Abstract
Introduction: The control of low-density lipoprotein (LDL) cholesterol is a critical concern, especially for patients with diabetes, where the use of statins is essential. Despite this necessity, actual treatment practices and the achievement of LDL cholesterol targets are often suboptimal. This study aimed to assess the rate of LDL cholesterol goal attainment and examine statin prescribing habits within the cardiology and endocrinology departments of a tertiary hospital in Ho Chi Minh City, Vietnam.
Methods: This retrospective study encompassed 515 diabetic patients. We performed cardiovascular risk stratification to set appropriate LDL cholesterol goals for each patient. Through both univariate and multivariate analyses, we identified factors that influence LDL cholesterol management. Additionally, we reviewed patients' statin prescriptions before and after LDL cholesterol evaluation to understand prescribing patterns.
Results: Our study found that all included patients were categorized as having high or very high cardiovascular risk. A significant majority, 88.2%, were prescribed statins at an intermediate intensity. However, only 15.3% achieved their LDL cholesterol targets—21.7% in the high-risk category and a mere 9.4% in the very high-risk group. Factors conducive to effective LDL cholesterol management included being female, belonging to the very high cardiovascular risk group, and concurrent use of fibrates. Noticeably, among patients not meeting their LDL cholesterol goals, only 10.1% had their statin dosage increased post-evaluation. It was also observed that endocrinologists tended to reduce or discontinue statin dosages more often than cardiologists.
Conclusions: The rate at which diabetic patients in Vietnam meet their LDL cholesterol targets is alarmingly low. Priority should be given to female patients and those at very high cardiovascular risk to improve target attainment rates. There is a clear need for targeted interventions to enhance statin prescribing practices and, by extension, the management of LDL cholesterol in this population.
Introduction
Dyslipidemia significantly increases the risk of cardiovascular diseases; therefore, effective management, specifically in controlling low-density lipoprotein (LDL) cholesterol, is imperative. Statins are the primary treatment for lowering LDL cholesterol due to their proven efficacy in reducing cardiovascular events. Recent guidelines emphasize the need for stricter LDL cholesterol targets for individuals at high or very high cardiovascular risks - a category that diabetic patients almost invariably fall into. This underscores the need for rigorous LDL cholesterol management in these individuals1, 2.
Despite clear guidelines, reaching LDL cholesterol targets often remains a challenge in clinical practice, even in developed countries. It is reported that only 35% and 14% of patients have met the LDL cholesterol targets recommended by the 2016 and 2019 ESC-EASD (European Society of Cardiology/European Association for the Study of Diabetes) guidelines, respectively3. This shortfall is attributed to various factors, including the prescription habits across different medical specialties.
Analysis shows a significantly lower rate of statin use among patients with type 2 diabetes in endocrinology departments compared to their counterparts in cardiology departments. Notably, the achievement of LDL cholesterol targets is significantly higher in patients managed within cardiology departments than those in endocrinology departments4. Hence, this study aims to investigate the real-world management of LDL cholesterol and the statin prescription patterns among diabetic patients attending cardiology and endocrinology outpatient clinics at a tertiary hospital in Vietnam. By identifying potential disparities between these specialties, the study seeks insights that could lead to enhanced management strategies. Additionally, it explores the risk factors that hinder the achievement of optimal LDL cholesterol control, aiming to find solutions to improve patient outcomes.
| Total (N = 515) | Cardiology clinic (N = 331) | Endocrinology clinic (N = 184) | P -value | |
|---|---|---|---|---|
| Demographic features | ||||
| Female | 290 (56.3) | 186 (56.2) | 104 (56.5) | 0.943 |
| Age | 66 (60-72) | 67 (61-73) | 64 (59-70) | < 0.05 |
| Comorbidities | ||||
| Hypertension | 500(97.1) | 318 (96.1) | 182 (98.9) | 0.066 |
| CCS | 246 (47.8) | 198 (59.8) | 48 (26.1) | < 0.05 |
| Heart failure | 25 (4.9) | 25 (7.6) | 0 | < 0.05 |
| Atrial fibrillation | 30 (5.9) | 29 (8.8) | 1 (0.5) | < 0.05 |
| Stroke | 13 (2.5) | 9 (2.7) | 4 (2.2) | 0.779 |
| PAD | 3 (0.6) | 3 (0.9) | 0 | 0.556 |
| CKD | 48 (9.3) | 21 (6.3) | 27 (14.7) | < 0.05 |
| Thyroid disease | 12 (2.4) | 7 (2.1) | 5 (2.7) | 0.664 |
| Lung disease | 5 (1.0) | 3 (0.9) | 2 (1.1) | 1.000 |
| Joint disease | 44 (8.5) | 19 (5.7) | 25 (13.6) | < 0.05 |
| Laboratory results | ||||
| Hemoglobin (g/L) | 135 (124-144) | 135 (124-143) | 135 (123-145) | 0.253 |
| HbA1C (%) | 6.6 (6-7.6) | 6.4 (5.9-7.5) | 6.9 (6.1-8.1) | 0.016 |
| Creatinine (umol/L) | 88.5 (76-104.4) | 88.9 (76.1-105) | 88.2 (75.5-104.2) | 0.125 |
| AST (IU/L) | 26 (21.8-32) | 26.2 (22-32) | 25 (21.4-31.6) | 0.644 |
| ALT (IU/L) | 25.6 (18.5-37.6) | 25.4 (19-36) | 25.6 (17.5-39.4) | 0.499 |
| Cholesterol (mmol/L) | 4.0 (3.4-4.9) | 4.0 (3.4-4.9) | 4.0 (3.3-4.8) | 0.305 |
| Triglyceride (mmol/L) | 1.8 (1.3-2.6) | 1.6 (1.3-2.6) | 1.8 (1.3-2.6) | 0.679 |
| HDL cholesterol (mmol/L) | 1.1 (1.0-1.3) | 1.2 (1-1.3) | 1.1 (1.0-1.3) | 0.349 |
| LDL cholesterol (mmol/L) | 2.2 (1.9-3.0) | 2.3 (1.9-3.0) | 2.2 (1.8-2.8) | 0.476 |
| TG/HDL | 1.7 (1.1-2.5) | 1.7 (1.0-2.5) | 1.7 (1.1-2.5) | 0.858 |
| TG/LDL | 0.8 (0.6-1.1) | 0.8 (0.5-1.1) | 0.8 (0.6-1.1) | 0.381 |
| Cardiovascular risk | ||||
| High | 249 (48.4) | 118 (35.7) | 131 (71.2) | < 0.05 |
| Very high | 266 (51.6) | 213 (64.3) | 53 (28.8) | |
| Medications | ||||
| ARNI | 2 (0.4) | 2 (0.6) | 0 | 0.540 |
| ACE inhibitors | 98 (19.0) | 66 (19.9) | 32 (17.4) | 0.480 |
| ARBs | 321 (62.3) | 227 (68.6) | 94 (51.1) | < 0.05 |
| BBs | 360 (69.9) | 263 (79.5) | 97 (52.7) | < 0.05 |
| CCBs | 264 (51.3) | 180 (54.4) | 84 (45.6) | 0.934 |
| Diuretics | 122 (23.7) | 92 (27.8) | 30 (16.3) | < 0.05 |
| MRA | 25 (4.9) | 25 (7.6) | 0 | < 0.05 |
| Aspirin | 73 (14.17) | 46 (13.9) | 27 (14.7) | 0.809 |
| P2Y12 inhibitors | 126 (24.5) | 101 (30.5) | 25 (13.6) | < 0.05 |
| VKAs | 10 (1.9) | 10 (3.0) | 0 | < 0.05 |
| DOACs | 20 (3.9) | 20 (6.0) | 0 | < 0.05 |
| Insulin | 70 (13.6) | 11 (3.3) | 59 (32.1) | < 0.05 |
| Metformin | 314 (61.0) | 160 (48.3) | 154 (83.7) | < 0.05 |
| SGLT2 inhibitors | 3 (0.58) | 1 (0.3) | 2 (1.1) | 0.291 |
| DPP4 inhibitors | 3 (0.58) | 0 | 3 (1.6) | < 0.05 |
| Sulfonylureas | 197 (38.3) | 102 (30.1) | 95 (51.3) | < 0.05 |
| Acarbose | 65 (12.6) | 13 (3.9) | 52 (28.3) | < 0.05 |
| Lipid-lowering agents | ||||
| Statins | ||||
| Atorvastatin | 345 (70.7) | 211 (65.3) | 134 (81.2) | < 0.05 |
| Rosuvastatin | 141 (28.9) | 112 (34.7) | 29 (17.6) | |
| Simvastatin | 2 (0.4) | 0 | 2 (1.2) | |
| Statin intensity | ||||
| High | 34 (6.6) | 33 (10.0) | 1 (0.5) | < 0.05 |
| Intermediate | 454 (88.2) | 290 (87.6) | 164 (89.1) | 0.671 |
| Others | ||||
| Fibrates | 30 (5.8) | 9 (2.7) | 21 (11.4) | < 0.05 |
| Ezetimibe | 6 (1.2) | 2 (0.6) | 4 (2.17) | 0.194 |
| Total (N = 515) | Cardiology clinic (N = 331) | Endocrinology clinic (N = 184) | P-value | |
|---|---|---|---|---|
| Total | 79 (15.3) | 45 (13.6) | 34 (18.5) | 0.141 |
| High risk | 54 (21.7) | 28 (23.7) | 26 (19.8) | 0.458 |
| Very high risk | 25 (9.4) | 17 (8.0) | 8 (15.1) | 0.112 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Cardiology clinic | 0.69 (0.43-1.13) | 0.142 | 0.85 (0.49-1.47) | 0.568 |
| Age | 0.85 (0.52-1.38) | 0.515 | -- | -- |
| Female gender | 0.64 (0.39-1.03) | 0.066 | 0.59 (0.36-0.97) | < 0.05 |
| Fibrates | 0.20 (0.02-1.51) | 0.119 | 0.12 (0.02-0.95) | < 0.05 |
| High intensity statin | 1.78 (0.77-4.08) | 0.175 | 2.16 (0.89-5.24) | 0.090 |
| Very high cardiovascular risk | 0.37 (0.22-0.62) | < 0.05 | 0.35 (0.20-0.61) | < 0.05 |
| Chronic kidney disease | 1.99 (0.99-4.02) | 0.055 | 1.96 (0.93-4.12) | 0.078 |
| Total (N = 436) | Cardiology clinic (N = 286) | Endocrinology clinic (N = 150) | P -value | |
| Increase | 44 (10.1) | 29 (10.1) | 15 (10.0) | 0.963 |
| Unchange | 306 (70.2) | 211 (73.8) | 95 (63.3) | < 0.05 |
| Decrease/stop | 86 (19.7) | 46 (16.1) | 40 (26.7) | < 0.05 |
| Drug modification | ||||
| Change to fibrates | 21 (4.8) | 11 (3.6) | 10 (6.7) | 0.191 |
| Combination | 3 (0.7) | 1 (0.4) | 2 (1.3) | 0.238 |
Methods
Study Design and Participants
This retrospective study encompassed 515 type 2 diabetes patients aged 18 and above who were receiving treatment at the cardiology (N = 331) and endocrinology (N = 184) clinics of Nhan Dan Gia Dinh Hospital in Ho Chi Minh City, Vietnam. Data collection was a part of regular clinical care, negating the need for separate patient consent for this process.
Data were gathered from May 2021 to May 2022, with analysis occurring from May to June 2023. During analysis, patients’ personal information was anonymized to ensure confidentiality. Exclusion criteria included those not treated with lipid-lowering medications for a minimum of three months, individuals with conditions that affect lipid absorption/metabolism, and pregnant women. Detailed characteristics of the study population are delineated in Table 1 and Table S1.
Outcome Definition and Data Collection
Cardiovascular risk levels and LDL cholesterol objectives were established according to Nhan Dan Gia Dinh Hospital’s protocols, aligning with contemporary international guidelines. Goals for LDL cholesterol levels were set at <1.8 mmol/L for high-risk and <1.4 mmol/L for very high-risk groups. Statin treatment intensity categories were defined as: low (atorvastatin <10 mg, rosuvastatin <5 mg), moderate (atorvastatin 10–20 mg, rosuvastatin 5–10 mg), and high (atorvastatin 40–80 mg, rosuvastatin 20–40 mg).
Lipid analyses and other laboratory evaluations were conducted at Nhan Dan Gia Dinh Hospital under stringent adherence to the Vietnamese Ministry of Health's guidelines. Also assessed was the statin prescription practice, focusing on adjustments in statin dosage or the incorporation of other lipid-lowering agents based on serum lipid outcomes.
Statistical Analysis
Descriptive data are presented as the mean ± SD, median, or percentage (%), as appropriate. The Shapiro-Wilk test assessed the normality of data distribution. Continuous variables, showing non-normal distribution, are reported as median with interquartile ranges (25th and 75th percentiles).
Group comparisons for categorical variables utilized the Chi-squared test, while differences in continuous variables between two groups were analyzed using the Mann-Whitney U test. The exploration of associated factors made use of univariate or multivariate logistic regression analyze, drawing on variables identified from preceding research5, 6, 7. Variables moving to multivariate analysis exhibited a P-value of <0.2 in univariate scrutiny.
Significance levels for all tests were set at a two-tailed P-value of <0.05, with confidence intervals calculated at the 95% level. Analyses were conducted utilizing STATA software version 17.0 (StataCorp, College Station, TX, USA).
Results
Baseline Characteristics of Participants
Upon comparing patient groups, those at the cardiology clinic were found to be older, had a higher prevalence of cardiovascular complications and atrial fibrillation, but showed fewer cases of joint diseases than their counterparts at the endocrinology clinic. This aligns with the observation that the cardiology clinic housed a greater number of patients classified as very high cardiovascular risk. Consequently, medications associated with very high cardiovascular risk—such as P2Y12 inhibitors, anticoagulants, angiotensin receptor blockers, beta blockers, diuretics, and mineralocorticoid receptor antagonists (MRAs)—were more commonly prescribed in the cardiology group. Conversely, the endocrinology group more frequently used insulin, metformin, dipeptidyl peptidase 4 (DPP4) inhibitors, sulfonylureas, and acarbose. Additionally, chronic kidney disease was more prevalent among patients in the endocrinology clinic (refer to Table 1).
No significant differences were observed in the lipid profile, specifically LDL cholesterol levels, between the two groups. The levels of triglycerides (TG), high-density lipoprotein (HDL) cholesterol, as well as the ratios of TG/HDL, and TG/LDL, were comparable across both specialties. However, in patients with uncontrolled LDL, the TG/LDL ratio was notably higher (refer to Table S1). Markers indicating liver injury, such as aspartate transaminase (AST) and alanine transaminase (ALT), did not present elevated levels. Notably, the endocrinology group exhibited higher HbA1C levels.
Regarding dyslipidemia treatment, statins were predominantly prescribed to the vast majority of patients. Only two individuals received simvastatin, and both were from the endocrinology group. Rosuvastatin and high-intensity statins were more frequently used in the cardiology group. Although fibrates were prescribed more in the endocrinology group, this difference did not reflect significant divergence in triglyceride levels between the two clinics (refer to Table 1).
LDL Cholesterol Achievement Rates and Associated Factors
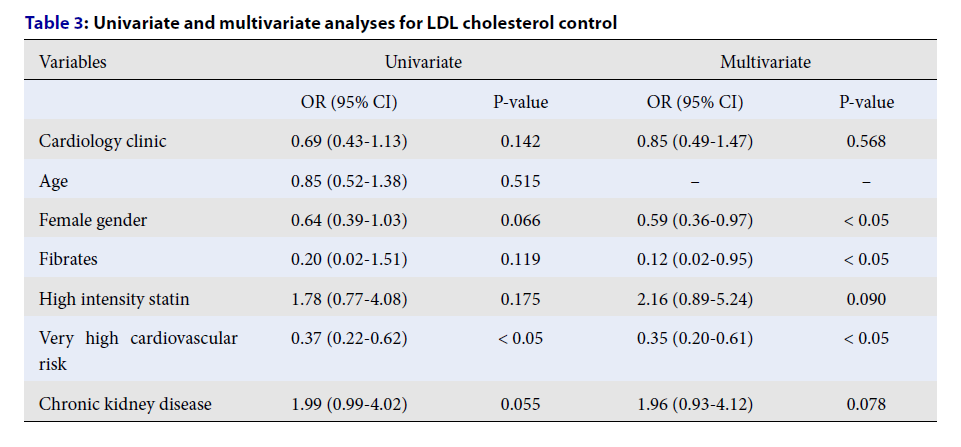
The overall control rate for LDL cholesterol stood at 15.3%, with no observable difference between the cardiology and endocrinology clinics. The group at very high cardiovascular risk demonstrated a significantly lower success rate in achieving LDL cholesterol objectives compared to the high-risk group (refer to Table 2). Analyses, both univariate and multivariate, identified female gender, fibrate prescription, and a classification of very high cardiovascular risk as factors adversely affecting LDL cholesterol management (refer to Table 3).
Lipid-lowering Treatment Modification in Patients Not Meeting LDL Cholesterol Targets
An in-depth investigation was conducted among patients who failed to meet the LDL cholesterol target (N = 436), focusing on clinicians' prescription practices. The increase of statin doses was conducted at a rate of 10.1%, distributed uniformly across both specialties. It is worth noting that endocrinologists showed a greater propensity to either reduce statin doses or discontinue their use altogether compared to cardiologists (refer to Table 4).
Discussion
Nhan Dan Gia Dinh Hospital, a tertiary general facility, adheres to the ESC/EASD 2019 guidelines, which specify lower LDL cholesterol targets for diabetic patients at high and very high cardiovascular risk1, 2. Our findings indicate a notably low success rate in meeting these LDL cholesterol goals, particularly among the very high-risk individuals. This trend aligns with recent studies focusing on the Asian demographic8, 9, suggesting that setting lower LDL cholesterol targets is a significant factor behind the low achievement rates10. A surprising finding from our study is that higher cardiovascular risk correlates with lesser success in LDL cholesterol management. Factors such as female gender were linked to suboptimal LDL cholesterol control, attributed to lower treatment adherence, lipid metabolism differences, and potential societal barriers affecting women11.
When examining other lipid parameters, such as the TG/HDL and TG/LDL ratios, no significant differences emerged between the two clinics studied. However, a noteworthy distinction was seen in patients with managed LDL cholesterol levels, where the TG/LDL ratio was substantially elevated compared to those with uncontrolled levels. This ratio is indicative of small, dense LDL particles in diabetic patients and suggests residual risk in dyslipidemia management. Therefore, attention to the TG/LDL ratio, along with other lipid markers, could enhance dyslipidemia control post-LDL cholesterol target achievement.
Regarding statin therapy, our analysis indicated a preference for moderate over high-intensity statin therapy among our patient cohort. Notably, we recorded neither complaints nor diagnoses pertaining to statin adverse effects. Some patients were on fibrates, either alone or in conjunction with statins. Our analysis revealed that fibrate use negatively impacted LDL cholesterol goal attainment. Given the atherogenic lipid profile commonly seen in diabetic patients—marked by prominent hypertriglyceridemia12 —fibrates' efficacy in reducing triglycerides does not translate well to lowering LDL cholesterol. Their benefits, particularly when compared to statins, remain debatable or marginally inferior13. While combining fibrates with statins is deemed safe, the evidence supporting outcome benefits is unconvincing14. This observation led us to speculate on the hesitancy in prescribing high-intensity statins alongside fibrates.
Moreover, our study highlighted a substantial oversight in statin dose adjustment among patients not meeting their lipid targets, with only 10.1% receiving an increase in their statin dosage. This reluctance to intensify treatment, known as clinical inertia, is linked to poor management of cardiometabolic conditions15. We observed a variation in therapeutic inertia across different clinical specialties; both clinics exhibited high rates of unchanged statin doses, whereas endocrinologists were more likely to reduce or discontinue statin therapy. This finding underscores the need for more aggressive statin therapy across specialties to combat inertia.
Factors contributing to clinical inertia include gaps in provider knowledge, discomfort with diagnostic ambiguities or treatment goals, and safety concerns. Patient-related factors such as male gender, older age, limited life expectancy, multiple comorbidities (especially psychiatric), medication load, and nearly achieved clinical targets (e.g., LDL cholesterol levels) also play a role16. The challenges of high patient volumes and time constraints, particularly prevalent in Vietnamese tertiary hospitals, exacerbate this issue16. Addressing clinical inertia necessitates a comprehensive approach that includes education for both clinicians and patients, team-based care, and population health management strategies17. Emphasizing guideline familiarity among physicians, especially regarding total cholesterol interpretation in the context of other cardiovascular risk factors, and integrating clinical information into healthcare systems are vital for improving prescribing practices18.
This study's limitations are its single-center, two-specialty focus, lack of investigation into lifestyle modifications impacting LDL cholesterol control, and the absence of data on patient treatment adherence. Addressing these gaps is crucial for future research initiatives.
Conclusions
The rate at which diabetic patients managed to reach their LDL cholesterol targets was notably low across two principal departments of a tertiary hospital in Vietnam. Special attention is needed for female patients and those at very high cardiovascular risk to enhance control over LDL cholesterol levels. To achieve better outcomes, it is crucial to implement targeted interventions aimed at refining clinicians' prescription practices. This includes a focus on the use of high-intensity statins, making proper dose adjustments, and avoiding the premature use of fibrates.
Abbreviations
LDL - Low-Density Lipoprotein, ESC-EASD - European Society of Cardiology/European Association for the Study of Diabetes, TG - Triglycerides, HDL - High-Density Lipoprotein, AST - Aspartate Transaminase, ALT - Alanine Transaminase, HbA1C - Hemoglobin A1c, DPP4 - Dipeptidyl Peptidase 4, MRAs - Mineralocorticoid Receptor Antagonists, SD - Standard Deviation
Acknowledgments
None.
Author’s contributions
SVN and DTMP were involved in the initial idea and design of this study. BDD, BQD and NTT collected the data. DHN and VVD analyzed the data. SVN, DHN, VVD, CQN and DTMP worked on the subsequent revisions and all authors contributed to the intellectual content of the paper. All authors have read and approved the final version of this manuscript.
Funding
None.
Availability of data and materials
Data are available upon reasonable request. To request access to the underlying research data, please contact Si Van Nguyen at [email protected].
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Nhan Dan Gia Dinh Hospital (37/NDGD-HDDD on March 3, 2022).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Mach
F.,
Baigent
C.,
Catapano
A.L.,
Koskinas
K.C.,
Casula
M.,
Badimon
L.,
Scientific Document Group
ESC,
2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal.
2020;
41
(1)
:
111-88
.
View Article PubMed Google Scholar -
Cosentino
F.,
Grant
P.J.,
Aboyans
V.,
Bailey
C.J.,
Ceriello
A.,
Delgado
V.,
Scientific Document Group
ESC,
2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European Heart Journal.
2020;
41
(2)
:
255-323
.
View Article PubMed Google Scholar -
Morieri
M.L.,
Avogaro
A.,
Fadini
G.P.,
the DARWIN-T2D Network of the Italian Diabetes Society
Cholesterol lowering therapies and achievement of targets for primary and secondary cardiovascular prevention in type 2 diabetes: unmet needs in a large population of outpatients at specialist clinics. Cardiovascular Diabetology.
2020;
19
(1)
:
190
.
View Article PubMed Google Scholar -
Chen
Z.,
Wang
X.,
Ding
Z.,
Fan
P.,
Ma
G.,
Differences in statin usage and target-goal achievement between departments at the same hospital. PLoS One.
2012;
7
(12)
.
View Article PubMed Google Scholar -
Kim
S.,
Han
S.,
Rane
P.P.,
Qian
Y.,
Zhao
Z.,
Suh
H.S.,
Achievement of the low-density lipoprotein cholesterol goal among patients with dyslipidemia in South Korea. PLoS One.
2020;
15
(1)
.
View Article PubMed Google Scholar -
Danchin
N.,
Almahmeed
W.,
Al-Rasadi
K.,
Azuri
J.,
Berrah
A.,
Cuneo
C.A.,
Investigators
ICLPS,
Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: The International ChoLesterol management Practice Study (ICLPS). European Journal of Preventive Cardiology.
2018;
25
(10)
:
1087-94
.
View Article PubMed Google Scholar -
Zhang
S.,
Li
Z.F.,
Shi
H.W.,
Zhang
W.J.,
Sui
Y.G.,
Li
J.J.,
Comparison of Low-Density Lipoprotein Cholesterol (LDL-C) Goal Achievement and Lipid-Lowering Therapy in the Patients With Coronary Artery Disease With Different Renal Functions. Frontiers in Cardiovascular Medicine.
2022;
9
.
View Article PubMed Google Scholar -
Yang
Y.S.,
Yang
B.R.,
Kim
M.S.,
Hwang
Y.,
Choi
S.H.,
Low-density lipoprotein cholesterol goal attainment rates in high-risk patients with cardiovascular diseases and diabetes mellitus in Korea: a retrospective cohort study. Lipids in Health and Disease.
2020;
19
(1)
:
5
.
View Article PubMed Google Scholar -
Wang
X.,
He
Y.,
Wang
T.,
Li
C.,
Ma
Z.,
Zhang
H.,
Lipid-Lowering Therapy and Low-Density Lipoprotein Cholesterol (LDL-C) Goal Achievement in High-Cardiovascular-Risk Patients in Fuzhou, China. Journal of Cardiovascular Pharmacology and Therapeutics.
2020;
25
(4)
:
307-15
.
View Article PubMed Google Scholar -
Vintila
A.M.,
Horumba
M.,
Cimpu
C.,
Dumitrescu
D.,
Miron
P.,
Alucai
A.,
Target achievement in very high risk patients in light of the new dyslipidemia guidelines. Journal of Hypertension.
2021;
39
.
View Article Google Scholar -
Russo
G.,
Pintaudi
B.,
Giorda
C.,
Lucisano
G.,
Nicolucci
A.,
Cristofaro
M.R.,
Age- and Gender-Related Differences in LDL-Cholesterol Management in Outpatients with Type 2 Diabetes Mellitus. International Journal of Endocrinology.
2015;
2015
.
View Article PubMed Google Scholar -
Hirano
T.,
Pathophysiology of Diabetic Dyslipidemia. Journal of Atherosclerosis and Thrombosis.
2018;
25
(9)
:
771-82
.
View Article PubMed Google Scholar -
Kim
N.H.,
Kim
S.G.,
Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes & Metabolism Journal.
2020;
44
(2)
:
213-21
.
View Article PubMed Google Scholar -
Ginsberg
H.N.,
Elam
M.B.,
Lovato
L.C.,
Crouse
J.R.,
Leiter
L.A.,
Linz
P.,
Study Group
ACCORD,
Effects of combination lipid therapy in type 2 diabetes mellitus. The New England Journal of Medicine.
2010;
362
(17)
:
1563-74
.
View Article PubMed Google Scholar -
Yan
X.,
Mudiganti
S.,
Husby
H.,
Hudnut
A.,
Gbotoe
M.,
Jones
J.B.,
Medication non-adherence and therapeutic inertia independently contribute to poor disease control for cardiometabolic diseases. Scientific Reports.
2022;
12
(1)
:
18936
.
View Article PubMed Google Scholar -
Usherwood
T.,
Therapeutic inertia. Australian Prescriber.
2024;
47
(1)
:
15-9
.
View Article PubMed Google Scholar -
Dixon
D.L.,
Sharma
G.,
Sandesara
P.B.,
Yang
E.,
Braun
L.T.,
Mensah
G.A.,
Therapeutic Inertia in Cardiovascular Disease Prevention: time to Move the Bar. Journal of the American College of Cardiology.
2019;
74
(13)
:
1728-31
.
View Article PubMed Google Scholar -
Irawati
S.,
Prayudeni
S.,
Rachmawati
R.,
Wita
I.W.,
Willfert
B.,
Hak
E.,
Key factors influencing the prescribing of statins: a qualitative study among physicians working in primary healthcare facilities in Indonesia. BMJ Open.
2020;
10
(6)
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 11 No 4 (2024)
Page No.: 6297-6304
Published on: 2024-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3551 times
- PDF downloaded - 1075 times
- Supplement downloaded - 796 times
- XML downloaded - 133 times
 Biomedpress
Biomedpress

