Abstract
Introduction: Given the limited research on the effect of L-arginine in preventing preeclampsia and intrauterine growth restriction (IUGR), coupled with the importance of preventing these conditions, this study was conducted to investigate the potential preventative role of L-arginine on preeclampsia and IUGR in primigravida women.
Methods: A single-blind randomized clinical trial was conducted with primigravida women attending Fatemieh Hospital in Hamadan city in 2021. An available sampling method was utilized to select the participants. The intervention group was treated with L-arginine at a dose of 1000 mg, along with prenatal supplements starting from the 20th week of pregnancy for twelve weeks, while the control group received only prenatal supplements. The occurrence of desired outcomes and preeclampsia, including blood pressure, proteinuria, and IUGR, was monitored and followed up until delivery.
Results: The mean age of mothers in the intervention and control groups was 35.8 ± 5.25 years and 39.4 ± 7.24 years, respectively (p = 0.31). The incidence of IUGR in the intervention group was significantly higher compared to the control group (12.66% vs. 2.7%, p = 0.033). Severe preeclampsia cases in the intervention group accounted for 5.41%, compared to 18.99% in the control group. Additionally, non-severe preeclampsia cases were more prevalent in the control group (p = 0.035).
Conclusion: In conclusion, the findings of this study suggest a potential role for L-arginine supplementation in reducing the incidence of preeclampsia and improving pregnancy outcomes. However, further research is needed to elucidate the underlying mechanisms, optimize treatment protocols, and evaluate long-term maternal and fetal outcomes.
Introduction
Preeclampsia is a multifaceted disorder during pregnancy that significantly compromises both maternal and fetal health. It manifests as high blood pressure and proteinuria, along with systemic complications. Despite extensive research, the root causes of preeclampsia remain poorly understood, and there is a notable absence of effective preventive measures. This condition presents a serious threat to the mother's health and also endangers the fetus, often resulting in premature birth, intrauterine growth restriction (IUGR), and in severe cases, death of both mother and child1.
The realm of preeclampsia research is fraught with ongoing controversies, especially concerning the fundamental mechanisms driving the disorder and the best approaches for its clinical management. While genetic factors and atypical immune responses have been proposed to contribute to its onset, recent studies have focused on the roles of vascular endothelial dysfunction and placental anomalies2, 3, 4, 5, 6. Furthermore, the observed variations in the occurrence and severity of preeclampsia among different racial and ethnic groups highlight the nuanced interconnection of genetic and environmental influences in its development.
Advancements in the understanding of preeclampsia have led to the identification of its pathophysiological underpinnings and potential avenues for intervention. Studies revealing immune dysregulation as a factor in preeclampsia's pathology underscore the promise of targeted immunomodulatory treatments7, 8. Additionally, the discovery of biomarkers such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) has been groundbreaking in facilitating the early detection and categorization of risk levels for preeclampsia, which supports more timely and effective treatment options, enhancing outcomes for pregnancies9.
Yet, the translation of these scientific insights into practical medical protocols remains challenging. There is still debate about the best management practices for preeclampsia. The safety and effectiveness of drug-based interventions, like aspirin and calcium supplements, are still under scrutiny, calling for more comprehensive research and clinical trials10, 11. Additionally, the potential effects of lifestyle and dietary changes, as well as emerging treatments like L-arginine supplementation, on the prevention and treatment of preeclampsia, need to be evaluated through rigorous scientific study.
The amino acid L-arginine, which is semi-essential, has gained attention as a possible preventative substance for hypertension-related conditions during pregnancy. L-arginine is integral to vasodilation and the synthesis of nitric oxide. When the demand for nitric oxide increases during pregnancy, the body's natural L-arginine production may not suffice, possibly leading to endothelial dysfunction and compromised vascular balance. Clinical evidence suggests that supplemental L-arginine can enhance endothelial functioning and decrease blood pressure, indicating its potential in staving off preeclampsia12.
Considering the pivotal role of nitric oxide bioactivity—or its absence—in the endothelial dysfunction characteristic of hypertension, supplementing to boost nitric oxide production appears to be a sound strategy. In hypercholesterolemic patients, L-arginine supplementation has improved endothelium-dependent vasodilation. This dysfunction and the resultant diminished activity of nitric oxide are significant indicators of the underlying pathophysiology in both cardiovascular diseases and hypertension. Hypertensive individuals often exhibit dilation of the epicardial arteries and a diminished response to nitric oxide-based agonists in their circulatory systems, which could contribute to altered vascular pressure regulation. Serving as the primary substrate for the synthesis of endothelial nitric oxide, L-arginine is indispensable13.
To address the critical need to prevent preeclampsia, our study investigates the efficacy of L-arginine supplementation in averting the onset of preeclampsia among first-time pregnant women attending the outpatient clinic of Fatemieh Hospital in Hamadan city.
Methods
In 2021, a single-blind randomized clinical trial was carried out with first-time pregnant women (primigravida) at Fatemieh Hospital's outpatient clinic in Hamadan city. An availability sampling method facilitated the patient selection process. Once suitable participants were identified, they were allocated to either the intervention or control group by employing a balanced block randomization technique, with blocks comprising four individuals each.
The study was informed by research from Camarena Pulido et al.14, which reported preeclampsia incidences of 0.61% in the L-arginine group and 2.34% in the placebo group. With a 0.05 Type I error rate and 80% test power, we calculated a necessary sample size of approximately 80 individuals per group.
The study's inclusion criteria specified participants be first-time pregnant women with no multiple pregnancies, chronic hypertension, thyroid conditions, diabetes, high blood pressure, or infections. Those on specific treatment protocols, including selenium-rich diets or selenium supplements, were excluded.
Following the ethical approval from Hamadan University of Medical Sciences and after securing informed consent from the participants, the intervention group was administered 1000 mg of L-arginine and prenatal supplements from the twentieth week of gestation for a duration of twelve weeks. The control group received solely the prenatal supplements. The intervention group underwent routine prenatal check-ups, adhering to national guidelines adjusted for gestational age. The monitoring process included regular assessments for preeclampsia symptoms, blood pressure, proteinuria, and Intrauterine Growth Restriction (IUGR). Uterine height was measured at each visit; a measurement below expected values led to an ultrasound to investigate potential growth restriction. Otherwise, standard pregnancy ultrasounds were conducted. Intrauterine growth restriction was defined by fetal weight or abdominal circumference below the 10th percentile or by abnormal Doppler findings, subsequently confirmed by the newborn's weight.
Care for both groups was uniform and followed a predetermined schedule. If increased blood pressure presented, with or without preeclampsia symptoms like urinary protein, headaches, blurred vision, epigastric pain, growth restriction, liver enzyme anomalies, or thrombocytopenia, a detailed preeclampsia assessment was conducted, and the findings were meticulously documented.
Participants were monitored up to childbirth, noting the weight of the newborns and the gestational age at birth. A tailored checklist was used for gathering demographic and clinical data, as well as information on any drug side effects observed in the study groups.
For the analysis, Stata software version 17 was utilized. The Student's t-test compared quantitative baseline variables between groups. The Chi-square test determined the variance in the incidence of preeclampsia, IUGR, and delivery timing between the groups. A P-value below 0.05 denoted statistical significance.
| Variable | Intervention group | Control group | P-Value* |
|---|---|---|---|
| Mother age (year) | 25.58 ± 5.38 | 24.77 ± 4.39 | 0.31 |
| BMI (kg/m 2 ) | 24.7 ± 3.11 | 25.15 ± 2.71 | 0.34 |
| Age at delivery (gr) | 2969.6 ± 667.6 | 2872.8 ± 777.4 | 0.41 |
| Gestational age at delivery (week) | 38.18 ± 3.19 | 38.76 ± 3.07 | 0.53 |
| Variables | Intervention group | Control group | P-Value* | |
|---|---|---|---|---|
| IUGR (%) | 2 (2.7) | 10 (12.66) | 0.033 | |
| Weigh less than 10 percentile (%) | 3 (4.05) | 9 (11.39) | 0.13 | |
| Cesarean delivery (%) | 28 (37.84) | 29 (36.71) | 0.89 | |
| Pre-term delivery (%) | 14 (18.92) | 21 (26.58) | 0.26 | |
| Pre-eclampsia | Sever | 4 (5.41) | 15 (18.99) | 0.035 |
| Non sever | 6 (8.11) | 7 (8.76) | ||
| No | 64 (86.49) | 57 (72.15) |
Results
Patient Selection and Follow-Up
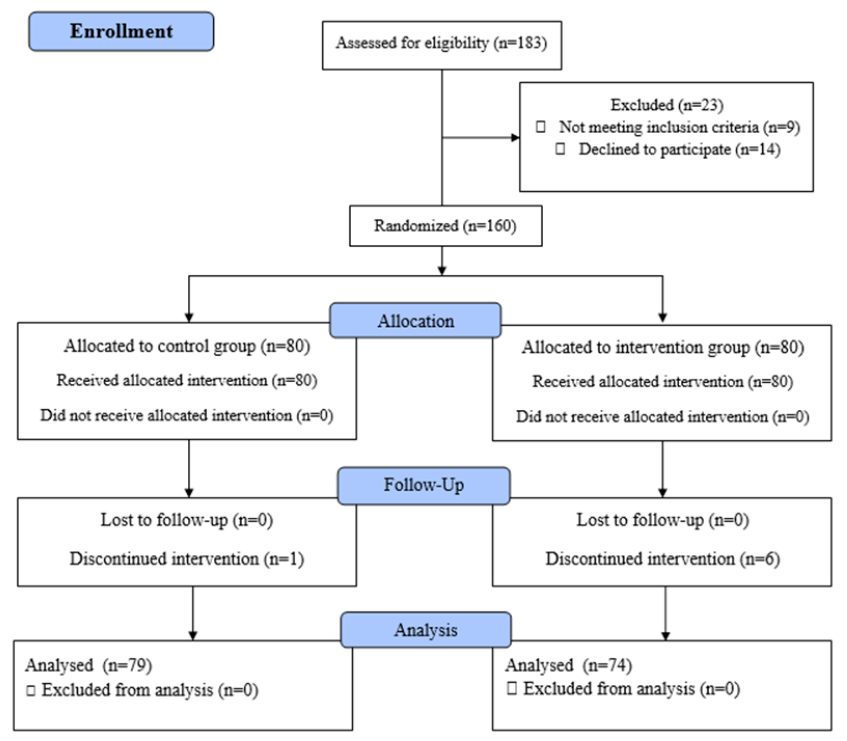
In this study, we evaluated 183 primigravida women at Fatemieh Hospital's prenatal clinic in Hamadan. Of these women, 14 did not provide informed consent, and 9 were excluded based on the study's inclusion criteria. Consequently, we randomly allocated the remaining 160 eligible women into two groups, intervention and control, with 80 participants in each, using block randomization. During the course of the study, attrition occurred as 6 women from the intervention group and 1 from the control group discontinued their participation (see Figure 1 for details).
Baseline Comparison Between Groups
Variables such as maternal age, neonatal birth weight, maternal BMI, and infant gender were compared across the intervention and control groups, as presented in Table 1. The average age of mothers in the intervention group was 38.5 ± 5.8 years, while it was 39.4 ± 7.7 years in the control group; this age discrepancy was statistically insignificant (p = 0.31). Likewise, no significant statistical differences were observed between the groups in terms of neonatal birth weight, maternal BMI, or the age of the mothers at delivery (P > 0.05).
Outcome Comparison Between Groups
Table 2 outlines the comparison of pregnancy outcomes for both the mothers and infants in the L-arginine treatment group and the control group. The intervention group showed a notably higher rate of Intrauterine Growth Restriction (IUGR), with 12.66% of cases, in contrast to 2.7% in the control group, a difference that was statistically significant (P = 0.033). Severe preeclampsia was observed in 5.41% of the intervention group compared to 18.99% in the control group. The prevalence of non-severe preeclampsia was also greater in the control group. The Chi-square test revealed a significant difference in the incidence of preeclampsia between the groups (P = 0.035). The intervention group had a lower percentage of preterm births (26.58%) compared to the control group (18.92%), yet this difference did not reach statistical significance (P = 0.26).
Side Effect Assessment in the Intervention
Group Among the intervention group, side effects following L-arginine administration were documented: nausea in three patients (4.05%), abdominal pain in two (2.7%), and diarrhea in one patient (1.35%).
Discussion
This study aimed to explore the preventive effects of L-arginine on preeclampsia in first-time pregnant women. Despite significant progress in medicine, preeclampsia still poses a challenge in both understanding and management. Our study adds to the evidence that supports the use of L-arginine supplementation as a strategy to decrease the risk of preeclampsia and enhance pregnancy outcomes.
Consistent with our research, a 2016 meta-analysis comprising nine studies with 576 patients also investigated L-arginine's impact on pregnancy. It found a notable rise in both fetal birth weight and the age of mothers receiving L-arginine15. Animal research suggests L-arginine positively affects intrauterine growth restriction (IUGR), improving fetal growth by aiding creatine production—essential for muscle development—and by stimulating the synthesis of skeletal muscle proteins16, 17. Yet, a 2024 meta-analysis showed no significant improvements in maternal and neonatal outcomes among groups treated with L-arginine18.
L-arginine is observed to induce the secretion of growth hormone-releasing hormone, which in turn raises plasma growth hormone levels, affecting overall growth19. The antioxidant properties of L-arginine may shield the fetus from oxidative stress—an imbalance between reactive oxygen species production and antioxidant defense—implicated in IUGR pathogenesis. L-arginine's ability to reduce oxidative stress could alleviate IUGR's impact on fetal development20.
A study by Pulido et al., in 2016, combined with our findings, suggests that L-arginine intake can lower preeclampsia risk by 26%14. A recent meta-analysis covering ten studies further supports L-arginine's superiority over placebo in reducing preeclampsia risk (OR: 0.36, 95% CI: 0.17- 0.77)18. Vadillo et al.'s research aligns with this, proposing an arginine deficiency as a possible preeclampsia cause21. A 2023 systematic review of 51 studies (25 involving humans and 26 involving animals) confirmed that L-arginine shows beneficial biological activity in pregnancies affected by hypertensive disorders and growth restrictions. The review endorses L-arginine as a safe intervention that could improve maternal and fetal outcomes, particularly in cases of moderate clinical disorders22.
Our study's findings indicate that L-arginine could offer a protective role against hypertensive pregnancy disorders, considering preeclampsia's complex origins—abnormal immune responses, endothelial dysfunction, and placental issues. L-arginine may exert broad-ranging effects, such as modulating inflammatory pathways, promoting angiogenesis, and enhancing trophoblast function in the placenta. Experimental studies point to L-arginine's immunomodulatory roles, like damping pro-inflammatory cytokines and bolstering regulatory T cell functions, which could be pivotal in countering preeclampsia23.
Importantly, no significant side effects followed L-arginine consumption in our study, a finding echoed by a systematic review that found no adverse reactions related to L-arginine in both human and animal studies, across various dosages22.
Nonetheless, our study faced limitations. Notably, six participants in the intervention group discontinued the study, which might affect the study's validity. Moreover, focusing exclusively on primiparous women limits the applicability of our results to all pregnant women.
Conclusions
In conclusion, this study's findings indicate that L-arginine supplementation may play a role in diminishing the risk of preeclampsia and in the enhancement of pregnancy outcomes. Nevertheless, there is a necessity for additional research. The purposes of future studies should include clarifying the mechanisms behind L-arginine's effects, refining treatment protocols for efficacy, and assessing the long-term health implications for both mother and child.
Abbreviations
BMI: Body Mass Index, CI: Confidence Interval, IUGR: Intrauterine Growth Restriction, mg: Milligrams, OR: Odds Ratio, p: Probability value (used in statistical tests), PlGF: Placental Growth Factor, sFlt-1: Soluble fms-like tyrosine kinase-1
Acknowledgments
The authors would like to appreciate the Hamadan University of Medical Sciences, Iran for financial support (Research Id: 140105043183). As well would like to acknowledge the efforts of health staff in Fatemieh hospital for their kind collaborations.
Author’s contributions
All authors significantly contributed to this work, read and approved the final manuscript.
Funding
The authors would like to appreciate the Hamadan University of Medical Sciences, Iran for financial support (Research Id: 140105043183).
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
We obtain the approval of the ethics committee of the Hamadan University of Medical Science.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Rahnemaei
F.A.,
Fashami
M.A.,
Abdi
F.,
Abbasi
M.,
Factors effective in the prevention of Preeclampsia:A systematic review. Taiwanese Journal of Obstetrics & Gynecology.
2020;
59
(2)
:
173-82
.
View Article PubMed Google Scholar -
Jain
S.,
Sharma
P.,
Kulshreshtha
S.,
Mohan
G.,
Singh
S.,
The role of calcium, magnesium, and zinc in pre-eclampsia. Biological Trace Element Research.
2010;
133
(2)
:
162-70
.
View Article PubMed Google Scholar -
Pathak
P.,
Kapil
U.,
Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian Journal of Pediatrics.
2004;
71
(11)
:
1003-5
.
View Article PubMed Google Scholar -
Ilekis
J.V.,
Reddy
U.M.,
Roberts
J.M.,
Preeclampsia a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reproductive Sciences (Thousand Oaks, Calif.).
2007;
14
(6)
:
508-23
.
View Article PubMed Google Scholar -
Sibai
B.,
Dekker
G.,
Kupferminc
M.,
Pre-eclampsia. Lancet.
2005;
365
(9461)
:
785-99
.
View Article PubMed Google Scholar -
Dadelszen
P. von,
Magee
L.,
What matters in preeclampsia are the associated adverse outcomes: the view from Canada. Current Opinion in Obstetrics & Gynecology.
2008;
20
(2)
:
110-5
.
View Article PubMed Google Scholar -
Mora-Palazuelos
C.,
Villegas-Mercado
C.E.,
Avendaño-Félix
M.,
Lizárraga-Verdugo
E.,
Romero-Quintana
J.G.,
López-Gutiérrez
J.,
The Role of ncRNAs in the Immune Dysregulation of Preeclampsia. International Journal of Molecular Sciences.
2023;
24
(20)
:
15215
.
View Article PubMed Google Scholar -
Laresgoiti-Servitje
E.,
A leading role for the immune system in the pathophysiology of preeclampsia. Journal of Leukocyte Biology.
2013;
94
(2)
:
247-57
.
View Article PubMed Google Scholar -
Dröge
L.A.,
Perschel
F.H.,
Stütz
N.,
Gafron
A.,
Frank
L.,
Busjahn
A.,
Prediction of preeclampsia-related adverse outcomes with the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor)-ratio in the clinical routine: a real-world study. Hypertension.
2021;
77
(2)
:
461-71
.
View Article PubMed Google Scholar -
Souza
E.V.,
Torloni
M.R.,
Atallah
A.N.,
Santos
G.M.,
Kulay
L.,
Sass
N.,
Aspirin plus calcium supplementation to prevent superimposed preeclampsia: a randomized trial. Brazilian Journal of Medical and Biological Research.
2014;
47
(5)
:
419-25
.
View Article PubMed Google Scholar -
Bujold
E.,
Hyett
J.,
Calcium supplementation for prevention of pre-eclampsia. Lancet.
2019;
393
(10169)
:
298-300
.
View Article PubMed Google Scholar -
Abukhodair
A.W.,
Abukhudair
W.,
Alqarni
M.S.,
The effects of L-arginine in hypertensive patients: A literature review. Cureus.
2021;
13
(12)
.
View Article PubMed Google Scholar -
Dorniak-Wall
T.,
Grivell
R.M.,
Dekker
G.A.,
Hague
W.,
Dodd
J.M.,
The role of L-arginine in the prevention and treatment of pre-eclampsia: a systematic review of randomised trials. Journal of Human Hypertension.
2014;
28
(4)
:
230-5
.
View Article PubMed Google Scholar -
Camarena Pulido
E.E.,
García Benavides
L.,
Panduro Barón
J.G.,
Pascoe Gonzalez
S.,
Madrigal Saray
A.J.,
García Padilla
F.E.,
Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: A double-blind, randomized, clinical trial. Hypertension in Pregnancy.
2016;
35
(2)
:
217-25
.
View Article PubMed Google Scholar -
Chen
J.,
Gong
X.,
Chen
P.,
Luo
K.,
Zhang
X.,
Effect of L-arginine and sildenafil citrate on intrauterine growth restriction fetuses: a meta-analysis. BMC Pregnancy and Childbirth.
2016;
16
(1)
:
225
.
View Article PubMed Google Scholar -
Kim
S.W.,
Wu
G.,
Wu
G.,
Dietary arginine supplementation enhances the growth of milk-fed young pigs. The Journal of Nutrition.
2004;
134
(3)
:
625-30
.
View Article PubMed Google Scholar -
Lacassie
H.J.,
Germain
A.M.,
Valdés
G.,
Fernández
M.S.,
Allamand
F.,
López
H.,
Management of Eisenmenger syndrome in pregnancy with sildenafil and L-arginine. Obstetrics and Gynecology.
2004;
103
(5 Pt 2)
:
1118-20
.
View Article PubMed Google Scholar -
Naderipour
F.,
Keshavarzi
F.,
Mirfakhraee
H.,
Dini
P.,
Jamshidnezhad
N.,
Abolghasem
N.,
Efficacy of L-arginine for preventing preeclampsia and improving maternal and neonatal outcomes in high-risk pregnancies: A systematic review and meta-analysis study. International Journal of Fertility & Sterility.
2024;
2024
:
Article in press
.
View Article Google Scholar -
Boo
H.A. de,
Eremia
S.C.,
Bloomfield
F.H.,
Oliver
M.H.,
Harding
J.E.,
Treatment of intrauterine growth restriction with maternal growth hormone supplementation in sheep. American journal of obstetrics and gynecology.
2008;
199
(5)
:
559. e1-e9
.
View Article Google Scholar -
Zhang
H.,
Sun
H.,
Peng
A.,
Guo
S.,
Wang
M.,
Loor
J.J.,
N-carbamylglutamate and l-arginine promote intestinal function in suckling lambs with intrauterine growth restriction by regulating antioxidant capacity via a nitric oxide-dependent pathway. Food & Function.
2019;
10
(10)
:
6374-84
.
View Article PubMed Google Scholar -
Vadillo-Ortega
F.,
Perichart-Perera
O.,
Espino
S.,
Avila-Vergara
M.A.,
Ibarra
I.,
Ahued
R.,
Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ (Clinical Research Ed.).
2011;
342
(may19 1)
:
d2901
.
View Article PubMed Google Scholar -
Menichini
D.,
Feliciello
L.,
Neri
I.,
Facchinetti
F.,
L-Arginine supplementation in pregnancy: a systematic review of maternal and fetal outcomes. The Journal of Maternal-Fetal & Neonatal Medicine.
2023;
36
(1)
.
View Article PubMed Google Scholar -
Ortiz-Cerda
T.,
Mosso
C.,
Alcudia
A.,
Vázquez-Román
V.,
González-Ortiz
M.,
Pathophysiology of Preeclampsia and L-Arginine/L-Citrulline Supplementation as a Potential Strategy to Improve Birth Outcomes. Advances in Experimental Medicine and Biology.
2023;
2023
(1428)
:
127--148
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 4 (2024)
Page No.: 6357-6362
Published on: 2024-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3583 times
- PDF downloaded - 1251 times
- XML downloaded - 130 times
 Biomedpress
Biomedpress


