Abstract
Background: Ovarian germ cell tumors (OGCTs) are the most common type of ovarian tumor, encompassing mature and immature teratoma and malignant tumors. The standard treatment for mature teratoma is surgery, traditionally involving oophorectomy or salpingo-oophorectomy. However, these procedures reduce ovarian tissue reserve and carry a high risk of bilateral tumor development. Therefore, ovarian tissue-sparing tumorectomy has emerged as a popular alternative. This procedure aims to remove the tumor while preserving as much healthy ovarian tissue as possible, mitigating the risk of bilateral tumor development and preserving future fertility. The primary treatment approach for malignant OGCTs is surgery followed by postoperative chemotherapy. This combined treatment strategy aims to achieve complete resection and increase the likelihood of a long-term cure. There are very few studies on pediatric OGCTs within the Asian population. Therefore, this study aimed to comprehensively review the clinical presentation, treatment modalities, and outcomes of pediatric OGCTs at a tertiary hospital in Vietnam.
Methods: This retrospective study examined patients aged 1 month to 15 years treated for OGCTs at Children's Hospital No.2 between January 2016 and January 2021. Their symptoms, age at diagnosis, imaging, tumor markers, treatment methods, and complications were recorded. The long-term outcomes were the rate of recurrence and mortality.
Results: This study comprised 162 patients with a mean age of 9.1 +/- 3.6 years. Most had mature teratomas (n = 137), followed by immature teratomas (n = 11), yolk sac tumors (n = 7), dysgerminomas (n = 5), and mixed germ cell tumors (n = 2). At admission, their most common complaint was abdominal pain (73.5%), followed by abdominal mass (20.4%). Alpha-fetoprotein (AFP) levels were elevated in nine patients with immature teratomas, all with yolk sac tumors, all with malignant mixed germ cell tumors, and one with dysgerminoma. Beta human chorionic gonadotrophin (b hCG) levels were elevated in two patients with dysgerminomas and all with malignant mixed germ cell tumors. The mean tumor size was 9.1 +/- 5.1 cm (2.8–32 cm). Our study identified size tumor 8 cm, solid mass, and positive tumor markers as important predictors of malignancy. The median follow-up was 3.3 years (3 months to 6.5 years). Among patients with mature teratomas, three (2.2%) experienced recurrence, and none died. Among patients with immature teratomas and malignant OGCTs, one (4%) experienced relapse and died.
Conclusions: Both benign and malignant OGCTs have a good prognosis. Imaging and tumor markers are important for initial diagnosis and treatment planning. Ovarian-sparing tumorectomy is a safe and effective management option for mature teratomas. Surgical resection combined with platinum-based chemotherapy achieves good outcomes for malignant OGCTs.
Introduction
Ovarian tumors are uncommon in childhood, with an estimated overall incidence of 2.6 per 100,000 prepubertal females. However, their incidence can vary by patient age and histological diagnosis1. Ovarian germ cell tumors (OGCTs) are the most common type of ovarian tumor, accounting for 47.3–87.7%2. Mature teratoma is the most frequent OGCT. Immature teratoma and malignant OGCTs, including yolk sac tumors, dysgerminoma, gonadoblastoma, embryonal carcinoma, and mixed germ cell tumors, are relatively rare.
The standard treatment for mature teratoma is surgery, often involving oophorectomy or salpingo-oophorectomy. However, these procedures can reduce ovarian tissue reserve and may lead to bilateral tumor development. Consequently, there is a growing preference for ovarian tissue-sparing tumorectomy. This procedure aims to remove the tumor while preserving as much healthy ovarian tissue as possible, minimizing the risk of bilateral tumor development and preserving future fertility3. The primary treatment approach for malignant OGCTs is surgery combined with adjuvant chemotherapy. Complete resection is essential to provide patients with a possible long-term cure4, 5.
Despite the significance of understanding and managing OGCTs in children, limited research is available, especially in the Asian population. Over the past decade, our center has made changes to treatment approaches. This study aimed to comprehensively review the clinical presentation, treatment modalities, and outcomes of pediatric OGCTs at a tertiary hospital in Viet Nam.
Methods
Setting and study design
We retrospectively reviewed all pediatric patients (aged ≤15 years at diagnosis) with OGCTs treated between January 2016 and January 2021 at Children’s Hospital No.2 in Ho Chi Minh City, Vietnam. We recorded their symptoms, age at diagnosis, imaging, tumor markers, treatment methods, and complications.
The OGCT diagnosis was based on a combination of clinical presentation, tumor markers (alpha-fetoprotein [AFP] and beta human chorionic gonadotrophin [βhCG]), and imaging characteristics. In cases where preoperative and intraoperative features suggested a benign tumor and its macroscopic appearance allowed for separating the ovarian tissue from the tumor, an ovarian tissue-sparing tumorectomy was performed. However, if there was any uncertainty about the benign nature of the tumor before surgery, a complete unilateral spingo-oophorectomy was performed. In situations where complete tumor resection risked injury to adjacent organs, resection was canceled, and neoadjuvant chemotherapy was administered. In such cases, a second surgery was performed to remove residual tumors and/or lymph nodes. Our study used the Children’s Oncology Group (COG) staging system. Immature teratomas were graded according to the Norris classification. The median follow-up was 3.3 years (3 months to 6.5 years).
This study was approved by the ethics committee of Children’s Hospital No.2 (225/QĐ-BVNĐ2) and accepted by The University of Medicine and Pharmacy at Ho Chi Minh City6.
Statistical analysis
All statistical analyses were performed using SPSS software (version 25.0; IBM, United States). Discrete variables are described as percentages. Continuous variables are described as means and standard deviations when normally distributed or medians with ranges when non-normally distributed. Normally distributed continuous variables were compared between groups using Student’s t-test, and non-normally distributed continuous variables were compared using the nonparametric Mann–Whitney U test. Discrete variables were compared between groups using Fisher’s exact test. A 95% confidence interval was used, and a p < 0.05 was considered statistically significant.
Overall survival (OS) was calculated as the time from the date of diagnosis to death from any cause. Event-free survival (EFS) was calculated as the time between diagnosis and any event (e.g., malignant germ cell recurrence and death). The last follow-up was censored for patients who remained alive. Survival was analyzed using the Kaplan–Meier method.
Results
Patient characteristics
Our study enrolled all 162 patients with a mean age of 9.1 ± 3.6 years (1–15 years). At admission, their most common complaint was abdominal pain (73.5%), followed by abdominal mass (20.4%). Among them, 23 (13%) were discovered incidentally with ultrasound. No patient with mature teratomas had elevated AFP or βhCG levels. However, AFP levels were elevated in 19 (11.7%) patients (47–105,031 ng/mL): nine with immature teratomas, seven with yolk sac tumors, one with dysgerminoma, and two with malignant mixed germ cell tumors. Four (2.5%) patients had elevated βhCG levels (60–16,638 mIU/mL): two with dysgerminomas and two with malignant mixed germ cell tumors (Table 1).
Cell surface-associated mucin 16 (MUC16/CA-125) levels were measured at diagnosis in 68 patients. They were elevated in 17 patients: seven with mature teratomas, five with immature teratomas, four with yolk sac tumors, and one with dysgerminoma.
| Parameters | Number (%) |
|---|---|
| Mean (range) | 9.1 (1-15 years) |
| Presenting symptoms | |
| Abdominal pain | 119 (73.5%) |
| Abdominal mass | 33 (20.4%) |
| Vomitting | 21 (13.0%) |
| Valginal bleeding | 5 (3.1%) |
| Urinary symptoms | 1 (0.6%) |
| Constipation | 1 (0.6%) |
| Prepubertal | 105 (64.8%) |
| Postpubertal | 57 (35.2%) |
| Presenting signs | |
| Palpable mass | 119 (73.5%) |
| Tumor markers | |
| Elevated | |
| AFP | 19 (11.7%) |
| hCG | 4 (2.5%) |
| AFP and bhCG | 3 (1.9%) |
| Normal | 142 (87.7%) |
| Imaging | |
| US | 162 (100%) |
| CT | 108 (66.7%) |
| Histopathology | n (%) |
|---|---|
| Mature teratoma | 137 (84.6%) |
| Immature teratoma | 11 (6.8%) |
| Grade 1 | 6 |
| Grade 2 | 1 |
| Grade 3 | 4 |
| Yolk sac | 7 (4.3%) |
| Dysgerminoma | 5 (3.1%) |
| Mixed germ cell | 2 (1.2%) |
| Total | 162 |
| Surgical procedure | n (%) | Malignant No. |
|---|---|---|
| Collection of ascites | 24 (96%) | 2 (8.3%) |
| Omentectomy | 22 (88%) | 8 (36.4%) |
| Lymph node sampling | 17 (68%) | 4 (23.5%) |
| Peritoneal biopsied | 8 (32%) | 4 (50%) |
| Contralateral ovarian biopsy | 2 (8%) | 0 |
| Total | 25 |
Diagnostic imaging
The tumor structure was reported to be cystic in 62 (38.3%) patients, solid in 18 (11.1%), and mixed cystic-solid in 82 (50.6%).
The median tumor size was 7.8 cm (2.8–32.0 cm). All malignant tumors exceeded 6 cm, with an average of 12 cm (6.5–25.0 cm), significantly larger than mature teratomas (mean = 7.3 cm, range = 2.8–32.0 cm, p < 0.001). We examined two different diameter thresholds to determine which would be the most helpful clinically: 6 cm (the size that all malignancies exceeded in our study) and 8 cm7. A threshold of ≥ 8 cm had a sensitivity of 84% and a specificity of 62.8% for malignant tumors. A threshold of ≥ 6 cm had a sensitivity of 100% and a specificity of 42% for malignant tumors.
Calcifications were observed in 108 (66.7%) patients. Fat nodes were observed in 91.7% of patients with teratomas and 25% with malignant OGCTs.
Histology
The predominant histopathologic subtype was mature teratoma (n = 137, 84.5%), followed by immature teratomas (n = 11, 6.8%) and malignant tumors (n = 14, 8.6%; Table 2).
Staging
Immature teratomas and malignant tumors were staged postoperatively. Seven patients had stage I, five had stage II, 17 had stage III, and none had metastasis at diagnosis.
Intraoperative characteristics
The tumors were right-sided in 90 (55.6%) patients, left-sided in 64 (39.5%), and bilateral in eight (4.9%). At operation, 35 (21.6%) patients had ovarian torsion: 33 with mature teratomas and two with malignant tumors. Seven patients experienced preoperative tumor rupture, and 11 experienced intraoperative rupture. Ruptures were more common with laparoscopy (13.2%) than laparotomy (2.3%, p = 0.011). The mean operating time was 83.8 ± 27.3 min. Operating time did not differ significantly between laparoscopy (76.5 ± 21.3 min) and laparotomy (83.1 ± 20.3 min) for mature teratomas (p = 0.067).
Treatment
Mature teratomas
All mature teratomas were treated with surgery alone. The initial operative approach was laparotomy in 68 (49.6%) patients and laparoscopy in 69 (50.4%). The procedures consisted of ovarian tissue-sparing tumorectomy (n = 99, 72.3%), oophorectomy (n = 18, 13.1%), and salpingo-oophorectomy (n = 20, 14.6%).
Immature teratomas and malignant OGCTs
All immature teratomas and malignant tumors were removed with open surgery. Operative staging procedures were performed in patients with immature teratomas and malignant germ cell tumors (Table 3).
Patients with immature teratomas at stage II or above received chemotherapy. All patients with malignant tumors received chemotherapy. One patient received chemotherapy before surgical excision, and 20 patients received postoperative chemotherapy. The JEB (carboplatin, etoposide, and bleomycin) and BEP (cisplatin, etoposide, and bleomycin) regimens were used.
Outcome: The median follow-up was 3.3 years (3 months to 6.5 years).
Mature teratomas
Metachronous disease in the contralateral ovary was observed in three (2.2%) patients with mature teratoma within five, 13, and 23 months after the first operation, respectively.
Recurrence was observed in three (2.2%) patients, and no patients died.
Immature teratomas and malignant OGCTs
One (4%) patient experienced recurrence and died. She was 12 years old, her AFP level was elevated (650,627 ng/mL), and her βhCG level was normal. Spingo-oophorectomy and omentectomy were performed intraoperatively. The histopathology was a yolk sac tumor. She was indicated for chemotherapy, but she was re-examined three months later. She received six cycles of chemotherapy with the JEB regimen. She was hospitalized with liver metastases ten months later; her AFP level was elevated (2,128 ng/mL), and her βhCG level was normal. She received palliative care and died two months later.
The three-year OS and EFS were 95.5% and 95.8%, respectively. The three-year OS and EFS rates were 100% and 100% for stage I-II and 90.9% and 91.7% for stage III, respectively.
Discussion
Previous studies have reported discrepant age distributions for malignancies. While some suggested a higher occurrence of malignancies at younger ages, with approximately 80% of ovarian tumors in children aged ≤9 years being malignant, De Backer found a more similar distribution of malignancy across different age groups, including an increase with age8. Our study also observed an even distribution of malignant tumors by age, with malignancy rates of 14.8% in the 0–5-year-old group, 14.9% in the 6–10-year-old group, and 15.4% in the 11–15-year-old group.
The presenting symptoms of ovarian tumors in children are often nonspecific, with abdominal pain and abdominal mass being the most common manifestations. Tumor markers such as AFP, βhCG, and CA-125 are important in diagnosing malignant ovarian tumors and monitoring their treatment. AFP and βhCG are commonly used and show high specificity for malignant OGCTs since mature teratomas are typically not positive for them. Patients with immature teratomas, yolk sac tumors, and malignant mixed germ cell tumors often present with elevated serum AFP levels.
In our study, most patients with malignant OGCTs had elevated tumor markers, with only four (16%) diagnosed with dysgerminoma and immature teratoma having normal marker levels. Four patients had AFP levels exceeding 10,000 ng/mL at diagnosis, with three classified as stage III and one as stage II. Additionally, CA-125, commonly associated with epithelial-type tumors, may also be elevated with OGCTs.
Imaging is crucial in the initial staging and treatment planning of ovarian tumors. Consistent with other studies, the risk of malignancy was directly associated with the tumor size in our study. In our study population, all malignancies exceeded 6 cm, and the 8 cm cutoff was significant for distinguishing malignancy. Solid masses also showed a higher risk of malignancy.
Like in the study by De Backe, the common histopathologic subtype in our study was mature teratoma.
Surgical resection is a vital component of the treatment plan for OGCTs, providing valuable information for staging. The surgical approach (laparotomy or laparoscopy) depends on the level of preoperative suspicion of malignancy, the size of the tumor, and the skill of the surgeon. The effectiveness of open or laparoscopic tumorectomy for mature teratoma is well-documented3, 9. Laparotomy allows for reducing the risk of spillage. Laparoscopic surgery is now generally accepted due to its tremendous advantages in intraoperative blood loss, postoperative recovery, and patients’ cosmetic satisfaction compared to traditional laparotomy. However, laparoscopy has been criticized for its associations with incorrect staging and a higher risk of intraperitoneal spillage. Therefore, we chose the laparoscopic approach for small mature ovarian teratomas (≤ 6 cm). Ultrasound and computed tomography scans can easily detect and characterize mature teratomas based on typical imaging features, including soft tissue, calcification or ossification, and fat, with no evidence of lymph node involvement or live metastases10.
Preserving fertility and gonadal function is important in the surgical treatment of pediatric ovarian tumors because their prognosis is good with a low recurrence rate and because there is evidence of bilaterality (synchronous or metachronous). Our study demonstrated that ovarian tumor recurrence and metachronous disease occur. Therefore, we strongly recommend that all pediatric patients undergo long-term follow-up after surgical resection of an ovarian mature teratoma.
There is a lack of consensus on the management strategy for ovarian immature teratoma, and there is an ongoing debate about their classification in malignant tumor analyses. We classified immature teratomas as malignant OGCTs because of their potential to recur as malignant tumors and because 30% of these patients have microscopic foci of malignant elements (specifically, immature teratoma with high AFP levels)11. If an immature teratoma is diagnosed histopathologically after an ovarian-sparing tumorectomy, chemotherapy or oophorectomy should be further discussed with the patient. Surgery alone may be curative for patients with resected stage I ovarian immature teratoma.
The COG established the current surgical staging of malignant OGCTs12. Ascite collection was the most common procedure performed (96%). Similarly, Elgendy et al. reported that this step was performed in 94.6% of their patients13. Omentectomy was performed in 88% of patients. The rate of omentectomy and other operative staging procedures is shown in Table 2.
Our study demonstrated good outcomes for malignant OGCTs with a multidisciplinary treatment strategy. Our result was similar to several national studies13, 14, 15.
Conclusion
Both benign and malignant OGCTs have a good prognosis. Imaging and tumor markers are important for initial diagnosis and treatment planning. Surgery is not only a treatment but also provides information for staging and pathological classification. Ovarian-sparing tumorectomy, also called fertility-sparing surgery, is a safe and effective option for managing mature teratomas. Surgical resection combined with platinum-based chemotherapy achieves good outcomes for malignant OGCTs. Close follow-up of patients with tumor markers and ultrasound scans is important for diagnosing recurrence and metachronous disease.
Abbreviations
COG: Children’s Oncology Group, EFS: Event-free survival, GCT: Germ cell tumor, OGCT: Ovarian germ cell tumor, OS: Overall survival
Acknowledgments
We are grateful for our colleagues at the Department of Hemato-Oncology and Department of General Surgery, Children’s Hospital #2, Ho Chi Minh City for their great assistance.
Author’s contributions
Truong Dinh Khai and Phung Nguyen Viet carried out the experiment. Phung Nguyen Viet Hung wrote the manuscript with support from Truong Dinh Khai and Tran Thi Phuong. Tran Van Hung and Nguyen Nguyen Thang helped collecting and interpreting data. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
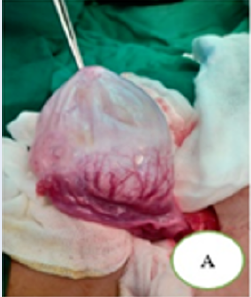
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Lindfors
O.,
Primary ovarian neoplasms in infants and children. A study of 81 cases diagnosed in Finland and Sweden. Annales Chirurgiae et Gynaecologiae Fenniae. Supplementum.
1971;
177
:
1-66
.
PubMed Google Scholar -
Vaysse
C.,
Delsol
M.,
Carfagna
L.,
Bouali
O.,
Combelles
S.,
Lemasson
F.,
Ovarian germ cell tumors in children. Management, survival and ovarian prognosis. A report of 75 cases. Journal of Pediatric Surgery.
2010;
45
(7)
:
1484-90
.
View Article PubMed Google Scholar -
Luczak
J.,
Baglaj
M.,
Ovarian teratoma in children: a plea for collaborative clinical study. Journal of Ovarian Research.
2018;
11
(1)
:
75
.
View Article PubMed Google Scholar -
Billmire
D.,
Vinocur
C.,
Rescorla
F.,
Cushing
B.,
London
W.,
Schlatter
M.,
Children's Oncology Group (COG)
Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. Journal of Pediatric Surgery.
2004;
39
(3)
:
424-9
.
View Article PubMed Google Scholar -
de Campos Vieira Abib
S.,
Chui
C.H.,
Cox
S.,
Abdelhafeez
A.H.,
Fernandez-Pineda
I.,
Elgendy
A.,
International Society of Paediatric Surgical Oncology (IPSO) Surgical Practice Guidelines. Ecancermedicalscience.
2022;
16
:
1356
.
View Article PubMed Google Scholar -
Chumpitazi
B.P.,
Fishman
S.J.,
Nurko
S.,
Long-Term Clinical Outcome After Botulinum Toxin Injection in Children With Nonrelaxing Internal Anal Sphincter. Official journal of the American College of Gastroenterology.
2009;
104
(4)
:
976-83
.
-
Oltmann
S.C.,
Garcia
N.,
Barber
R.,
Huang
R.,
Hicks
B.,
Fischer
A.,
Can we preoperatively risk stratify ovarian masses for malignancy?. Journal of Pediatric Surgery.
2010;
45
(1)
:
130-4
.
View Article PubMed Google Scholar -
Backer
A. De,
Madern
G.C.,
Oosterhuis
J.W.,
Hakvoort-Cammel
F.G.,
Hazebroek
F.W.,
Ovarian germ cell tumors in children: a clinical study of 66 patients. Pediatric Blood & Cancer.
2006;
46
(4)
:
459-64
.
View Article PubMed Google Scholar -
Templeman
C.L.,
Hertweck
S.P.,
Scheetz
J.P.,
Perlman
S.E.,
Fallat
M.E.,
The management of mature cystic teratomas in children and adolescents: a retrospective analysis. Human Reproduction (Oxford, England).
2000;
15
(12)
:
2669-72
.
View Article PubMed Google Scholar -
Chabaud-Williamson
M.,
Netchine
I.,
Fasola
S.,
Larroquet
M.,
Lenoir
M.,
Patte
C.,
Ovarian-sparing surgery for ovarian teratoma in children. Pediatric Blood & Cancer.
2011;
57
(3)
:
429-34
.
View Article PubMed Google Scholar -
Olson
T.A.,
Schneider
D.T.,
Perlman
E.J.,
Principles and Practice of Pediatric Oncology. In: Philip AP, David GP, editors. Germ Cell Tumors. 7th ed: LWW; 2015. p. 1045-67. 2015
.
-
Frazier
A.L.,
Hale
J.P.,
Rodriguez-Galindo
C.,
Dang
H.,
Olson
T.,
Murray
M.J.,
Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. Journal of Clinical Oncology.
2015;
33
(2)
:
195-201
.
View Article PubMed Google Scholar -
Elgendy
A.,
Mostafa
M.,
Salem
M.A.,
Ali
A.,
Khairi
A.,
Shehata
S.,
Surgical resection and outcome of malignant ovarian germ cell tumors in children-a national multicentric study compared to international results. Pediatric Surgery International.
2020;
36
(9)
:
1067-75
.
View Article PubMed Google Scholar -
Terenziani
M.,
Bisogno
G.,
Boldrini
R.,
Cecchetto
G.,
Conte
M.,
Boschetti
L.,
Malignant ovarian germ cell tumors in pediatric patients: The AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) study. Pediatric Blood {&}amp; Cancer.
2017;
64
(11)
:
1-8
.
View Article PubMed Google Scholar -
Park
J.Y.,
Kim
D.Y.,
Suh
D.S.,
Kim
J.H.,
Kim
Y.M.,
Kim
Y.T.,
Outcomes of pediatric and adolescent girls with malignant ovarian germ cell tumors. Gynecologic Oncology.
2015;
137
(3)
:
418-22
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 11 (2023)
Page No.: 6057-6064
Published on: 2023-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4094 times
- PDF downloaded - 1149 times
- XML downloaded - 135 times
 Biomedpress
Biomedpress






