Abstract
Introduction: Pelvic varicosity, a subset of pelvic venous incompetence (PVI), is considered a multifactorial, chronic disease with a progressive course. One effective therapeutic approach may be the use of drugs that inhibit oxidative stress (OS) reactions. The aim of this study was to evaluate the effect of an antioxidant complex on the state of the lipid peroxidation system and venous hemodynamic parameters in the treatment of patients with pelvic varicose veins.
Methods: One hundred fifty patients with PVI were divided into two groups of seventy-five each, comparable in basic characteristics. Treatment for both groups included standard therapy with one of the venotropic drugs for 60 days. Additionally, the patients in the second group received an antioxidant complex application (ACA) for 30 days (one course), with a total of three courses over two months. Spectrophotometric and immunoenzymatic research methods were used.
Results: In patients with PVI, the application of an antioxidant complex in combination with baseline venotropic therapy resulted in a statistically significant decrease in levels of LH, CDs, TBARs, and an increase in Catalase, SOD, GPO, GR, GST, and GSH after treatment. Additionally, there was an increase in blood flow velocity in varicose pelvic veins (iliac, ovarian, and arcuate), as well as a decrease in the duration of retrograde discharge to 0.3 cm.
Conclusion: The use of antioxidant drugs (superoxide dismutase, acetyl glutathione, astaxanthin) in combination with venotropic therapy for the treatment of PVI significantly improves the indices of the lipid peroxidation-antioxidant defense system and venous hemodynamics in the pelvic organs of this patient cohort. The advantages of this complex treatment are evident both in comparison with the data before treatment and with the data from patients on venotropic therapy alone.
Introduction
Pelvic varicosity is a distinct subgroup of pelvic venous incompetence (hereinafter referred to as PVI), classified under ICD-10 code I86.2. It is considered a multifactorial, chronic disease with a progressive course (PVI frequency varies widely, from 5 to 80%)1, 2. The primary clinical features of PVI include menstrual disorders, chronic pelvic pain, psycho-emotional disorders, and infertility. These symptoms significantly impair both the work capacity and quality of life of affected female patients3, 4. Typically, PVI progresses chronically and worsens in the absence of adequate treatment4. Most experts link PVI to prolonged exposure to dynamic hypervolemia and hypertension on the venous walls, which underlie the pathological process5, 6. Consequently, there are morphological changes in the venous bed that lead to retrograde blood flow, perfusion disorders, and pelvic phlebohypertension7. However, viewing the pathogenesis of PVI solely through the lens of venous hemodynamic disorders does not fully explain all the mechanisms of disease progression, which hinders the effectiveness of treatment8.
It is believed that conservative treatment should not be considered pathogenetic, as no single drug addresses the morphological basis of the disease or venous congestion in the pelvis, resulting in only a short-term clinical effect when using various pharmacological groups (venotropic, vasoactive, microcirculation-improving drugs, non-steroidal anti-inflammatory drugs)9. The primary role in therapeutic and preventive measures for PVI may be the management of oxidative stress (OS) reactions, which support impaired blood and lymph flow in the pelvis and trigger trophic processes10, 11. The high intensity of OS reactions in PVI is attributed to pelvic venous discirculatory disorders, hypoxemia, and ischemia of organs, which accelerate the formation of toxic lipid peroxidation (LPO) products while simultaneously diminishing their neutralization processes12. As a result, one of the effective therapeutic approaches could be the use of drugs that inhibit OS reactions and regulate antioxidant status13, 14, 15, 16. To date, studies of this nature have not been conducted. Given the foregoing, a comprehensive therapeutic approach to normalize hemodynamics in the pelvic venous system and the disturbed pro- and antioxidant interactions appears very promising. The goal was to evaluate the effect of an antioxidant complex on the state of the lipid peroxidation system and venous hemodynamic parameters in the treatment of patients with pelvic varicose veins.
Materials and Methods
Study Design
The study included patients diagnosed with PVI (main group, n=150) who had not received antioxidants, venotonics, angioprotective drugs, or synthetic analogues of female sex hormones (combined oral contraceptives) for the last 6 months. The initial diagnosis was based on ultrasound duplex angioscanning of pelvic veins, and the final diagnosis was confirmed by histological examination of vein biopsy material taken during treatment and diagnostic laparoscopy.
Inclusion Criteria
Inclusion criteria for the main group were as follows: patients aged between 20 and 45 years; clinical symptomatology of complaints indicating persistent dysmenorrhea and/or chronic pelvic pain resistant to non-steroidal anti-inflammatory drugs and antispasmodics; informed consent for inclusion in the study.
Exclusion Criteria
Exclusion criteria included age under 18 or over 45 years; pregnancy and the postpartum period (less than 6 months); concurrent gynecological or other pelvic pathologies; acute inflammatory diseases; severe somatic pathologies; and surgical interventions on pelvic organs in the history (less than 6 months ago).
Patients were divided into two groups of 75 each (groups 1 and 2), comparable by sex, age, presentation of complaints, and degree of PVI severity. Treatment for both groups included standard therapy with one venotropic drug, Detralex 1000 (Diosmin 900 mg + Hesperidin 100 mg, Servier Rus LLC, Russia), prescribed as 500 mg (1/2 tablet) once a day for 60 days. Additionally, group 2 received an antioxidant complex (ACA) for 30 days (1 course), with a frequency of 3 courses in 2 months. ACA included the following drugs: superoxide dismutase (SOD) - 1 capsule (250 mg) twice a day for 30 days with repeated courses at 2 and 4 months; acetyl-glutathione - 1 tablet (100 mg) twice a day for 30 days with repeated courses at 2 and 4 months; astaxanthin - 1 capsule (400 mg) per day for 30 days with repeated courses at 2 and 4 months.
Patient Examination
The examination of the patients included a questionnaire survey, analysis of medical records, collection of anamnesis data, and general and gynecological examinations. The effectiveness of treatment was assessed by analyzing the state of the lipid peroxidation (LPO) - antioxidant defense (AOD) system after treatment, and regional phlebohemodynamics in women with PVI after treatment (peak systolic speed blood flow in the iliac, ovarian, and arcuate veins, and duration of retrograde discharge by the ovarian veins).
Ethics Approval
All women signed informed consent in accordance with the World Medical Association Declaration of Helsinki (1964, 2013 ed.). The Ethics Committee (Scientific Centre for Family Health and Human Reproduction Problems) approved the research (No. 1 dated January 15, 2019).
Biochemical Measurements
To determine the state of pro- and antioxidant systems, blood was collected from the ulnar vein on an empty stomach, following generally accepted methods. Plasma and erythrocyte hemolysates were used as test materials. LPO parameters such as lipid hydroperoxides (LH), conjugated dienes (CDs) were determined by spectrophotometric method, and the level of TBA-reactive products (TBARs) by fluorometric method15. The state of the AOD system was determined by the activity of superoxide dismutase (SOD)15, reduced glutathione (GSH) content15, as well as catalase, glutathione peroxidase (GPO), glutathione reductase (GR), and glutathione-S-transferase (GST) activity using commercial kits by Randox (UK). Measurements were made using the spectrofluorophotometer “Shimadzu RF-1501” and spectrophotometer “Shimadzu RF-1650” (Japan). Enzyme immunoassay was performed on a MultiSkan ELX808 microplate reader (Biotek, USA). The equipment of the Collective Usage Centre “Center for the Development of Progressive Personalized Health Technologies” was used.
Statistical procedure
Statistical analysis of data was performed using Statistica 10.0 programme (Statsoft Inc., USA). The visual-graphical method and the Kolmogorov-Smirnov agreement criteria with the Lilliefors and Shapiro-Wilk correction were used to determine the normality of the distribution of the quantitative features. Due to the fact that the distribution in the studied samples differed from normal, the non-parametric Mann-Whitney test was used. Categorical data were described with absolute values and percentages, quantitative data were described with median (Me) and lower and upper quartiles (Q1; Q3). The critical level of significance was taken as 5% (0.05).
| Parameters | Group 1 | Group 2 | ||
| before treatment | after treatment | before treatment | after treatment | |
| LH, units | 6.25 (6.10 6.41) | 6.05 (5.71;6.11) | 6.51 (6.38 6.77) | 5.22 **, # (5.14;5.43) |
| CDs, μmol/l | 2.80 (2.73;2.90) | 2.73 (2.60;2.82) | 2.79 (2.66 2.94) | 2,45 **, # (2.40;2.61) |
| TBARs, μmol/l | 4.35 (4.18;4.52) | 4.28 * (4.10;4.33) | 4.47 (4.30;4.65) | 3.90 **, # (3.74 4.02) |
| Parameters | Group 1 | Group 2 | ||
| before treatment | after treatment | before treatment | after treatment | |
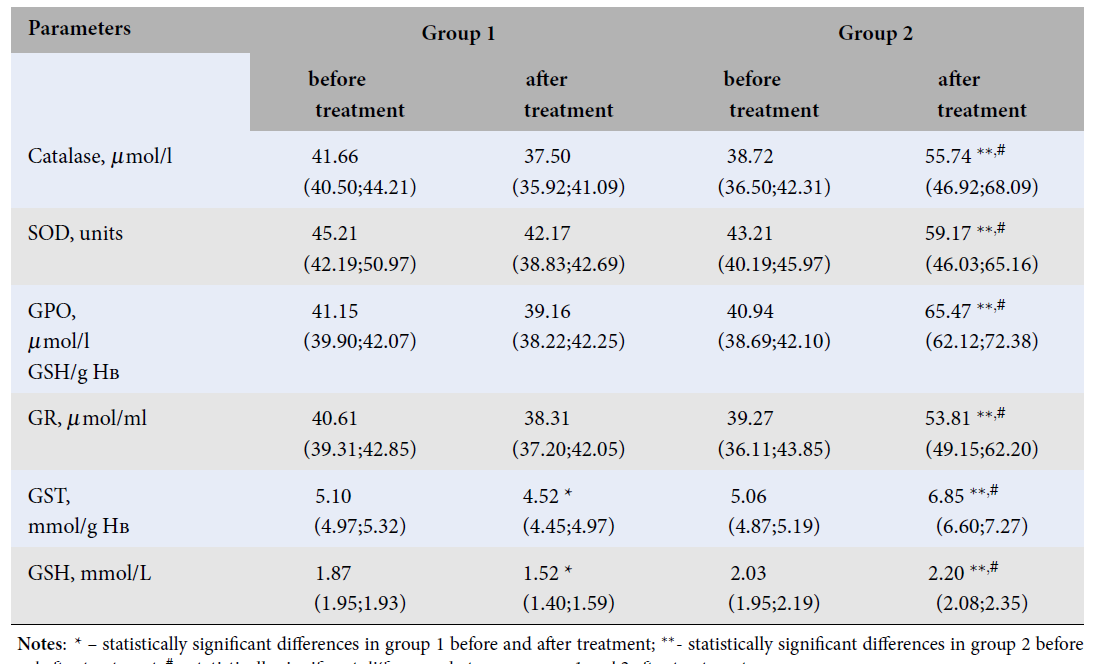
| Catalase, μmol/l | 41.66 (40.50;44.21) | 37.50 (35.92;41.09) | 38.72 (36.50;42.31) | 55.74 **, # (46.92;68.09) |
| SOD, units | 45.21 (42.19;50.97) | 42.17 (38.83;42.69) | 43.21 (40.19;45.97) | 59.17 **, # (46.03;65.16) |
| GPO, μmol/l GSH/g Нв | 41.15 (39.90;42.07) | 39.16 (38.22;42.25) | 40.94 (38.69;42.10) | 65.47 **, # (62.12;72.38) |
| GR, μmol/ml | 40.61 (39.31;42.85) | 38.31 (37.20;42.05) | 39.27 (36.11;43.85) | 53.81 **, # (49.15;62.20) |
| GSТ, mmol/g Нв | 5.10 (4.97;5.32) | 4.52 * (4.45;4.97) | 5.06 (4.87;5.19) | 6.85 **, # (6.60;7.27) |
| GSH, mmol/L | 1.87 (1.95;1.93) | 1.52 * (1.40;1.59) | 2.03 (1.95;2.19) | 2.20 **, # (2.08;2.35) |
| Parameters | Group 1 | Group 2 | ||
| before treatment | after treatment | before treatment | after treatment | |
| Peak systolic speed blood flow, сm/sec: | ||||
| iliac veins- | 11.0 (10.0 13.0) | 11.5 (10.5 12.5) | 12.0 (11.2 15.4) | 15.0 **, # (13.5 16.0) |
| in the ovarian veins - | 7.4 (6.2 8.0) | 8.0 (7.0 8.5) | 7.6 (6.5 8.0) | 8.5 **, # (7.6 9.0) |
| arcuate vei - | 3.4 (2.8 3.7) | 3.5 (3.1 4.0) | 3.5 (3.0 4.2) | 4.0 **, # (3.6 4.7) |
| Duration retrograde discharge by ovarian veins, sec | 0.7 (0.5 1.1) | 0.5 (0.3 0.6) | 0.6 (0.4 7.0) | 0.3 ** (0.2 0.4) |
Results
Assessment of the lipid peroxidation system parameters in group 1, which received only venotropic therapy, showed a statistically significant decrease in TBARS (p = 0.041) compared to data before treatment (Table 1).
In group 2, which received ACA in combination with baseline venotropic therapy, there was a statistically significant decrease in LH (p < 0.0001), CDs (p = 0.001), and TBARS (p < 0.0001) (Table 1 ). Differences between groups 1 and 2 in these parameters after treatment were noted, with lower LH (p < 0.0001), CDs (p < 0.0001), and TBARS (p < 0.0001) in group 2 compared to group 1 (Table 1 ).The AOD system in group 1 was characterized by a statistically significant decrease in GST (p = 0.001) and GSH (p < 0.001) after treatment (Table 2 ).Group 2 showed an increase in the values of Catalase (p < 0.0001), SOD (p < 0.0001), GPO (p < 0.0001), GR (p < 0.0001), GST (p = 0.002), and GSH (p = 0.032) after treatment. Differences between groups 1 and 2 in these parameters after treatment included higher levels of Catalase (p < 0.0001), SOD (p < 0.0001), GPO (p < 0.0001), GR (p < 0.0001), GST (p < 0.0001), and GSH (p = 0.004) in group 2 compared to group 1 (Table 2 ).
Next, hemodynamic parameters in the studied groups wereevaluated (Table 3 ).After complex antioxidant treatment in group 2, there was an increase in blood flow velocity in varicose pelvic veins (iliac (p = 0.003), ovarian (p = 0.041), and arcuate (p = 0.040)), as well as a decrease in the duration of retrograde discharge to 0.3 cm (Table 3 ). In group 1, no statistically significant changes were observed with venotropic treatment alone (p > 0.05). Intergroup differences included higher blood flow velocities in the iliac (p = 0.029), ovarian (p = 0.033), and arcuate (p = 0.041) veins in group 2 compared with group 1 (Table 3 ).
Discussion
PVI is a chronic disease that is difficult to treat due to a wide variety of factors acting on the venous wall1, 2. The exact mechanism of this syndrome's pathogenesis is not yet fully understood. One of the most important pathogenetic factors influencing the development and progression of PVI may be the activation of OS reactions in cell biomembranes10, 16, 17, 18. Thus, there is evidence that women with PVI have a higher incidence of pelvic pain and various types of disorders that were shown to be associated with OS19. In particular, TBARs accumulation and decreased antioxidant activity are positively correlated with the intensity of pelvic pain20, 21.
Our study shows a significant decrease in the level of LPO products in women with PVI in group 2, against the background of complex antioxidant therapy in combination with basal therapy with a venotropic drug. It is known that in excessive concentrations, LPO products are highly toxic and have a damaging effect on the structural and functional state of cells17, 22, 23. At the molecular level, LPO free-radical products formed at different stages of the chain reaction (lipid hydroperoxides, CDs, aldehydes, etc.) negatively affect the metabolism of proteins, enzymes, and nucleic acids, which can cause cell apoptosis, inhibiting cell proliferation, maturation, and growth24, 25. In this case, negative hemodynamic changes, development of tissue ischemia, and hypoxia are possible with regard to venous blood flow9, 21. Morphological changes in the venous wall caused by these processes can also lead to reflux, which further aggravates hemodynamic disorders, while the regulatory antioxidant function of endothelial cells in the venous wall can be impaired11. Therefore, the prescription of drugs with a combined action, offering both antioxidant and phlebotropic therapeutic effects, is reasonable5, 17.
SOD is an endogenous acceptor of reactive oxygen species that determine the development of oxygen-dependent pathological processes—hypoxia, intoxication, inflammation17. This enzyme catalyzes the reaction of superoxide-anion radical dismutation, prevents the formation of hydroxyl radical and singlet oxygen; it participates in the suppression of inflammation factors; normalizes oxidative processes, preventing oxidative modification of proteins, as well as the destruction of cell biomembranes associated with the activation of LPO26, 27. Acetyl-glutathione is a thiol-disulfide compound that performs antioxidant defense, actively removes toxic substances and heavy metals from the body, improves lung and liver function, reduces inflammation activity, increases immune defense, and promotes repair of damaged tissues28. Astaxanthin is one of the strongest natural antioxidants29. The astaxanthin molecule has a special structure that allows it to protect at the level of biomembranes, participate in the normalization of metabolism at the cellular level, and repair damaged tissues30.
We observed a concomitant stimulating effect on the AOD system in patients with PVI after treatment with this complex of antioxidant agents. It was expressed in the increase in the values of antioxidant enzymes—Catalase, SOD,components of glutathione status. The obtained data confirm the reversibility of systemic damage to cell membranes with the timely start of treatment.
Parallel to the decrease in the intensity of free-radical reactions and activation of the AOD system, there were processes that contributed to normalizing the hemodynamic parameters in the pelvic venous basin. Before the treatment, the regional hemodynamics parameters showed signs of moderate blood stasis in the pelvic organs, which in turn indicated tissue hypoxia. After the treatment, there was an increase in blood flow velocity in the pelvic veins, as well as a decrease in the duration of retrograde discharge in them. These changes may indicate a significant improvement in venous outflow from the pelvic organs in the group of patients with PVI after treatment.
The main issues in the study could relate to potential limitations in the study design, such as sample size and selection criteria. Moreover, it was not possible to comprehensively assess the state of the antioxidant defense system in patients with PVI, including the analysis of the content of the parameters of the non-enzymatic link—water and fat-soluble vitamins, the level of total antioxidant activity of the blood, etc. This is associated with additional financial costs for the implementation of this study.
Conclusions
Our results showed that using a complex therapeutic intervention to improve pelvic venous system hemodynamics and normalize impaired pro- and antioxidant interactions in women with pelvic varicose veins seems promising. An additional component that enhances the results of basic venotropic therapy for women with PVI can be adaptive metabolic therapy involving the application of an antioxidant complex. The use of antioxidant drugs in combination with venotropic therapy for the treatment of PVI significantly improves the indices of the lipid peroxidation-antioxidant defense system and venous hemodynamics in the pelvic organs in patients of this cohort.
Abbreviations
ACA: Antioxidant Complex Application, AOD: Antioxidant Defense, CDs: Conjugated Dienes, GPO: Glutathione Peroxidase, GR: Glutathione Reductase, GSH: Glutathione, GST: Glutathione-S-Transferase, ICD-10: International Classification of Diseases, Tenth Revision, LH: Lipid Hydroperoxides, LPO: Lipid Peroxidation, OS: Oxidative Stress, PVI: Pelvic Venous Incompetence, SOD: Superoxide Dismutase, TBARs: Thiobarbituric Acid Reactive Substances
Acknowledgments
None.
Author’s contributions
Research concept and design: Andrey A. Semendyaev, Dmitriy A. Stupin; Collection and processing of material: Dmitriy A. Stupin, Andrey A. Semendyaev, Daria V. Tukhieva; Text writing: Marina A. Darenskaya, Andrey A. Semendyaev, Sergey I. Kolesnikov; Editing: Sergey I. Kolesnikov, Lyubov I. Kolesnikova; Approval of the final version of the article: Sergey I. Kolesnikov, Lyubov I. Kolesnikova. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The Institutional review board approved the study, and a participant provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Khilnani
N.M.,
Meissner
M.H.,
Learman
L.A.,
Gibson
K.D.,
Daniels
J.P.,
Winokur
R.S.,
Research Priorities in Pelvic Venous Disorders in Women: Recommendations from a Multidisciplinary Research Consensus Panel. Journal of Vascular and Interventional Radiology.
2019;
30
(6)
:
781-9
.
View Article PubMed Google Scholar -
Bendek
B.,
Afuape
N.,
Banks
E.,
Desai
N.A.,
Comprehensive review of pelvic congestion syndrome: causes, symptoms, treatment options. Current Opinion in Obstetrics & Gynecology.
2020;
32
(4)
:
237-42
.
View Article PubMed Google Scholar -
Campbell
B.,
Goodyear
S.,
Franklin
I.,
Nyamekye
I.,
Poskitt
K.,
Investigation and treatment of pelvic vein reflux associated with varicose veins: current views and practice of 100 UK vascular specialists. Phlebology.
2020;
35
(1)
:
56-61
.
View Article PubMed Google Scholar -
Hansrani
V.,
Dhorat
Z.,
McCollum
C.N.,
Diagnosing of pelvic vein incompetence using minimally invasive ultrasound techniques. Vascular.
2017;
25
(3)
:
253-9
.
View Article PubMed Google Scholar -
Dahikar
G.D.,
Giradkar
D.D.,
Khan
S.A.,
Ganjiwale
R.O.,
A review on remedies used in treatment of varicose veins and varicocele. GSC Biological and Pharmaceutical Sciences.
2022;
18
(2)
:
244-52
.
View Article Google Scholar -
Gus
A.I.,
Kolesnikova
L.I.,
Semendyaev
A.A.,
Stupin
D.A.,
Shcherbatykh
A.V.,
Kalyagin
A.N.,
Optimizing management strategy in women with pelvic varicose veins. Obstetrics and Gynecology.
2019;
4
:
58-64
.
View Article Google Scholar -
Khilnani
N.M.,
Winokur
R.S.,
Scherer
K.L.,
Meissner
M.H.,
Clinical Presentation and Evaluation of Pelvic Venous Disorders in Women. Techniques in Vascular and Interventional Radiology.
2021;
24
(1)
.
View Article PubMed Google Scholar -
Semendyaev
A.A.,
Stupin
D.A.,
Darenskaya
M.A.,
Kolesnikov
S.I.,
Pesterev
K.V.,
Kolesnikova
L.I.,
Successful treatment of pelvic venous incompetence by a single-stage combined laparoscopic intervention: a case report. Biomedical Research and Therapy.
2023;
10
(5)
:
5666-70
.
View Article Google Scholar -
Yetkin
E.,
Ileri
M.,
Dilating venous disease: pathophysiology and a systematic aspect to different vascular territories. Medical Hypotheses.
2016;
91
:
73-6
.
View Article PubMed Google Scholar -
Heydari
L.,
Mugahi
S.M.,
Fazelipour
S.,
Koruji
M.,
Alizadeh
R.,
Abbasi
N.,
Effects of Ovarian Varicose Vein on Mitochondrial Structure, Malondialdehyde and Prooxidants: Antioxidants Balance in Rat Ovaries. International Journal of Morphology.
2015;
33
(3)
:
930-5
.
View Article Google Scholar -
Castro-Ferreira
R.,
Cardoso
R.,
Leite-Moreira
A.,
Mansilha
A.,
The role of endothelial dysfunction and inflammation in chronic venous disease. Annals of Vascular Surgery.
2018;
46
(46)
:
380-93
.
View Article PubMed Google Scholar -
Saribal
D.,
Kanber
E.M.,
Hocaoglu-Emre
F.S.,
Akyolcu
M.C.,
Effects of the oxidative stress and genetic changes in varicose vein patients. Phlebology.
2019;
34
(6)
:
406-13
.
View Article PubMed Google Scholar -
Donnez
J.,
Binda
M.M.,
Donnez
O.,
Dolmans
M.M.,
Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertility and Sterility.
2016;
106
(5)
:
1011-7
.
View Article PubMed Google Scholar -
Darenskaya
M.A.,
Semendyaev
A.A.,
Stupin
D.A.,
Kolesnikov
S.I.,
Semenova
N.V.,
Pesterev
K.V.,
Blood cytokines of the ovarian vein basin in different stages of pelvic varicose veins. Bulletin of Experimental Biology and Medicine.
2023;
175
(3)
:
311-4
.
View Article PubMed Google Scholar -
Kolesnikova
L.I.,
Semendyaev
A.A.,
Stupin
D.A.,
Darenskaya
M.A.,
Grebenkina
L.A.,
Natyaganova
L.V.,
The intensity of lipid peroxidation processes in women with primary varicose veins of the small pelvis depending on the stage of the disease. Annals of the Russian Academy of Medical Sciences..
2018;
73
(4)
:
229-35
.
View Article Google Scholar -
Darenskaya
M.A.,
Stupin
D.A.,
Semendyaev
A.A.,
Kolesnikov
S.I.,
Shcherbatykh
A.V.,
Tolkachev
K.S.,
Pelvic venous insufficiency: lipid peroxidation levels in ovarian venous blood. Biomedical Research and Therapy.
2022;
9
(2)
:
4884-91
.
View Article Google Scholar -
Kolesnikova
L.I.,
Darenskaya
M.A.,
Kolesnikov
S.I.,
Free radical oxidation: a pathophysiologist's view. Bulletin of Siberian Medicine.
2017;
16
(4)
:
16-29
.
View Article Google Scholar -
Darenskaya
M.A.,
Stupin
D.A.,
Semendyaev
A.A.,
Kolesnikov
S.I.,
Semenova
N.V.,
Kolesnikova
L.I.,
Cytokine profile and oxidative stress parameters in women with initial manifestations of pelvic venous insufficiency. AIMS Medical Science.
2022;
9
(3)
:
414-23
.
View Article Google Scholar -
Khatri
G.,
Khan
A.,
Raval
G.,
Chhabra
A.,
Diagnostic evaluation of chronic pelvic pain. Physical Medicine and Rehabilitation Clinics of North America.
2017;
28
(3)
:
477-500
.
View Article PubMed Google Scholar -
Guo
R.,
Yin
L.,
The relationship between oxidative stress and estrogen receptor levels in ectopic endometrium and pelvic pain. Tianjin Yi Yao.
2016;
44
:
88-90
.
-
Naoum
J.J.,
Hunter
G.C.,
Woodside
K.J.,
Chen
C.,
Current advances in the pathogenesis of varicose veins. The Journal of Surgical Research.
2007;
141
(2)
:
311-6
.
View Article PubMed Google Scholar -
Darenskaya
M.A.,
Grebenkina
L.A.,
Sholokhov
L.F.,
Rashidova
M.A.,
Semenova
N.V.,
Kolesnikov
S.I.,
Lipid Peroxidation Activity in Women with Chronic Viral Hepatitis. Free Radical Biology and Medicine.
2016;
100
(2)
:
S192
.
View Article Google Scholar -
Kolesnikova
L.I.,
Rychkova
L.V.,
Kolesnikova
L.R.,
Darenskaya
M.A.,
Natyaganova
L.V.,
Grebenkina
L.A.,
Coupling of lipoperoxidation reactions with changes in arterial blood pressure in hypertensive ISIAH rats under conditions of chronic stress. Bulletin of Experimental Biology and Medicine.
2018;
164
(6)
:
712-5
.
View Article PubMed Google Scholar -
Zeliger
H.I.,
Oxidative Stress Index as a Public Health Survey Instrument. Eur. J. Med. Health Sci..
2019;
1
(2)
.
View Article Google Scholar -
Niki
E.,
Lipid peroxidation products as oxidative stress biomarkers. BioFactors (Oxford, England).
2008;
34
(2)
:
171-80
.
View Article PubMed Google Scholar -
Saxena
P.,
Selvaraj
K.,
Khare
S.K.,
Chaudhary
N.,
Superoxide dismutase as multipotent therapeutic antioxidant enzyme: role in human diseases. Biotechnology Letters.
2022;
44
(1)
:
1-22
.
View Article PubMed Google Scholar -
Bairova
T.A.,
Kolesnikov
S.I.,
Kolesnikova
L.I.,
Pervushina
O.A.,
Darenskaya
M.A.,
Grebenkina
L.A.,
Lipid peroxidation and mitochondrial superoxide dismutase-2 gene in adolescents with essential hypertension. Bulletin of Experimental Biology and Medicine.
2014;
158
(2)
:
181-4
.
View Article PubMed Google Scholar -
Raj Rai
S.,
Bhattacharyya
C.,
Sarkar
A.,
Chakraborty
S.,
Sircar
E.,
Dutta
S.,
Glutathione: role in oxidative/nitrosative stress, antioxidant defense, and treatments. ChemistrySelect.
2021;
6
(18)
:
4566-90
.
View Article Google Scholar -
Ekpe
L.,
Inaku
K.,
Ekpe
V.,
Antioxidant effects of astaxanthin in various diseases\textemdashA review. J. Mol. Pathophysiol..
2018;
7
(1)
:
1-6
.
View Article Google Scholar -
Sztretye
M.,
Dienes
B.,
Gönczi
M.,
Czirják
T.,
Csernoch
L.,
Dux
L.,
Keller-Pintér
A.,
Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxidative Medicine and cellular longevity.
2019;
2019
:
3849692
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 3 (2024)
Page No.: 6282-6288
Published on: 2024-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2948 times
- PDF downloaded - 1032 times
- XML downloaded - 110 times
 Biomedpress
Biomedpress


