Abstract
Introduction: Type 2 diabetes mellitus (T2DM) is a serious metabolic disorder characterized by hyperglycemia and insulin resistance. Long-standing T2DM may lead to various macro- and microvascular complications such as diabetic nephropathy, neuropathy, and retinopathy. Currently available treatments for T2DM target high plasma glucose levels but do not address T2DM-associated complications. In this report, the therapeutic application of mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) transplantation in improving diabetic blood monitoring parameters among selected T2DM patients was investigated.
Methods: Five Filipino patients with T2DM diagnosed for more than five years agreed to participate in the autologous bone marrow-derived stem cell transplantation. Five milliliters (mL) per kilogram (kg) of bone marrow was collected from the patients following standard procedures, and bone marrow-derived stem cells underwent quantification, genetic typing, microbial analysis, and quality control before being infused into the patients. MSCs and EPCs were intravenously transfused into the patients once a month for 6 months. Fasting blood glucose (FBG), blood urea nitrogen (BUN), glycated hemoglobin (HbA1c), and creatinine (CREA) levels were recorded pre- and post-stem cell transplantation.
Results: The findings of the study revealed that the administration of autologous bone marrow-derived stem cells showed no adverse effects and improved or controlled the blood monitoring levels in most patients. Four out of five patients showed a reduction in their BUN (mean reduction = 2.246) and HbA1c (mean reduction = 0.74%) and maintained their creatinine levels within the normal range following the 6 months of infusion. Meanwhile, three out of five patients showed a decrease in FBG levels (mean reduction = 1.484 mmol/L).
Conclusion: This preliminary report suggests the potential of autologous bone marrow-derived stem cell transplantation for the treatment and management of T2DM. Future studies may focus on examining other parameters such as C-peptide levels and evaluate the efficacy and safety of autologous MSCs and EPCs in the long-term management of T2DM.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a metabolic disorder caused by beta cell dysfunction, a failed response of insulin-sensitive tissues to insulin, and uncompensated insulin resistance1. It affects primarily adults aged 45 years old or older and is often characterized by a high body fat percentage in the abdominal region and/or obesity. Risk factors include a sedentary lifestyle, age, population aging, family history, and a high-caloric diet2. T2DM may present with multiple-systemic complications, both microvascular (diabetic retinopathy, neuropathy, and nephropathy) and macrovascular (cardiovascular diseases, peripheral vascular disease, etc.), primarily caused by a chronic and unregulated hyperglycemic state3. Diagnosis of T2DM includes laboratory testing (fasting blood glucose level, glycated hemoglobin percentage, and oral glucose tolerance test), assessment of risk factors (BMI, race, family history, lifestyle), and clinical symptoms4. The management of T2DM is aimed at maintaining good metabolic and glycemic control as well as near-normal levels of glycated hemoglobin to reduce the development or progression of diabetes complications5.
T2DM affected 536.6 million people globally in 2021 and is expected to rise to about 783.2 million people by 20456. In the Western Pacific, the Philippines ranks fifth—behind China, Indonesia, Japan, and Thailand—in the number of diabetic patients. With the current population of over 100 million, local experts estimate that the Philippines has more than 5 million diagnosed diabetics. A similar number will likely remain undiagnosed or have prediabetes7. T2DM is the most common type of diabetes in the Philippines, with the highest prevalence rate found among the richest in the wealth index, those living in urban areas, and within the 60- to 69-year age group in both sexes. It also continues to be the fourth leading cause of mortality in the Philippines, following ischemic heart disease, cerebrovascular diseases, and neoplasms8. Continuing efforts are made to develop anti-diabetic drugs, which help to delay the various complications. However, due to the progressive nature of the disease, most patients eventually respond less to these drugs9.
Recent progress in regenerative medicine, especially stem cell therapy, has suggested several novel and potential cures for T2DM. Various sources of stem cells exist, with mesenchymal stem cells (MSCs) being the most common. MSCs have been determined to be efficacious in reducing fasting blood sugar and glycated hemoglobin levels among T2DM patients10. They also showed potential in mitigating insulin resistance and dependence observed in diabetic patients11. Another cell type used for T2DM management is the endothelial progenitor cells (EPCs). EPCs are used to alleviate vascular complications observed in T2DM patients12. However, there are limited studies exploring the synergistic effects of stem cell therapy in combination with other cell-based therapies. In this report, we present the application of autologous MSCs with endothelial progenitor cell (EPC) therapy in treating Filipino T2DM patients at the Lung Center of the Philippines, Quezon City, Philippines.
| Fasting blood sugar (mmol/L) | HbA1c (%) | Blood urea nitrogen (mmol/L) | Creatinine (µmol/L) | |
|---|---|---|---|---|
| Patient 1 | 9.7 | 7.40 | 14.4 | 314 |
| Patient 2 | 5.98 | 5.30 | 7.1 | 112 |
| Patient 3 | 5.95 | 5.1 | 7.6 | 93 |
| Patient 4 | 9.7 | 7.4 | 14.4 | 314 |
| Patient 5 | 8.51 | 7 | 6.3 | 84 |
Case Presentation
Patient Enrollment
Five T2DM Filipino patients aged 53-68 years old, diagnosed according to American Diabetes Association13 criteria, were enrolled in the study. The inclusion criteria were: (1) must be Filipino diabetic patients over 45 years old; (2) diagnosed with T2DM for at least the past 10 years; and (3) may have an existing diabetic complication that is stable and non-progressive. Patients with malignancies, acute or chronic infections, hematological illnesses or coagulopathies, or immunosuppressive diseases were excluded from the trial. Patients were clinically assessed by a physician, and clinical clearances were obtained prior to their enrollment in the trial. All patients had an HbA1c over 5.0% for the year preceding the enrollment, with stable hepatic, renal, and cardiopulmonary status. The summary of the baseline characteristics is shown in Table 1.
Ethical Considerations
The study protocol was approved by the Technical Review Board Committee of the Lung Center of the Philippines. Patient cases were assessed by the Stem Cell Ethics Committee of the Lung Center of the Philippines to determine the eligibility of patients who would benefit from the cellular therapy. All patients were informed of the risks, potential benefits, and formulations of the stem cell therapy to be administered, provided written informed consent, and confirmed their willingness to be treated by autologous bone marrow-derived stem cell transplantation.
Stem Cell Collection, Expansion, Identification, and Quality Check
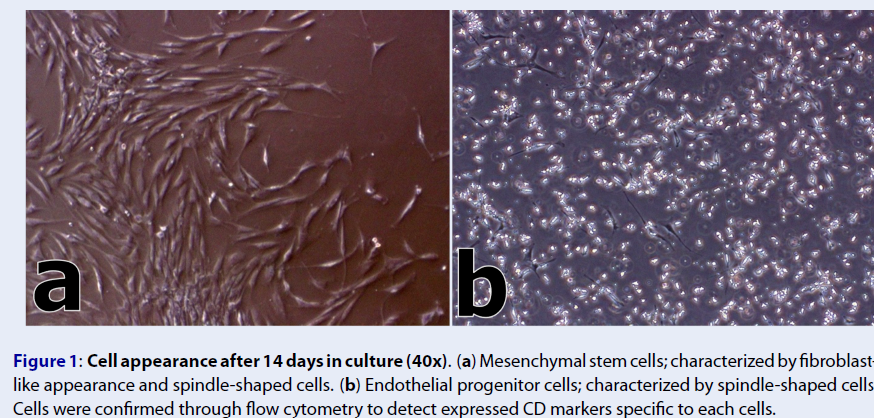
Bone marrow samples were collected from each patient under general anesthesia in a sterile environment. Approximately 70 – 75 mL of bone marrow was collected from the posterior iliac crests using Jamshidi biopsy needles (gauge 8) into a syringe containing 10% sodium heparin. For the isolation of bone marrow-derived stem cells, the Ficoll-Paque density gradient centrifugation method was used following the protocol of Gudleviciene and colleagues (2015)14. Mesenchymal stem cells were expanded in MesenPRO RS™ medium (Thermo Fisher Scientific), while Iscove's modified Dulbecco's medium (Sigma-Aldrich) supplemented with fibroblast growth factors (FGFs) and vascular endothelial growth factors (VEGFs) was used for endothelial progenitor cell (EPC) expansion.Cells were characterized based on their morphology fourteen (14) days following the cell expansion (Figure 1). Cellular identities were confirmed through detection of expressed cluster of differentiation (CD) markers using the flow cytometer (BD FACSCalibur™). MSCs were positive for CD90 and CD105 markers, while EPCs were positive for the CD133 marker. Both cell cultures were negative for the CD45 marker. Finally, microbiologic control testing revealed that all cells were negative for mycoplasma, endotoxins, bacterial, and fungal infections.
Immune Cell Therapy Infusion and Monitoring Expanded cells were washed, transferred to a syringe, and reconstituted in 10 milliliters of lactated Ringer’s solution for cell therapy infusion. The cell count for each cell therapy was set at approximately 5 million MSCs and 1 million EPCs. The patients were given cell infusion under the supervision of the cell therapy physician, anesthesiologist, and cardiologist to ensure the safety of the treated patients. Each patient received a total of 30 to 35 million MSCs and 6 to 7 million EPCs. All patients underwent one cell therapy intravenous infusion per month for six (6) months. No adverse effects were observed in the patients receiving the therapy. Peripheral blood samples were collected from patients before cell therapy and after the 6th cell infusion for close monitoring of hemoglobin A1c (HbA1c), fasting blood sugar (FBS), blood urea nitrogen (BUN), and creatinine levels. It is important to note that some patients continued with their prescribed or herbal medication when they received the cells.
Case Studies
Patient 1
A 70-year-old female Filipino patient has been diagnosed with type-2 diabetes since 2003. She presented with numbness in her fingers to the Lung Center of the Philippines in 2013. Her recent medical examination revealed increased blood sugar levels. The patient has a family history of liver disease and hypertension. The patient was referred to a cardiologist to manage her hypertension. At the time of consultation, the patient was taking only herbal medicines for a year to manage her diabetes and hypertension.
The patient received a total of 6 monthly intravenous infusions of EPCs (1.0 x 106 cells) and MSCs (5.0 x 106 cells) at the Lung Center of the Philippines. Cellular therapy response monitoring was performed following the 6th infusion treatment and compared against her baseline blood chemistry results (Table 1). Findings showed improvements in FBS and HbA1c (Figure 2A-B), with a significant impact on her BUN and creatinine levels (Figure 2C-D).
Patient 2
In 2008, a 74-year-old male Filipino businessman was diagnosed with T2DM at the Lung Center of the Philippines. He presented elevated blood sugar accompanied by muscular spasms. He has been treated with metformin (an antihyperglycemic drug; 500 mg) and tamsulosin (an α-1 adrenoreceptor antagonist; 400 mg) for the past 5 years. Medical evaluation prior to therapy revealed that his FBS level was 5.98 mmol/L and his HbA1c was 5.30%. His BUN level was 7.1 mmol/L, and he had a creatinine level of 112 µmol/L (Table 1).He received a total of 6 monthly intravenous infusions of EPC and MSC cellular therapy at the Lung Center of the Philippines. Following post-treatment monitoring after 6 months, the patient’s FBS and HbA1c levels unfortunately showed no improvements (Figure 2 A-B).
Patient 3
A 66-year-old male Filipino patient was admitted to the Lung Center of the Philippines and was diagnosed with T2DM in 2013. Medical evaluation prior to his enrollment in the study revealed that his FBS level was 5.95 mmol/L and his HbA1c was 5.1%. Besides this, his BUN levels pre-treatment were 7.6 mmol/L, and his creatinine level was 93 µmol/L. The patient presented some diabetic complications such as renal function impairment and hypertension.6 cells) and MSC (5.0 x 106 cells) were given to the patient. All of the patient’s tested blood parameters showed slight improvements (Figure 2 B-D) except for the FBS levels (Figure 2 A).
Patient 4
A 69-year-old male Filipino working as an ophthalmologist presented with an elevated blood sugar level to the Lung Center of the Philippines in 2013. Aside from hypertension, which is one of the common diabetic complications, the patient also presented with chronic kidney disease. The patient was then referred to a cardiologist and nephrologist to manage his hypertension and CKD. His past medical history revealed an increase in blood sugar level, BUN, and creatinine. The patient did not mention any medications for managing his preexisting conditions.
He received a total of 6 infusions intravenously of EPC (1.0 x 106 cells) and MSC (5.0 x 106 cells) every month at the Lung Center of the Philippines (LCP). All cellular therapy products were tested and cleared for any fungal, bacterial, Mycoplasma, and endotoxin contamination.
Cellular therapy response monitoring was performed pre-treatment and post-6th treatment. Results showed a decrease in blood parameters such as FBS, HbA1c, BUN, and creatinine (Figure 2). The patient expired due to T2DM and CKD complications.
Patient 5
A 71-year-old male Filipino patient was diagnosed with T2DM in 1980. In 2017, he was also diagnosed with hepatocellular carcinoma and was treated with chemotherapy. Furthermore, his past medical history revealed an increase in blood sugar level. He has a family history of cancer and diabetes mellitus. His initial symptoms include frequent urination with high blood sugar levels. The patient was then referred to an oncologist to manage his hepatocellular carcinoma.
The patient received 6 infusions intravenously of EPC (1.0 x 106 cells) and MSC (5.0 x 106 cells) every month at LCP. All cellular therapy products underwent quality assurance procedures to avoid any complications associated with contaminated blood products. Cellular therapy response monitoring was performed pre-treatment and post-6th treatment. Findings suggest decreased levels in tested blood parameters such as FBS, HbA1c, and creatinine (Figure 2 A, B, D) with the exception of the BUN (Figure 2 C).
Discussion
Type 2 diabetes mellitus (T2DM) represents a significant public health concern, particularly given the prevalence of individuals exhibiting resistance to the conventional therapies available15. Moreover, these conventional therapies were not designed to prevent the diabetic complications that are often chronic and difficult to manage. The development of cellular-based therapies offers an alternative approach in the management of T2DM and the prevention of its known complications. Over the years, cellular-based therapies have been explored as treatments for some of the known DM complications, such as diabetic foot ulcers and retinopathy, among others16.
Diabetic patients exhibit impaired mobilization of adult stem cells from the bone marrow, along with dysfunctional circulating progenitor cells. A growing body of evidence has demonstrated that DM is associated with a generalized reduction in circulating endothelial progenitor cells (EPC), and that this decline is linearly correlated with the severity of DM, in terms of HbA1c and blood glucose levels. It can be inferred that an increased number of circulating mesenchymal stem cells (MSC) and EPC in the system can directly correlate with improvements in glycated hemoglobin and fasting blood glucose levels17. In this case series, most of the patients demonstrated improved glucose parameters following the treatment. Although there is little or no clear evidence of in vivo stem cell differentiation into pancreatic beta cells, other potential mechanisms to explain this improvement include neovascularization, endothelial repair, modulation of the inflammatory environment, and stimulation of endogenous stem cells through paracrine mediators18. Additionally, this case series has presented the capability of autologous MSC and EPC therapy in improving creatinine levels in patients following six monthly infusions, supporting current studies regarding the feasibility of autologous combined MSC and EPC therapy in the management of diabetic kidney disease19.
Combined stem cell therapy for T2DM management has been performed in recent years. Most of these studies were conducted in vivo using murine models, and its application in humans led to the discovery of its actual efficacy and associated safety10. Some literature has investigated the synergistic effects of growth factors with MSC therapy in managing T2DM microvascular complications20. Other studies on the combinatory application of different cellular types, such as hematopoietic and MSCs, were applied to type-1 diabetes mellitus (T1DM)21. This study demonstrates the safe and effective application of combined MSC and EPC therapy in managing T2DM.
In the Philippines, bone marrow aspiration (BMA) is the primary method used for harvesting stem cells. BMA has been found to yield a sufficient quantity of cells required for cellular therapy infusion, though the quality varies according to the patient's age22. Proper cellular processing of bone marrow-derived MSCs must be achieved to counteract its significant reduction in proliferative capacity, differentiation potential, and clonal expandability with age, gender, and seeding density. This case series has demonstrated a standardized protocol for immune cell therapy conducted solely for research purposes, in accordance with national health guidelines on cellular-based therapies23. While attempts have been made to establish a bioethics advisory board to regulate cellular-based therapies in the Philippines24, there have been no updates regarding the establishment of laws regulating and supporting cellular-based therapies for clinical and research applications. This case series elucidates the potential of autologous cellular-based therapies for disease management and alternative medicine. Future studies might explore its application for long-term stem cell therapy, safety monitoring, and T2DM management on a larger sample size and the integration of controls. Statistical treatment could also be applied to describe its therapeutic value and dosing.
Conclusion
The six-monthly infusions of combined autologous mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) therapy through the intravenous route in this case series were safe and well-tolerated in patients with T2DM. This case series provides clear preliminary evidence of the potential benefits of autologous immune cell therapy in controlling T2DM, while the optimal dosage, administration, and cell types are yet to be determined.
This case series has observed that both MSCs and EPCs obtained from bone marrow aspiration (BMA) are easy to prepare, generally economical, and readily available in most tertiary hospitals in the country. The methodology for stem cell preparation adapted in this case series has demonstrated a safe and humane procedure aligned with the current standardized procedures for stem cell collection and infusion. Likewise, this can also serve as a framework for designing large-scale randomized studies to determine the optimal stem cell type and corresponding cell dosage. Subsequent studies related to this case series may investigate the most effective intervention time for treatment and explore other routes of cell infusion administration. Application of this intervention on a larger sample size, along with statistical treatment and the incorporation of controls, could further maximize the potential of combined stem cell therapy for T2DM. Finally, prolonged cellular infusion, patient monitoring, and follow-up interventions can be applied to further elucidate the potential of combined cellular therapy in T2DM management.
Abbreviations
BMA: Bone Marrow Aspiration, BUN: Blood Urea Nitrogen, CD: Cluster of Differentiation, CKD: Chronic Kidney Disease, CREA: Creatinine, EPCs: Endothelial Progenitor Cells, FBG: Fasting Blood Glucose, FBS: Fasting Blood Sugar, FGFs: Fibroblast Growth Factors, HbA1c: Hemoglobin A1c, kg: Kilogram, LCP: Lung Center of the Philippines, mL: Milliliters, MSCs: Mesenchymal Stem Cells, T2DM: Type 2 Diabetes Mellitus, VEGFs: Vascular Endothelial Growth Factors
Acknowledgments
The proponents would like to thank Mr. Marlon Echavez for assisting in cell preparations, Ms. Pauline Fernandez and Mrs. Abigail Bilbao for providing clinic support during cell therapy infusions.
Author’s contributions
M. S. A. De Guzman, M. P. B. Apelado, and J. P. Panuelos designed the experiment and performed the bone marrow collection, extraction of stem cells and quality control; M. S. A. De Guzman, M. P. B. Apelado, J. P. Panuelos, F. M. Heralde, III, P. R. Relacion. N. S. Tan-Liu, and M. T. A. Barzaga performed the analysis of the data and manuscript writing; M. S. A. De Guzman, M. P. B. Apelado, J. P. Panuelos, A. B. Bilbao performed the patient recruitment, infusion of autologous bone marrow-derived stem cells to the patients and monitored the patients during the transplantation. All of the authors read and approved the final version of the manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
The study protocol was approved by the technical review Board Committee of the Lung Center of the Philippines. Patient cases were assessed by the Stem Cell Ethics Committee, Lung Center of the Philippines to determine the eligibility of the patients that would benefit from the cellular therapy.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare no competing interests.
References
-
Roden
M.,
Shulman
G.I.,
The integrative biology of type 2 diabetes. Nature.
2019;
576
(7785)
:
51-60
.
View Article PubMed Google Scholar -
Padhi
S.,
Nayak
A.K.,
Behera
A.,
Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine and Pharmacotherapy.
2020;
131
.
View Article PubMed Google Scholar -
Peng
B.Y.,
Dubey
N.K.,
Mishra
V.K.,
Tsai
F.C.,
Dubey
R.,
Deng
W.P.,
Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. Journal of Diabetes Research.
2018;
2018
.
View Article PubMed Google Scholar -
Smyth
A.,
Jenkins
M.,
Dunham
M.,
Kutzer
Y.,
Taheri
S.,
Whitehead
L.,
Systematic review of clinical practice guidelines to identify recommendations for sleep in type 2 diabetes mellitus management. Diabetes Research and Clinical Practice.
2020;
170
.
View Article PubMed Google Scholar -
Alam
S.,
Hasan
M.K.,
Neaz
S.,
Hussain
N.,
Hossain
M.F.,
Rahman
T.,
Diabetes Mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology.
2021;
2
(2)
:
36-50
.
View Article Google Scholar -
Cho
N.H.,
Shaw
J.E.,
Karuranga
S.,
Huang
Y.,
da Rocha Fernandes
J.D.,
Ohlrogge
A.W.,
IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice.
2018;
138
:
271-81
.
View Article PubMed Google Scholar -
Saeedi
P.,
Petersohn
I.,
Salpea
P.,
Malanda
B.,
Karuranga
S.,
Unwin
N.,
Diabetes Atlas Committee
IDF,
Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice.
2019;
157
.
View Article PubMed Google Scholar -
Philippine Statistics Authority (PSA). Causes of Deaths in the Philippines. 2023. Available from https://psa.gov.ph/content/2022-causes-deaths-philippines-preliminary-28-february-2023. 2023
.
-
Tan
G.H.,
Diabetes Care in the Philippines. Annals of Global Health.
2015;
81
(6)
:
863-9
.
View Article PubMed Google Scholar -
Mathur
A.,
Taurin
S.,
Alshammary
S.,
The Safety and Efficacy of Mesenchymal Stem Cells in the Treatment of Type 2 Diabetes- A Literature Review. Diabetes, Metabolic Syndrome and Obesity.
2023;
16
:
769-77
.
View Article PubMed Google Scholar -
Nguyen
L.T.,
Hoang
D.M.,
Nguyen
K.T.,
Bui
D.M.,
Nguyen
H.T.,
Le
H.T.,
Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Translational Medicine.
2021;
10
(9)
:
1266-78
.
View Article PubMed Google Scholar -
Wils
J.,
Favre
J.,
Bellien
J.,
Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacology & Therapeutics.
2017;
170
:
98-115
.
View Article PubMed Google Scholar -
Sun
H.,
Saeedi
P.,
Karuranga
S.,
Pinkepank
M.,
Ogurtsova
K.,
Duncan
B.B.,
IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice.
2022;
183
.
View Article PubMed Google Scholar -
Gudleviciene
Z.,
Kundrotas
G.,
Liudkeviciene
R.,
Rascon
J.,
Jurga
M.,
Quick and effective method of bone marrow mesenchymal stem cell extraction. Open Medicine (Warsaw).
2014;
10
(1)
:
44-9
.
PubMed Google Scholar -
Scheen
A.J.,
Pharmacotherapy of `treatment resistant' type 2 diabetes. Expert Opinion on Pharmacotherapy.
2017;
18
(5)
:
503-15
.
View Article PubMed Google Scholar -
Fan
X.L.,
Zhang
Y.,
Li
X.,
Fu
Q.L.,
Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cellular and Molecular Life Sciences.
2020;
77
(14)
:
2771-94
.
View Article PubMed Google Scholar -
Yu
S.,
Cheng
Y.,
Zhang
L.,
Yin
Y.,
Xue
J.,
Li
B.,
Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Research & Therapy.
2019;
10
(1)
:
333
.
View Article PubMed Google Scholar -
Ceriello
A.,
Monnier
L.,
Owens
D.,
Glycaemic variability in diabetes: clinical and therapeutic implications. The Lancet. Diabetes & Endocrinology.
2019;
7
(3)
:
221-30
.
View Article PubMed Google Scholar -
Sávio-Silva
C.,
Beyerstedt
S.,
Soinski-Sousa
P.E.,
Casaro
E.B.,
Balby-Rocha
M.T.,
Simplício-Filho
A.,
Mesenchymal stem cell therapy for diabetic kidney disease: a review of the studies using syngeneic, autologous, allogeneic, and xenogeneic cells. Stem Cells International.
2020;
2020
.
View Article PubMed Google Scholar -
Kim
J.W.,
Luo
J.Z.,
Luo
L.,
Bone Marrow Mesenchymal Stem Cells as a New Therapeutic Approach for Diabetes Mellitus. InA Roadmap to Non-Hematopoietic Stem Cell-based Therapeutics 2019 Jan 1 (pp. 251-273). Academic Press..
.
View Article Google Scholar -
Madani
S.,
Amanzadi
M.,
Aghayan
H.R.,
Setudeh
A.,
Rezaei
N.,
Rouhifard
M.,
Investigating the safety and efficacy of hematopoietic and mesenchymal stem cell transplantation for treatment of T1DM: a systematic review and meta-analysis. Systematic Reviews.
2022;
11
(1)
:
82
.
View Article PubMed Google Scholar -
Chu
D.T.,
Phuong
T.N.,
Tien
N.L.,
Tran
D.K.,
Thanh
V.V.,
Quang
T.L.,
An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. International Journal of Molecular Sciences.
2020;
21
(3)
:
708
.
View Article PubMed Google Scholar -
Department of Health – Philippines. Rules and Regulations Governing the Accreditation of Health Facilities Engaging in Human Stem Cell and Cell-Based or Cellular Therapies in the Philippines. 2013. Available from https://www.fda.gov.ph/wp-content/uploads/2021/04/Administrative-Order-No.-2013-0012.pdf. 2013
.
-
Senate of the Philippines. An act intensifying stem cell research and therapy in the Philippines and for other purposes. 2017. Available from https://hrep-website.s3.ap-southeast-1.amazonaws.com/legisdocs/basic_18/HB01761.pdf . 2017
.
Comments
Article Details
Volume & Issue : Vol 11 No 4 (2024)
Page No.: 6326-6332
Published on: 2024-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3868 times
- PDF downloaded - 1059 times
- XML downloaded - 115 times
 Biomedpress
Biomedpress




