Abstract
Introduction: This study aimed to demonstrate the cytotoxic effect of a bitter leaf (Vernonia amygdalina Del.) ethanol extract on myeloid leukemia cells.
Methods: The plant extract was prepared using the maceration method. The toxicity assays used the trypan blue exclusion method. Flow cytometry and reverse transcription PCR methods were used to deduce the mechanism of action.
Results: The V. amygdalina Del. extract strongly affected K562 cells, with a half-maximal inhibitory concentration of 8.78 ? 2.224 ?g/mL. The extract could induce apoptosis and arrest the cell cycle in K562 cells. The extract increased the mRNA levels of caspase 3 (CASP3), baculoviral IAP repeat containing 5 (BIRC5/survivin), and phosphatidylinositol 3-kinase (PI3K) and decreased the mRNA levels of retinoblastoma transcriptional corepressor 1 (RB1/pRB), B cell lymphoma/leukemic 2 (BCL2), BCL2-like 1 (BCL2L1/BCL-XL), caspase 9 (CASP9), and the breakpoint cluster region (BCR)-Abelson (ABL) fusion gene.
Conclusion: The V. amygdalina Del. extract strongly inhibited the acute myeloid leukemia cell line K562. It was found to arrest the cell cycle and induce apoptosis by regulating the expression of related genes that predicted targeting BCR-ABL downregulation.
Introduction
Cancer is a leading cause of mortality worldwide, with >19 million new cases and nearly 10 million deaths1, 2. Unfortunately, cancer cases are expected to increase significantly over the next decade3. The economic burden on patients and their families is enormous, significantly affecting public health, the national economy, and social security4. Therefore, medical research is racing to develop effective cancer treatments to prolong patient lives. However, current treatments remain largely ineffective5. One of the least treatable cancers is leukemia, which causes > 250,000 deaths and nearly 500,000 new diagnoses1, 2. Despite advances in knowledge and medical techniques, leukemia-related mortality remains high6.
Phytochemical compounds are being explored as potential treatments for blood cancer7, 8. Various plant compounds have shown inhibitory effects on leukemia cell proliferation. For example, maytansinoids and their derivatives extracted from Maytenus serrata inhibited tubulin, alvocidib extracted from Dysoxylum binectariferum inhibited cyclin-dependent kinase 9 (CDK9) activity, and omacetaxine mepesuccinate extracted from Cephalotaxus harringtonia has been approved by the US Food and Drug Administration9, 10, 11.
Bitter leaf (Vernonia amygdalina Del.) is among the major sources of compounds with scientifically demonstrated anticancer activity. Bitter leaf extract disrupts the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) signaling pathway, the mitogen-activated protein kinase (MAPK) pathway, and fms-related receptor tyrosine kinase 3 (FLT3) phosphorylation, inhibiting cancer cell proliferation12, 13, 14, 15. Studies have found the bitter leaf to be cytotoxic in breast cancer (half-maximal inhibitory concentration [IC50]: MCF-7 = 50.36 µg/mL, 4T1 = 25.04 ± 0.36 µg/mL, and T47D = 59.19 ± 0.55 µg/mL), neuroblastoma (IC50: U-87 = 18.80 ± 1.11 µg/mL), prostate cancer (IC50: PC-3 = 196.60 µg/mL and DU145 = 40.10 ± 4.30 µg/mL), and acute myeloblastic leukemia (IC50: HL-60 = 5.58 µg/mL, THP-1 = 24.17 ± 3.33 µg/mL, MOLM-13 = 11.45 ± 2.12 µg/mL, and MV4-11 = 16.08 ± 1.21 µg/mL) cells16, 17, 18, 19, 20, 21. However, few studies have examined bitter leaf’s effect on leukemia cells, especially chronic myeloid leukemia, one of the four main leukemia groups. Therefore, this study aimed to investigate the cytotoxicity of a bitter leaf ethanol extract on chronic myeloid leukemia cell line K562 and determine its mechanism of action.
Methods
Plant extraction
The bitter leaves were harvested from Xuyen Moc district, Ba Ria–Vung Tau province, Vietnam. Dang Le Anh Tuan, Ph.D., of the Botany Laboratory in the Department of Ecology and Evolutionary Biology, Faculty of Biology and Biotechnology, University of Science, Vietnam National University, Ho Chi Minh City, performed the botanical identification (voucher: PHH0004908; Supplementary Figure 1). After washing and thoroughly drying at 40oC, the leaves were ground to a powder, which was then suspended in 96% ethanol (1:10 w/v). The plant extract was collected and rotary evaporated to obtain a crude extract. Dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA) was used to dissolve the crude V. amygdalina extract (VAE) into a solution for use, which was stored at −20oC until needed.
Cell culture
The human leukemia cell line K562 was obtained from Prof. Yuko Sato (Tokyo, Japan)22, 23. The K562 cells were cultured in Roswell Park Memorial Institute 1640 (RPMI 1640) medium (Sigma-Aldrich, USA) supplemented with 10% inactivated fetal bovine serum (Thermo Fisher Scientific, USA), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Sigma-Aldrich, USA) at 37oC with 5% CO2. Fibroblast cells were cultured in Dulbecco’s modified Eagle’s medium (StemCell, Singapore) prepared similarly to RPMI 1640.
Cytotoxicity effect of VAE extract
The toxicity of the VAE on K562 cells was evaluated using the trypan blue exclusion method in a six-well plate24. Briefly, 1500 µL of K562 cells at a density of 2×105 cells/mL was added to each experimental well before the same volume of VAE at 0 to 100 µg/mL was added. The plates were incubated for 72 hours at 37oC with 5% CO2. Then, cell viability was calculated as the percentage difference between the treated and negative control groups.
The toxicity of the VAE on fibroblasts was determined using the 3-(4 5-dimethylthiazol-2-yl)-2 5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, USA). Briefly, 100 µL of fibroblasts at a density of 2×105 cells/mL was added to each experimental well and incubated in a cell culture incubator. After 24 hours, 100 µL of the VAE at 0 to 200 µg/mL was added. After 72 hours, the viability of the fibroblasts was measured using the MTT assay.
Untreated cells were used as negative controls. Moreover, the effect of the DMSO (Protide, USA) was evaluated at 0.1%, corresponding to the solvent content in the highest VAE treatment.
Annexin V/PI analysis
K562 cells at a density of 105 cells/mL were exposed to the VAE at 50 and 100 µg/mL. After 24 hours, the K562 cells were collected and washed twice with phosphate-buffered saline (PBS; TBR company, Vietnam). Then, the cells were stained according to the ANNEX100B protocol (BioRad, USA). Briefly, cell pellets were resuspended in 195 µL of 1× binding buffer before adding 5 µL of Annexin V. After incubation for 15 minutes in the dark, the cells were washed with 200 µL of binding buffer and resuspended in 190 µL of binding buffer before adding 10 µL of propidium iodide (PI). The stained cells were analyzed using a BD Accuri C6 Plus Flow Cytometer (BD Biosciences, USA).
mRNA expression analysis
K562 cells at a density of 105 cells/mL were exposed to the VAE at 50 and 100 µg/mL. After 16 hours, the K562 cells were collected and washed with PBS. Next, their RNA was extracted according to the TRIzol reagent guidelines (Thermo Fisher Scientific, USA). Then, mRNA expression was detected using the SensiFAST SYBR No-ROX One-Step Kit (Meridian Bioscience, USA) with the primers listed in Table 1. Gene expression was determined using reverse transcription quantitative PCR and the 2−ΔΔCT method25.
| Gene | Sequences (5’ – 3’) | Reference |
|---|---|---|
| TP53 | TGTGGAGTATTTGGATGACA | Kang Pa Lee, et al . 26 |
| GAACATGAGTTTTTTATGGC | ||
| pRB | ACTCCGTTTTCATGCAGAGACTAA | Deborah L. Burkhart, et al . 27 |
| GAGGAATGTGAGGTATTGGTGACA | ||
| Bcl-XL | TTGGACAATGGACTGGTTGA | Suresh Kumar, et al . 28 |
| GTAGAGTGGATGGTCAGTG | ||
| Bcl-2 | AAGATTGATGGGATCGTTGC | M. Jaberipour, et al . 29 |
| GCGGAACACTTGATTCTGGT | ||
| Bax | TGGCAGCTGACATGTTTTCTGAC | Kostas V Floros, et al . 30 |
| TCACCCAACCACCCTGGTCTT | ||
| Survivin | GTTGCGCTTTCCTTTCTGTC | Sang Il Kim, et al . 31 |
| TCTCCGCAGTTTCCTCAAAT | ||
| Caspase-3 | GAACTGGACTGTGGCATTGA | Sadia Perveen, et al . 32 |
| CCTTTGAATTTCGCCAAGAA | ||
| Caspase-9 | GGTGATGTCGGTGCTCTTGA | IDT, Inc. |
| CGACTCACGGCAGAAGTTCA | ||
| BCR-ABL | CGGGAGCAGCAGAAGAAGTTGTTC | Nga Nguyen, et al. 33 |
| CAGGCACGTCAGTGGTGTCTCTGTG | ||
| MAPK | TGAAATGACAGGCTACGTGG | Liping Jiang, et al . 34 |
| GACTTCATCATAGGTCAGGC | ||
| Pi3K | GGTTGTCTGTCAATCGGTGACTGT | Ismael Riquelme, et al . 35 |
| GAACTGCAGTGCACCTTTCAAGC | ||
| GADPH | GAAGGTGAAGGTCGGAGTC | Qiuying Chen, et al . 36 |
| GAAGATGGTGATGGGATTTC |
Data Analysis
Experiments were repeated at least three times, and data are presented as mean ± standard deviation. Statistical analyses were conducted using GraphPad Prism software (version 9.0.0) with α = 0.05.
Results
VAE strongly inhibited myelocytic leukemia cells
K562 cell viability was greater with (116.90% ± 16.92%) than without 0.1% DMSO (P = 0.0002). In contrast, 0.1% DMSO did not significantly affect fibroblast viability (P = 0.0786). Therefore, 0.1% DMSO was considered benign for evaluating cell growth (Figure 1). The VAE significantly decreased leukemia cell proliferation but did not significantly affect fibroblast proliferation (Figure 2 and Supplemental Figure 2). The IC50 values for the VAE were 8.78 ± 2.22 µg/mL for K562 cells and > 200 µg/mL for fibroblasts. The effects of VAE on K562 cells were classified as selective based on an estimated selective index (SI) of 22.78.
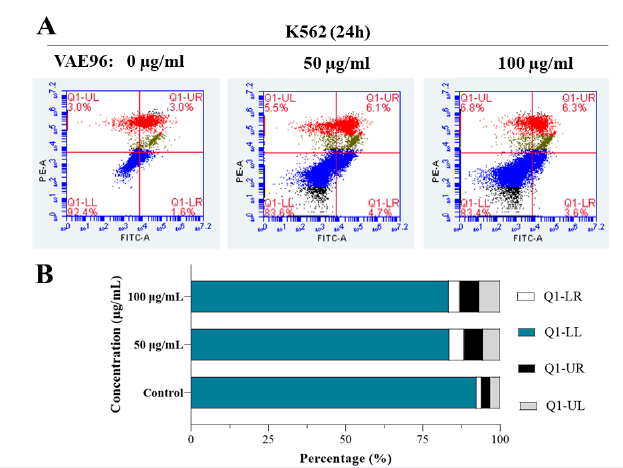
VAE induced apoptosis in K562 cells
K562 cells were further examined by staining with PI and Annexin V after 24 hours of VAE exposure. Cell death increased with VAE concentration. The percentages of Annexin V-positive and PI-positive cells were higher among cells treated with 50 or 100 µg/mL VAE than among untreated control cells (P < 0.0001). Most cells died due to apoptosis (6.10% ± 0.10% for 50 µg/mL and 6.50% ± 0.44% for 100 µg/mL VAE) or necrosis (5.52% ± 0.50% for 50 µg/mL and 6.59% ± 0.52% for 100 µg/mL VAE; Figure 3). In addition, the VAE tended to arrest cells at G2/M (9.95% ± 0.84% for 50 µg/mL and 10.67% ± 0.40% for 100 µg/mL VAE) or S (2.08% ± 0.07% for 50 µg/mL and 3.26% ± 0.03% for 100 µg/mL VAE) checkpoints since the percentage of cells in these phases increased after exposure in a dose-dependent manner (Figure 4).
VAE regulates mRNA expression in K562 cells
We examined the expression of genes involved in apoptosis, the cell cycle, and breakpoint cluster region (BCR)-Abelson (ABL) pathway signaling (Figure 5). The control group comprised healthy cells at the same density as the experimental groups. When 2−ΔΔCT values were compared between the 50 and 100 µg/mL VAE experimental groups, the mRNA levels of the following target genes decreased with increasing VAE concentration: retinoblastoma transcriptional corepressor 1 (RB1/pRB; from 5.59 ± 2.63 to 1.24 ± 0.88), B cell lymphoma-leukemia 2 (BCL2; from 1.00 ± 0.46 to 0.60 ± 0.15), BCL2-like 1 (BCL2L1/BCL-XL; from 2.44 ± 0.51 to 1.16 ± 0.61), BCL2-associated X apoptosis regulator (BAX; from 1.75 ± 0.17 to 1.23 ± 0.12), caspase 9 (CASP9; from 9.57 ± 1.40 to 2.84 ± 0.32), and the BCR-ABL fusion gene (from 2.73 ± 0.13 to 1.84 ± 0.33). In contrast, the mRNA levels of the following target genes increased with increasing VAE concentration: baculoviral IAP repeat containing 5 (BIRC5/survivin; from 1.04 ± 0.15 to 2.00 ± 0.67), caspase 3 (CASP3; from 1.21 ± 0.29 to 1.78 ± 0.51), and PI3K from 0.71 ± 0.40 to 1.00 ± 0.33). However, VAE did not affect tumor protein p53 (TP53) and MAPK mRNA levels.
Discussion
DMSO is widely used in herbal pharmacology37. However, DMSO easily permeates cells at high concentrations, causing hemolytic toxicity38, 39. In this study, 0.1% DMSO showed no cytotoxicity, leading to no data distortion. The VAE, which had an IC50 of 8.78 ± 2.22 µg/mL for the K526 cell line, is a potential cytotoxic crude extract according to the US National Cancer Institute criteria40. Its toxicity has also been reported in several other cancer cell lines16, 17, 18, 19, 20.
A V. amygdalina Del. extract was previously reported to have a prominent inhibitory effect on the acute myeloid leukemia cell line HL-60, with an IC50 of 5.58 µg/mL41. Another V. amygdalina Del. extract was reported to have a strong cytotoxic effect on acute myeloid leukemia cell lines THP-1 (IC50 = 24.17 ± 3.33 µg/mL), MOLM-13 (IC50 = 11.45 ± 2.12 µg/mL), and MV4-11 (IC50 = 16.08 ± 1.21 µg/mL)21. Moreover, a V. amygdalina Del. root extract had a remarkably toxic effect in a clinical trial, killing 50%–75% of acute myeloid and lymphocytic leukemia patient-derived tumor cells42. However, leukemic inhibitory activity has also been reported for extracts from other plants of the same Vernonia genus, such as V. condensate, which had an IC50 of 24.20 µg/mL for HL-60 cells43, 44.
Its diversity of phytochemical compounds may contribute to the anti-leukemia effects of V. amygdalina Del., including vernodalin, which requires further in-depth research45. Furthermore, the effects of the VAE appear highly selective based on its SI of 22.78, facilitating its further evaluation46. A previous study reported that a VAE could prevent the phosphorylation of FLT3. Inhibiting the FLT3 pathway could reduce cell proliferation and enhance cell death through apoptosis15. Interestingly, V. amygdalina Del. induced apoptosis in breast cancer cells by regulating the expression procaspases and the BCL2 family18, 19. In our study, the concomitant increases in PI-positive and Annexin V-positive cells after VAE treatment suggests that VAE induces apoptosis in K562 cells. Moreover, PI stain analysis suggests VAE induces cell cycle arrest in K562 cells, consistet with its reported affects on MCF-7 and MDA-MB-231 cells18, 19.
Decreased pRB expression has been closely associated with cell cycle arrest47. In addition, the decreased expression of genes such as BCL-XL and BCL2 and the increased expression of CASP3 were found to promote apoptosis in K562 cells48. Moreover, V. amygdalina Del. extract has been shown to prevent tyrosine kinase receptor phosphorylation activity15. We found that the VAE decreased the expression of the BCR-ABL fusion gene, suggesting that it injured K562 cells through the BCR-ABL pathway. However, since changes in mRNA levels indirectly reflect changes in the protein levels and activities49, 50, 51, further in-depth research is required to confirm our results at the protein level.
CONCLUSIONS
The V. amygdalina Del. ethanol extract showed a potent, selective inhibitory effect on the chronic myeloid leukemia cell line K562. Our results show that its mechanism of action was via apoptosis induction, which evidence suggests is through the BCR-ABL pathway.
Abbreviations
DMSO: Dimethyl sulfoxide, IC50: The half maximal inhibitory concentration, SI: Selective index, VAE: Vernonia amygdalina Del. ethanol extract
Acknowledgments
We appreciate Ms. Pham Hoai Linh and Mr. Van Duc Huy for helping with the data analysis.
Author’s contributions
NTQ, BTKL, and HTC designed the study, NTQ performed the experiments and data acquisition, and all authors read and approved the final manuscript.
Funding
This study was funded by the Vietnam National Foundation for Science and Technology Development (grant no. 106.02 2019.50).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sung
H.,
Ferlay
J.,
Siegel
R.L.,
Laversanne
M.,
Soerjomataram
I.,
Jemal
A.,
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal for Clinicians.
2021;
71
(3)
:
209-49
.
View Article PubMed Google Scholar -
Bộ Y tế. Tình hình Ung thư tại Việt Nam. Cổng thông tin điện tử Bộ Y Tế. 2021. 2021;
:
(In Vietnamese)
.
-
Sung
H.,
Ferlay
J.,
Siegel
R.L.,
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 2021;71(3):209-49. 2021
.
View Article Google Scholar -
Siegel
R.L.,
Miller
K.D.,
Fuchs
H.E.,
Jemal
A.,
Cancer Statistics, 2021. CA: a Cancer Journal for Clinicians.
2021;
71
(1)
:
7-33
.
View Article PubMed Google Scholar -
Bauman
J.R.,
Temel
J.S.,
The integration of early palliative care with oncology care: the time has come for a new tradition. Journal of the National Comprehensive Cancer Network : JNCCN.
2014;
12
(12)
:
1763-71
.
View Article PubMed Google Scholar -
Huang
J.,
Chan
S.C.,
Ngai
C.H.,
Lok
V.,
Zhang
L.,
Lucero-Prisno
D.E.,
Disease Burden, Risk Factors, and Trends of Leukaemia: A Global Analysis. Frontiers in Oncology.
2022;
12
:
904292
.
View Article PubMed Google Scholar -
Hussein
R.,
El-Anssary
A.,
Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants, Herbal medicine, Philip F. Builders, IntechOpen. 2019
.
-
Lowe
H.I.,
Daley-Beckford
D.,
Toyang
N.J.,
Watson
C.,
Hartley
S.,
Bryant
J.,
The Anti-cancer Activity of Vernonia divaricata Sw against Leukaemia, Breast and Prostate Cancers In Vitro. The West Indian Medical Journal.
2014;
63
(4)
:
285-8
.
View Article PubMed Google Scholar -
Kim
W.,
Whatcott
C.,
Siddiqui-Jain
A.,
Anthony
S.,
Bearss
D.J.,
Warner
S.L.,
The CDK9 Inhibitor, Alvocidib, Potentiates the Non-Clinical Activity of Azacytidine or Decitabine in an MCL-1-Dependent Fashion, Supporting Clinical Exploration of a Decitabine and Alvocidib Combination. Blood.
2018;
132
(Supplement 1)
:
4355
.
View Article Google Scholar -
Chen
K.T.J.,
Militao
G.G.C.,
Anantha
M.,
Witzigmann
D.,
Leung
A.W.Y.,
Bally
M.B.,
Development and characterization of a novel flavopiridol formulation for treatment of acute myeloid leukemia. Journal of controlled release : official journal of the Controlled Release Society.
2021;
333
:
246-57
.
View Article Google Scholar -
Karp
J.E.,
Ross
D.D.,
Yang
W.,
Tidwell
M.L.,
Wei
Y.,
Greer
J.,
Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clinical Cancer Research.
2003;
9
(1)
:
307-15
.
PubMed Google Scholar -
Mitina O. Src kinases and Flt3: phosphorylation, interference with receptor maturation and mechanism of association (Doctoral dissertation, lmu).. 2005
.
-
Hayakawa
F.,
Towatari
M.,
Kiyoi
H.,
Tanimoto
M.,
Kitamura
T.,
Saito
H.,
Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene.
2000;
19
(5)
:
624-31
.
View Article PubMed Google Scholar -
Mizuki
M.,
Fenski
R.,
Halfter
H.,
Matsumura
I.,
Schmidt
R.,
Müller
C.,
Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood.
2000;
96
(12)
:
3907-14
.
View Article PubMed Google Scholar -
H.T.
Chi,
B.T.
Ly,
Screening for the Potent FMS-Like Tyrosine Kinase 3 (FLT3) Inhibitors from Vietnamese Traditional Herbal Medicines. International Journal of Pharmaceutical Research.
2022;
13
(2)
:
2890-2900
.
View Article Google Scholar -
Johnson
W.,
Tchounwou
P.B.,
Yedjou
C.G.,
Therapeutic Mechanisms of Vernonia amygdalina Delile in the Treatment of Prostate Cancer. Molecules (Basel, Switzerland).
2017;
22
(10)
:
1594
.
View Article PubMed Google Scholar -
L.A. Olufunmilayo,
M.J. Oshiobugie,
A.I. Iyobosa,
Gas chromatography-mass spectrometry (GC-MS) analysis of phytocomponents in the root, stem nark and leaf of Vernonia amygdalina. World Journal of Pharmaceutical Research.
2017;
6
(2)
:
35-49
.
View Article Google Scholar -
Hasibuan
P.A.,
Harahap
U.,
Sitorus
P.,
Satria
D.,
The anticancer activities of Vernonia amygdalina Delile. Leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon.
2020;
6
(7)
:
e04449
.
View Article PubMed Google Scholar -
Wong
F.C.,
Woo
C.C.,
Hsu
A.,
Tan
B.K.H.,
The anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One.
2013;
8
(10)
:
e78021-e
.
View Article Google Scholar -
Joseph
J.,
Lim
V.,
Rahman
H.,
Othman
H.,
Samad
N.,
Anti-cancer effects of Vernonia amygdalina: A systematic review. Tropical Journal of Pharmaceutical Research.
2020;
19
(8)
:
1775-84
.
View Article Google Scholar -
Quân
N.T.,
Tâm
P.T.M.,
S
H.K.,
Chí
H.T.,
Đánh giá tác động gây độc tế bào ung thư máu cấp dòng tuỷ của cao chiết từ cây lá đắng. Tạp chí Khoa học và Công nghệ - Đại học Đà Nẵng.
2022;
2022
:
49-53 (In Vietnamese)
.
-
Grosveld
G.,
Verwoerd
T.,
Agthoven
T. van,
Klein
A. de,
Ramachandran
K.L.,
Heisterkamp
N.,
The chronic myelocytic cell line K562 contains a breakpoint in bcr and produces a chimeric bcr/c-abl transcript. Molecular and Cellular Biology.
1986;
6
(2)
:
607-16
.
View Article PubMed Google Scholar -
Tsuchiya
S.,
Yamabe
M.,
Yamaguchi
Y.,
Kobayashi
Y.,
Konno
T.,
Tada
K.,
Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). International Journal of Cancer.
1980;
26
(2)
:
171-6
.
View Article PubMed Google Scholar -
Ly
B.T.,
Chi
H.T.,
Yamagishi
M.,
Kano
Y.,
Hara
Y.,
Nakano
K.,
Inhibition of FLT3 expression by green tea catechins in FLT3 mutated-AML cells. PLoS One.
2013;
8
(6)
:
e66378
.
View Article PubMed Google Scholar -
Livak
K.J.,
Schmittgen
T.D.,
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.).
2001;
25
(4)
:
402-8
.
View Article PubMed Google Scholar -
Kumar
S.,
Sharma
V.K.,
Yadav
S.,
Dey
S.,
Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chemistry Central Journal.
2017;
11
(1)
:
56
.
View Article PubMed Google Scholar -
Nga
N.T.H.,
Xinh
P.T.,
Khảo sát đột biến gen BCR/ABL gây kháng Imatinib trên bệnh nhân bạch cầu mạn dòng tuỷ (CML) Bằng phương pháp giải trình tự chuỗi DNA. Bệnh viện Truyền máu Huyết học, Tp. Hồ Chí Minh; 2013.. Đề tài nghiên cứu khoa học Sở Khoa học và Công nghệ Tp. HCM.
2013;
:
(In Vietnamese)
.
-
Chen
Q.,
Kirk
K.,
Shurubor
Y.I.,
Zhao
D.,
Arreguin
A.J.,
Shahi
I.,
Rewiring of Glutamine Metabolism Is a Bioenergetic Adaptation of Human Cells with Mitochondrial DNA Mutations. Cell Metabolism.
2018;
27
(5)
.
View Article PubMed Google Scholar -
Riquelme
I.,
Tapia
O.,
Espinoza
J.A.,
Leal
P.,
Buchegger
K.,
Sandoval
A.,
The Gene Expression Status of the PI3K/AKT/mTOR Pathway in Gastric Cancer Tissues and Cell Lines. Pathology Oncology Research.
2016;
22
(4)
:
797-805
.
View Article PubMed Google Scholar -
Jiang
L.,
Tang
Z.,
Expression and regulation of the ERK1/2 and p38 MAPK signaling pathways in periodontal tissue remodeling of orthodontic tooth movement. Molecular Medicine Reports.
2018;
17
(1)
:
1499-506
.
View Article PubMed Google Scholar -
Burkhart
D.L.,
Ngai
L.K.,
Roake
C.M.,
Viatour
P.,
Thangavel
C.,
Ho
V.M.,
Regulation of RB transcription in vivo by RB family members. Molecular and Cellular Biology.
2010;
30
(7)
:
1729-45
.
View Article PubMed Google Scholar -
Kim
S.I.,
Yeo
S.G.,
Gen
Y.,
Ju
H.R.,
Kim
S.H.,
Park
D.C.,
Differences in autophagy-associated mRNAs in peritoneal fluid of patients with endometriosis and gynecologic cancers. European Journal of Obstetrics & Gynecology and Reproductive Biology.
2019;
2
:
100016
.
View Article PubMed Google Scholar -
Floros
K.V.,
Thomadaki
H.,
Florou
D.,
Talieri
M.,
Scorilas
A.,
Alterations in mRNA expression of apoptosis-related genes BCL2, BAX, FAS, caspase-3, and the novel member BCL2L12 after treatment of human leukemic cell line HL60 with the antineoplastic agent etoposide. Annals of the New York Academy of Sciences.
2006;
1090
(1)
:
89-97
.
View Article PubMed Google Scholar -
Jaberipour
M.,
Habibagahi
M.,
Hosseini
A.Z.,
Abbasi
M.,
Sobhani-lari
A.,
Talei
A-r,
Detection of B cell lymphoma 2, tumor protein 53, and FAS gene transcripts in blood cells of patients with breast cancer. Indian Journal of Cancer.
2010;
47
(4)
:
412-7
.
View Article Google Scholar -
Lee
K.P.,
Baek
S.,
Yoon
M.S.,
Park
J.S.,
Hong
B.S.,
Lee
S.J.,
Potential anticancer effect of aspirin and 2'-hydroxy-2,3,5'-trimethoxychalcone-linked polymeric micelles against cervical cancer through apoptosis. Oncology Letters.
2022;
23
(1)
:
31
.
View Article PubMed Google Scholar -
Perveen
S.,
Ashfaq
H.,
Shahjahan
M.,
Manzoor
A.,
Tayyeb
A.,
Citrullus colocynthis regulates de novo lipid biosynthesis in human breast cancer cells. Journal of Cancer Research and Therapeutics.
2020;
16
(6)
:
1294-301
.
View Article PubMed Google Scholar -
Kelava
T.,
Cavar
I.,
Culo
F.,
Biological actions of drug solvents. Periodicum Biologorum.
2011;
113
:
311-20
.
-
Gad
S.E.,
Sullivan
D.W.,
Dimethyl Sulfoxide (DMSO). Encyclopedia of Toxicology (Third Edition), Academic Press, Oxford 2014, p.166-168.
.
View Article Google Scholar -
Cell
H.P.,
Hematopoietic Stem Cell Collections and Cellular Therapies. Clinical Principles of Transfusion Medicine. 2018 Feb 5:151.. 2018;
:
151-67
.
View Article Google Scholar -
Boik J. Natural compounds in cancer therapy. Oregon Medical Press, Minnesota, USA, 2001.. 2001
.
-
Iweala
E.,
Liu
F.F.,
Cheng
R.R.,
Li
Y.,
Omonhinmin
C.,
Zhang
Y.J.,
Anti-Cancer and Free Radical Scavenging Activity of Some Nigerian Food Plants in vitro. International Journal of Cancer Research.
2015;
11
(1)
:
41-51
.
View Article Google Scholar -
Khalafalla
M.M.,
Abdellatef
E.,
Daffalla
H.M.,
Nassrallah
A.A.,
Aboul-Enein
K.M.,
Lightfoot
D.A.,
Antileukemia activity from root cultures of Vernonia amygdalina. Journal of Medicinal Plants Research.
2009;
3
(8)
:
556-62
.
-
Khay
M.,
Toeng
P.,
Mahiou-Leddet
V.,
Mabrouki
F.,
Sothea
K.,
Ollivier
E.,
HPLC analysis and cytotoxic activity of Vernonia cinerea. Natural Product Communications.
2012;
7
(10)
:
1259-62
.
View Article PubMed Google Scholar -
Thomas
E.,
Gopalakrishnan
V.,
Somasagara
R.R.,
Choudhary
B.,
Raghavan
S.C.,
Extract of Vernonia condensata, Inhibits Tumor Progression and Improves Survival of Tumor-allograft Bearing Mouse. Scientific Reports.
2016;
6
(1)
:
23255
.
View Article PubMed Google Scholar -
I.I. Ijeh,
C.E. Ejike,
Current perspectives on the medicinal potentials of Vernonia amygdalina Del. Journal of Medicinal Plants Research.
2010;
5
(7)
:
1051-1061
.
-
Peña-Morán
O.A.,
Villarreal
M.L.,
Álvarez-Berber
L.,
Meneses-Acosta
A.,
Rodríguez-López
V.,
Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules (Basel, Switzerland).
2016;
21
(8)
:
1013
.
View Article PubMed Google Scholar -
Giacinti
C.,
Giordano
A.,
RB and cell cycle progression. Oncogene.
2006;
25
(38)
:
5220-7
.
View Article PubMed Google Scholar -
Fulda
S.,
Debatin
K.M.,
Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
2006;
25
(34)
:
4798-811
.
View Article PubMed Google Scholar -
Barrett
L.W.,
Fletcher
S.,
Wilton
S.D.,
Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cellular and Molecular Life Sciences.
2012;
69
(21)
:
3613-34
.
View Article PubMed Google Scholar -
Alberts B. Molecular biology of the cell. Garland science; 2017 Aug 7..
.
-
Panda
S.,
A Review on Regulation of Gene in Eukaryotes. International Journal of Bioassays.
2016;
5
(8)
:
4729
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 10 No 8 (2023)
Page No.: 5855-5863
Published on: 2023-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4877 times
- PDF downloaded - 1298 times
- XML downloaded - 332 times
- Supplement downloaded - 963 times
 Biomedpress
Biomedpress







