kalligeros
M.,
Tashima
K.T.,
Mylona
E.K.,
Rybak
N.,
Flanigan
T.P.,
Farmakiotis
D.,
Remdesivir Use Compared With Supportive Care in Hospitalized Patients With Severe COVID-19: A Single-Center Experience. Open Forum Infectious Diseases.
2020;
7
(10)
.
View Article PubMed Google Scholar -
Shinkai
M.,
Tsushima
K.,
Tanaka
S.,
Hagiwara
E.,
Tarumoto
N.,
Kawada
I.,
Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infectious Diseases and Therapy.
2021;
10
(4)
:
2489-509
.
View Article PubMed Google Scholar -
Solaymani-Dodaran
M.,
Ghanei
M.,
Bagheri
M.,
Qazvini
A.,
Vahedi
E.,
Hassan Saadat
S.,
Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. International Immunopharmacology.
2021;
95
:
107522
.
View Article PubMed Google Scholar -
Ohl
M.E.,
Miller
D.R.,
Lund
B.C.,
Kobayashi
T.,
Richardson Miell
K.,
Beck
B.F.,
Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19. JAMA Network Open.
2021;
4
(7)
:
e2114741
.
View Article PubMed Google Scholar -
Almaghlouth
N.K.,
Anyiam
F.E.,
Shah
S.,
Haq
S.,
Attia
M.J.,
Guevara
R.,
The Use of Single Therapy With Tocilizumab Versus Combination Therapy With Remdesivir and Tocilizumab in SARS-CoV-2 Patients in El Paso, Texas. Cureus.
2021;
13
(7)
:
e16351
.
View Article PubMed Google Scholar -
Padilla
R.,
Arquiette
J.,
Mai
Y.,
Singh
G.,
Galang
K.,
Liang
E.,
Clinical Outcomes of COVID-19 Patients Treated with Convalescent Plasma or Remdesivir Alone and in Combination at a Community Hospital in California's Central Valley. Journal of Pharmacy & Pharmaceutical Sciences.
2021;
24
:
210-9
.
View Article PubMed Google Scholar -
Flisiak
R.,
Zar\kebska-Michaluk
D.,
Berkan-Kawińska
A.,
Tudrujek-Zdunek
M.,
Rogalska
M.,
Piekarska
A.,
Remdesivir-based therapy improved the recovery of patients with COVID-19 in the multicenter, real-world SARSTer study. Pol Arch Intern Med.
2021;
131
(1)
:
103-10
.
PubMed Google Scholar -
Olender
S.A.,
Walunas
T.L.,
Martinez
E.,
Perez
K.K.,
Castagna
A.,
Wang
S.,
Remdesivir Versus Standard-of-Care for Severe Coronavirus Disease 2019 Infection: An Analysis of 28-Day Mortality. Open Forum Infectious Diseases.
2021;
8
(7)
.
View Article PubMed Google Scholar -
Kuno
T.,
Miyamoto
Y.,
Iwagami
M.,
Ishimaru
M.,
Takahashi
M.,
Egorova
N.N.,
The association of remdesivir and in-hospital outcomes for COVID-19 patients treated with steroids. The Journal of Antimicrobial Chemotherapy.
2021;
76
(10)
:
2690-6
.
View Article PubMed Google Scholar -
Gupta
V.,
Ingawale
S.,
Bhondve
A.,
Khot
W.,
Salagre
S.,
Sonawale
A.,
Clinical Study of Use of Remdesivir and Tocilizumab in Severely Ill COVID-19 Patients. The Journal of the Association of Physicians of India.
2021;
69
(7)
:
14-9
.
PubMed Google Scholar -
Pan
H.,
Peto
R.,
Henao-Restrepo
A.M.,
Preziosi
M.P.,
Sathiyamoorthy
V.,
Abdool Karim
Q.,
Solidarity Trial Consortium
WHO,
Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. The New England Journal of Medicine.
2021;
384
(6)
:
497-511
.
View Article PubMed Google Scholar -
Wang
Y.,
Zhang
D.,
Du
G.,
Du
R.,
Zhao
J.,
Jin
Y.,
Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet.
2020;
395
(10236)
:
1569-78
.
View Article PubMed Google Scholar -
Almoosa
Z.,
Saad
M.,
Qara
S.,
Mustafa
M.,
Mansour
A.,
Alshab
D.,
Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study. Journal of Infection and Public Health.
2021;
14
(9)
:
1247-53
.
View Article PubMed Google Scholar -
Pasquini
Z.,
Montalti
R.,
Temperoni
C.,
Canovari
B.,
Mancini
M.,
Tempesta
M.,
Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. The Journal of Antimicrobial Chemotherapy.
2020;
75
(11)
:
3359-65
.
View Article PubMed Google Scholar -
Lamb
Y.N.,
Remdesivir: first Approval. Drugs.
2020;
80
(13)
:
1355-63
.
View Article PubMed Google Scholar -
Liu
W.,
Zhou
P.,
Chen
K.,
Ye
Z.,
Liu
F.,
Li
X.,
Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. Canadian Medical Association Journal.
2020;
192
(27)
:
734-44
.
View Article PubMed Google Scholar -
Hassanipour
S.,
Arab-Zozani
M.,
Amani
B.,
Heidarzad
F.,
Fathalipour
M.,
Martinez-de-Hoyo
R.,
The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Scientific Reports.
2021;
11
(1)
:
11022
.
View Article PubMed Google Scholar -
Prakash
A.,
Singh
H.,
Kaur
H.,
Semwal
A.,
Sarma
P.,
Bhattacharyya
A.,
Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients. Indian Journal of Pharmacology.
2020;
52
(5)
:
414-21
.
View Article PubMed Google Scholar -
Gordon
C.J.,
Tchesnokov
E.P.,
Woolner
E.,
Perry
J.K.,
Feng
J.Y.,
Porter
D.P.,
Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. The Journal of Biological Chemistry.
2020;
295
(20)
:
6785-97
.
View Article PubMed Google Scholar -
Verdugo-Paiva
F.,
Acuña
M.P.,
Solá
I.,
Rada
G.,
Working Group
COVID-19 L\cdotOVE,
Remdesivir for the treatment of COVID-19: a living systematic review. Medwave.
2020;
20
(11)
:
e8080
.
View Article PubMed Google Scholar -
Al-Abdouh
A.,
Bizanti
A.,
Barbarawi
M.,
Jabri
A.,
Kumar
A.,
Fashanu
O.E.,
Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Contemporary Clinical Trials.
2021;
101
:
106272
.
View Article PubMed Google Scholar -
Jiang
Y.,
Chen
D.,
Cai
D.,
Yi
Y.,
Jiang
S.,
Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: A network meta-analysis. Journal of Medical Virology.
2021;
93
(2)
:
1171-4
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 5 (2023)
Page No.: 5701-5716
Published on: 2023-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4573 times
- PDF downloaded - 1525 times
- XML downloaded - 170 times
 Biomedpress
Biomedpress










