Abstract
Background: Cytokines can be key factors in the pathogenesis of coronary artery disease (CAD). This systematic review and meta-analysis aimed to assess the levels of interleukin-10 (IL-10), an anti-inflammatory cytokine, in the serum/plasma of patients with CAD.
Methods: An exhaustive search was conducted across the Web of Science, PubMed/Medline, Scopus, and Cochrane Library databases up to March 25, 2022. Review Manager 5.3 software was used to calculate the effect sizes, presenting the standardized mean difference (SMD) along with a 95% confidence interval (CI). STRING software, which maps protein-protein interactions (PPI), was utilized to explore the functional interactions among the genes under study.
Results: From the 1130 records retrieved from the databases, 26 articles were included in the meta-analysis. The pooled SMD for CAD cases compared to controls was 0.33 (p = 0.15). The sample size was adequate for comparing blood IL-10 levels in CAD patients versus controls.
Conclusion: The findings suggest there was no significant difference in the serum/plasma levels of IL-10 between CAD patients and controls. Hence, the pathogenesis of CAD can be multifactorial and complex.
Introduction
Heart diseases (HDs) are the leading cause of death worldwide, with the majority of fatalities, approximately 80%, occurring in low- to middle-income countries. If current trends continue, it is projected that by 2030, cardiovascular diseases will claim the lives of about 23.6 million individuals, predominantly through heart attacks and strokes1, 2. Ischemic HD is recognized as a significant threat in the 21st century3, also known as coronary artery disease (CAD) or coronary HD. A large number of individuals with CAD live with chronic disabilities and impaired quality of life4.
One of the most significant risk factors associated with HD is a family history of HD5. The increase in HD risk due to family history can be attributed to shared genetic, environmental, and lifestyle factors. The importance of genetics becomes more apparent with the early onset of HD in the family and the number of family members affected6. It is believed that an imbalance between pro- and anti-inflammatory activities plays a crucial role in the development of atherosclerosis7. Inflammation contributes to the early stages of HD, and therefore, may drive the progression of this disease8, 9.
CAD is the primary cause of deaths related to cardiovascular issues10, and atherosclerosis is the most common reason for CAD, which is a longstanding inflammatory condition of the arterial walls that arises from an inappropriate inflammatory response and an imbalance in lipid metabolism11. A multitude of evidence, including both clinical trials and experimental studies, collectively indicates that inflammation is integral to all phases of atherosclerosis development9, 12, 13.
Several signaling pathways have been reported and linked to CAD pathogenesis14, 15, 16, 17. Cytokines, which are part of the extracellular signaling proteins, are secreted by both immune and non-immune cells, including cells of the vascular endothelium18. Increased levels of inflammatory cytokines in the plasma have been documented in patients with CAD, especially in those with unstable disease conditions10. Conversely, the presence of anti-inflammatory mediators is less well documented. Interleukin-10 (IL-10) is a potent anti-inflammatory cytokine that plays a vital and often indispensable role in warding off inflammatory and autoimmune conditions19, 20, 21, 22. The gene for human IL-10 is located on chromosome 1, specifically at the juncture of regions 1q31 and 1q3223. IL-10 can reduce the likelihood of atherosclerosis development and improve the progression of atherosclerosis and vascular complications24. Studies have documented the role of plasma/serum IL-10 levels in CAD patients but with varying and contradictory results25, 26, 27. The interactions of IL-10 are complex and can vary depending on the specific context and conditions28, 29, 30.
To our knowledge, this topic has not been the subject of a meta-analysis. Therefore, the goal of this meta-analysis was to assess the levels of IL-10 in the blood of patients with CAD to obtain better and more accurate results and to identify the possible reasons for these discrepancies between the results of individual studies. Another aim was to understand the pathogenesis, protein-protein interactions (PPI), and patient-specific factors as research gaps.
Methods
Design and Registration
This study adhered to the guidelines set forth by PRISMA31. Additionally, the protocol for this meta-analysis was registered in the PROSPERO database under the registration number CRD42022335594. The question posed in terms of PECO was: Is there an association between serum/plasma levels of IL-10 and the risk of CAD in studies with a case-control design?
Article Discovery
An author of the study, M.S., carried out a comprehensive search in databases such as PubMed/MEDLINE, Web of Science, Scopus, and Cochrane Library up until March 25, 2022, without imposing any restrictions, to collect relevant articles. M.S. also reviewed the titles and abstracts of these articles. Subsequently, the full texts of the articles that met the selection criteria were obtained. The search strategy included keywords/title/abstract: ("coronary atherosclerotic heart disease" or "coronary heart disease" or "coronary artery disease" or "ischemic heart disease" or "myocardial infarction" or "acute coronary syndrome" or "angina pectoris") and ("interleukin-10" or "IL-10" or "IL10" or "interleukin 10") and ("plasma" or "serum" or "blood") and ("control" or "normal" or "healthy"). The bibliographies of the retrieved articles were scrutinized to ensure no significant studies were missed. Another author, R.H.M., verified the search and selection procedures. In the event of any discrepancies between the two authors, a third author, N.S., intervened in the resolution.
Criteria for Selection and Rejection
The inclusion criteria were as follows: 1) Any study that reported the levels of IL-10 in the serum or plasma of CAD patients and control subjects. 2) Studies that included more than 10 cases in both the case and control groups. 3) CAD was defined based on the criteria reported in Alshammary's study32, and Table 1 shows the criteria for each study. 4) CAD patients without any other systemic diseases and control subjects who were in good health. 5) CAD patients with or without medical treatment, such as statins. Conversely, review articles, meta-analyses, articles with missing data, studies conducted on animals, articles lacking a control group, commentary papers, conference papers, book chapters, duplicate studies, studies that included disease-afflicted controls, and studies involving cases under treatment were excluded.
| First author, publication year | CAD definition |
|---|---|
| Mazzone, 1999 33 | Standard progressive changes in electrocardiography linked with a rise in CK values exceeding twice the upper normal limit and alterations in the ST-segment |
| Mizia-Stec, 2002 34 | Coronangiography |
| Mizia-Stec, 2003 35 | Coronangiography was performed if there was a constriction of the diameter by 75% or more in at least one of the three primary epicardial coronary arteries |
| Lee, 2006 36 | Cardiac catheterization |
| Nilsson, 2006 37 | Angiography |
| Szodoray, 2006 38 | Angiography |
| Paulsson, 2008 39 | Angiography |
| Cheng, 2009 40 | Scanning with radioactive thallium or coronary angiogram |
| Jha, 2009 41 | Angiography |
| Jha, 2010 42 | Angiography |
| Khan, 2011 43 | Angiography revealing a stenosis of more than 70% in at least one coronary vessel |
| Tapp, 2012 44 | European Society of Cardiology definition |
| Karu, 2013 45 | NR |
| Li, 2015 46 | Clinical symptoms, ECG alterations, coronary angiography, and cardiac troponin tests |
| Mirhafez, 2015 47 | Angiography |
| Cheng, 2016 48 | Coronary stenosis with at least one main coronary vessel with 50% luminal narrowing |
| Liang, 2016 49 | A narrowing of the lumen by 50% or more was observed in at least one primary coronary artery or its main branches |
| Bergström, 2017 25 | Non-segment elevation myocardial infarction identified through characteristic ECG alterations and increased levels of troponins |
| Tajfard, 2017 50 | An occlusion of 50% or more in at least one coronary artery |
| Xu, 2017 51 | Stenosis exceeding 50% in at least one primary vessel |
| Boles, 2018 26 | Angiography |
| Kharaeva, 2018 27 | Angiography |
| Kumari, 2018 18 | NR |
| Ansari, 2019 52 | Angiography revealing stenosis exceeding 70% in a single coronary artery |
| Shipulin, 2020 53 | Left ventricular ejection fraction ≤ 40%, stenosis left main or proximal part of the left descending artery or two or more epicedial vessels ≥ 75% |
| Nowrouzi-Sohrabi, 2022 54 | Angiography confirmed stenosis of more than 50% in at least one coronary artery |
Data Summary
The authors, M.S. and M.R., independently extracted data from the studies included in the meta-analysis. Extracted data encompassed authors' names, publication year, study country, case ethnicity, the number of coronary artery disease (CAD) patients and control subjects, sample size, average age, IL-10 levels in serum or plasma, and the quality score.
Quality Evaluation
The quality assessment was conducted by one author, M.S., using the Newcastle-Ottawa Scale (NOS) tool to evaluate the quality and potential bias in case-control studies55. The highest possible score on the NOS is nine, with scores of seven or higher indicative of high quality.
Statistical Analyses
The Review Manager 5.3 (RevMan 5.3, The Cochrane Collaboration, Oxford, UK) software was utilized to calculate effect sizes, providing the standardized mean difference (SMD) and a 95% confidence interval (CI) for IL-10 levels in the blood of CAD cases and controls. The Z-test determined the significance of the pooled SMD, considering a two-sided p-value of less than 0.05 significant. If heterogeneity was significant, indicated by a Pheterogeneity value of less than 0.1 and an I2 value greater than 50%, a random-effects model56 was used. Conversely, a fixed-effect model57 wad used in cases of insignificant heterogeneity.
Subgroup analysis, random meta-regression analysis, and sensitivity analysis ("one-study-removed" and "cumulative" analyses) were performed using the Comprehensive Meta-Analysis version 2.0 (CMA 2.0; Biostat Inc., Englewood, NJ, USA). Publication bias was assessed using Egger's58 and Begg’s tests59, with a 2-sided p < 0.10 indicating the presence of publication bias.
The NCSS 2021 version 21.0.2 (NCSS, Kaysville, UT, USA) software generated two plots (Radial and L'Abbé plots). The Radial, or Galbraith, plot displays the z-statistic60, and the L’Abbé plot illustrates event rates in cases compared to control groups61, 62, with a p-value less than 0.05 indicating statistically significant heterogeneity.
Trial Sequential Analysis (TSA) was conducted using TSA software (version 0.9.5.10 beta) (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark)63. The required information size (RIS) was calculated with an alpha risk of 5%, a beta risk of 20%, and a two-sided boundary type. The studies were considered to have included sufficient cases if the Z-curve intersected the RIS line, adhered to the boundary line, or entered the futility area. Otherwise, additional information and further studies were deemed necessary.
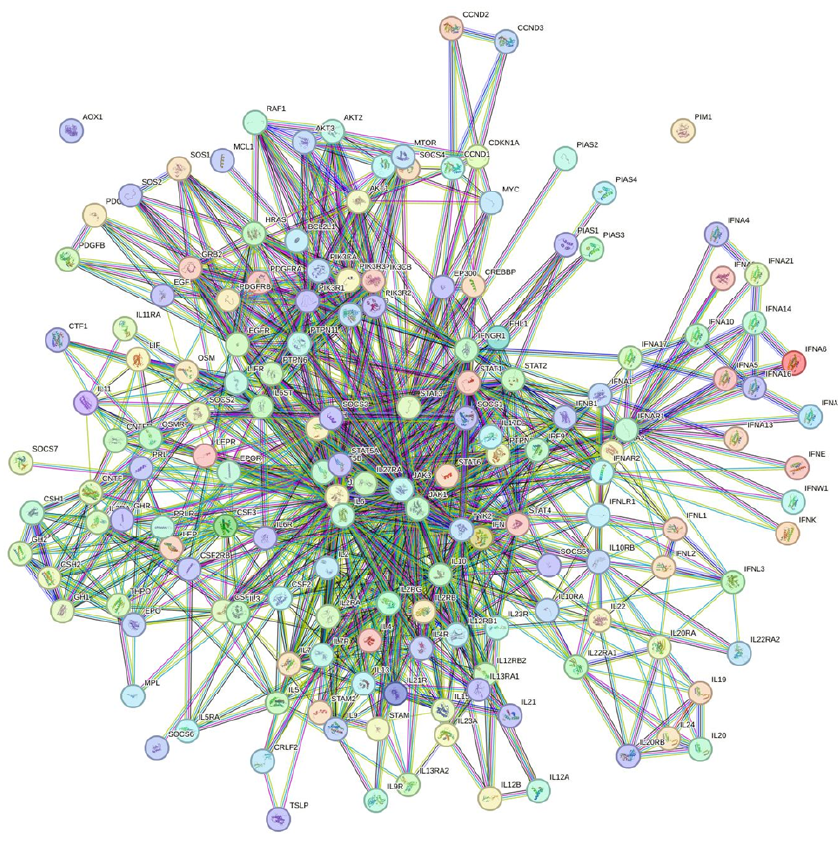
Functional interactions between examined genes were studied using the STRING software, a protein-protein interaction network tool accessed at https://string-db.org/ (accessed on 5 August 2023)64. Interaction settings were limited to "Homo sapiens" and required an interaction score threshold of more than 0.900. In the resulting networks, proteins are represented by nodes, and interactions are indicated by edges. STRING was used to identify potential interactions between differentially expressed genes (DEGs) in various tissues, with KEGG analysis obtained from the STRING software.
Results
Choice of Studies
After removing duplicates and records that were not relevant, 63 out of the 1130 records that were initially retrieved from the databases met the criteria for inclusion as full-text articles (Figure 1). Subsequently, 37 full texts were removed for reasons. At last, 26 articles including 27 studies were entered into the meta-analysis.
Attributes of the Study
The main features of 26 articles18, 25, 26, 27, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 incorporated in the meta-analysis are shown in Table 2. The articles were disseminated from 1999 to 2022. Seventeen articles25, 26, 27, 33, 34, 35, 37, 38, 39, 43, 44, 45, 47, 50, 51, 52, 53, 54 were reported in Caucasians and nine18, 36, 40, 41, 42, 46, 48, 49, 51 in Asians. Eleven articles25, 26, 33, 37, 41, 42, 44, 46, 51, 53, 54 were reported IL-10 level in plasma and fifteen18, 27, 34, 35, 36, 38, 39, 40, 43, 45, 47, 48, 49, 50, 52 in serum. Other variables and the quality score of each article are shown in Table 2 and Table 3, respectively. A few studies in some cases reported the CAD patients under statin therapy.
| First author, publication year | Country | Ethnicity | Sample | Groups | Number | Mean age, years | IL-10 level (Mean ± SD) | Detection assay |
|---|---|---|---|---|---|---|---|---|
| Mazzone, 1999 33 | Italy | Caucasian | Plasma | CAD | 42 | 61 | 10.8 ± 1.8 | ELISA |
| CO | 39 | 55 | 1.9 ± 1.1 | |||||
| Mizia-Stec, 2002 34 | Poland | Caucasian | Serum | CAD | 100 | 58.0 | 52.37 ± 106.15 | ELISA |
| CO | 20 | 55.3 | 14.3 ± 28.5 | |||||
| Mizia-Stec, 2003 35 | Poland | Caucasian | Serum | CAD | 33 | 60.9 | 68.0 ± 152.5 | ELISA |
| CO | 20 | 55.4 | 14.3 ± 28.5 | |||||
| Lee, 2006 36 | Taiwan | Asian | Serum | CAD | 30 | 65.5 | 1.56 ± 1.37 | ELISA |
| CO | 73 | 63 | 1.22 ± 0.85 | |||||
| Nilsson, 2006 37 | Sweden | Caucasian | Plasma | CAD | 65 | 56.9 | 2.58 ± 2.04 | ELISA |
| CO | 28 | 52.5 | 2.4 ± 2.5 | |||||
| Szodoray, 2006 38 | Hungary | Caucasian | Serum | CAD | 62 | 64.2 | 32.3 ± 95.3 | ELISA |
| CO | 58 | 72.3 | 6.95 ± 15.38 | |||||
| Paulsson, 2008 39 | Sweden | Caucasian | Serum | CAD | 19 | 61 | 0.73 ± 0.67 | Immunoassay |
| CO | 19 | 60 | 0.55 ± 0.49 | |||||
| Cheng, 2009 40 | Taiwan | Asian | Serum | CAD | 138 | 65.5 | 2.1 ± 0.2 | ELISA |
| CO | 74 | 63 | 1.2 ± 0.1 | |||||
| Jha, 2009 41 | India | Asian | Plasma | CAD | 192 | - | 1.83 ± 0.16 | ELISA |
| CO | 192 | - | 1.95 ± 0.19 | |||||
| Jha, 2010 42 | India | Asian | Plasma | CAD (male) | 148 | - | 1.83 ± 0.15 | ELISA |
| CAD (female) | 42 | - | 1.82 ± 0.16 | |||||
| CO (male) | 142 | - | 1.94 ± 0.17 | |||||
| CO (female) | 50 | - | 1.94 ± 0.18 | |||||
| Khan, 2011 43 | Pakistan | Caucasian | Serum | CAD | 98 | 40 | 2.07 ± 1.70 | ELISA |
| CO | 74 | 35 | 1.7 ± 1.85 | |||||
| Tapp, 2012 44 | UK | Caucasian | Plasma | CAD | 40 | 60.4 | 0.55 ± 0.93 | Flow cytometry |
| CO | 40 | 59.5 | 0.88 ± 1.16 | |||||
| Karu, 2013 45 | Estonia | Caucasian | Serum | CAD | 39 | 64 | 0.53 ± 0.19 | High-sensitivity array |
| CO | 39 | 62 | 0.65 ± 0.21 | |||||
| Li, 2015 46 | China | Asian | Plasma | CAD | 29 | 66.9 | 18.74 ± 20.84 | ELISA |
| CO | 11 | 62.3 | 20.92 ± 14.69 | |||||
| Mirhafez, 2015 47 | Iran | Caucasian | Serum | CAD | 289 | 59.1 | 0.75 ± 0.29 | Sandwich Chemiluminescence |
| CO | 89 | 58.7 | 0.84 ± 0.39 | |||||
| Cheng, 2016 48 | China | Asian | Serum | CAD | 52 | 60 | 7.42 ± 3.81 | ELISA |
| CO | 50 | 59 | 17.46 ± 5.01 | |||||
| Liang, 2016 49 | China | Asian | Serum | CAD | 128 | 65.3 | 134.43 ± 38.24 | ELISA |
| CO | 106 | 64.7 | 164.38 ± 36.45 | |||||
| Bergström, 2017 25 | Sweden | Caucasian | Plasma | CAD | 57 | 66 | 0.32 ± 0.12 | ELISA |
| CO | 41 | 67 | 1.03 ± 0.13 | |||||
| Tajfard, 2017 50 | Iran | Caucasian | Serum | CAD | 231 | 59.5 | 0.76 ± 0.29 | Biochip array |
| CO | 120 | 53.3 | 0.83 ± 0.34 | |||||
| Xu, 2017 51 | China | Asian | Plasma | CAD | 264 | 59 | 98.65 ± 34.79 | ELISA |
| CO | 186 | 58 | 32.18 ± 12.15 | |||||
| Boles, 2018 26 | Sweden | Caucasian | Plasma | CAD | 69 | 64.5 | 0.29 ± 0.21 | ELISA |
| CO | 140 | 58.6 | 0.25 ± 0.15 | |||||
| Kharaeva, 2018 27 | Russia | Caucasian | Serum | CAD | 27 | 57 | 53.6 ± 3.2 | ELISA |
| CO | 20 | 55 | 10.0 ± 3.0 | |||||
| Kumari, 2018 18 | India | Asian | Serum | CAD | 290 | 51.6 | 5.33 ± 3.34 | ELISA |
| CO | 290 | 51.7 | 5.83 ± 2.63 | |||||
| Ansari, 2019 52 | Pakistan | Caucasian | Serum | CAD | 340 | 42 | 0.83 ± 0.53 | ELISA |
| CO | 310 | 39 | 0.87 ± 0.36 | |||||
| Shipulin, 2020 53 | Russia | Caucasian | Plasma | CAD | 26 | 59.2 | 25.16 ± 4.07 | ELISA |
| CO | 14 | 58.6 | 20.5 ± 4.44 | |||||
| Nowrouzi-Sohrabi, 2022 54 | Iran | Caucasian | Plasma | CAD | 15 | 58.9 | 1.98 ± 0.73 | ELISA |
| CO | 15 | 56.2 | 1.87 ± 0.92 |
| First author, publication year | Selection # | Comparability & | Exposure $ | Total score |
|---|---|---|---|---|
| Mazzone, 1999 33 | *** | * | ** | 6 |
| Mizia-Stec, 2002 34 | **** | ** | ** | 8 |
| Mizia-Stec, 2003 35 | **** | ** | ** | 8 |
| Lee, 2006 36 | **** | ** | ** | 8 |
| Nilsson, 2006 37 | **** | ** | ** | 8 |
| Szodoray, 2006 38 | **** | ** | ** | 8 |
| Paulsson, 2008 39 | **** | ** | ** | 8 |
| Cheng, 2009 40 | **** | ** | ** | 8 |
| Jha, 2009 41 | **** | ** | ** | 8 |
| Jha, 2010 42 | **** | ** | ** | 8 |
| Khan, 2011 43 | *** | ** | ** | 7 |
| Tapp, 2012 44 | **** | ** | ** | 8 |
| Karu, 2013 45 | **** | ** | ** | 8 |
| Li, 2015 46 | **** | ** | ** | 8 |
| Mirhafez, 2015 47 | **** | ** | ** | 8 |
| Cheng, 2016 48 | **** | ** | ** | 8 |
| Liang, 2016 49 | **** | ** | ** | 8 |
| Bergström, 2017 25 | **** | ** | ** | 8 |
| Tajfard, 2017 50 | **** | ** | ** | 8 |
| Xu, 2017 51 | **** | ** | ** | 8 |
| Boles, 2018 26 | *** | ** | ** | 7 |
| Kharaeva, 2018 27 | **** | ** | ** | 8 |
| Kumari, 2018 18 | **** | ** | ** | 8 |
| Ansari, 2019 52 | *** | ** | ** | 7 |
| Shipulin, 2020 53 | **** | ** | ** | 8 |
| Nowrouzi-Sohrabi, 2022 54 | *** | ** | ** | 7 |
Quality Score
The quality scores for case-control studies incorporated in the meta-analysis are shown in Table 3. Most studies involved a high quality (total score ≥ 7).
Pooled Analysis for All Studies
As Figure 2 shows, the pooled SMD for 85 studies reporting CAD patients was 0.33 (95%CI: ˗ 0.12, 0.78; p = 0.15; I2 = 98%). Based on the result, the two groups (patients with CAD and controls) did not exhibit a significant difference in blood IL-10 levels.
Subgroup analysis
The subgroup analysis derived from ethnicity, blood sample, and sample size is shown in Table 4. The findings reported that sample size could be an effective factor in the pooled result.
| Variable | Number of studies | SMD | 95%CI | p-value | Heterogeneity, % |
|---|---|---|---|---|---|
| Ethnicity | |||||
| Caucasian | 18 | 0.30 | ˗ 0.16, 0.76 | 0.20 | 96 |
| Asian | 9 | ˗ 0.29 | ˗ 1.07, 0.49 | 0.46 | 99 |
| Sample | |||||
| Serum | 15 | 0.42 | ˗ 0.08, 0.93 | 0.10 | 97 |
| Plasma | 12 | 0.12 | ˗ 0.77, 1.01 | 0.78 | 99 |
| Sample size | |||||
| ≥200 | 11 | 0.93 | 0.24, 1.63 | 0.008 | 99 |
| <200 | 16 | ˗ 0.08 | ˗ 0.72, 0.56 | 0.81 | 96 |
Sensitivity Analysis
The results of "one-study-removed" and "cumulative" analyses indicated that the combined analysis was stable.
Publication Bias
Figure 3 indicates funnel plots of blood IL-10 levels in controls compared to CAD patients. With regards to blood IL-10 levels, the p-values of tests were Egger’s: 0.274 and Begg’s: 0.128 for the CAD patients compared to controls. Publication bias was not observed.
Trial Sequential Analysis
Figure 4 presents the outcome of the TSA for blood IL-10 levels in CAD patients versus controls. The data indicates that there are ample cases for comparing blood IL-10 levels in CAD patients and controls.
Radial and L'Abbé plots
The results of both radial and L'Abbé plots for blood IL-10 levels in the CAD patients versus the controls are identified in Figure 5 and Figure 6, respectively. The radial plot indicated that one possible cause of heterogeneity in the initial analysis could be outliers. Also, the L'Abbé plot shows evidence of high heterogeneity (p < 0.001).
Meta-regression
Table 5 represents the random-effects meta-regression of IL-10 levels for CAD patients compared to controls. The results indicated that the publication year and blood sample were moderator factors in the pooled initial analyses (p < 0.01).
| Variable | Data | |
|---|---|---|
| Publication year | Point Estimate | ˗ 0.00065 |
| SE | 0.00020 | |
| Lower Limit | ˗ 0.00103 | |
| Upper Limit | ˗ 0.00026 | |
| Z | ˗ 3.30123 | |
| 0.00096 | ||
| Sample Size | Point Estimate | 0.00023 |
| SE | 0.00016 | |
| Lower Limit | ˗ 0.00008 | |
| Upper Limit | 0.00053 | |
| Z | 1.45301 | |
| 0.14622 | ||
| Blood sample | Point Estimate | ˗ 0.26738 |
| SE | 0.06390 | |
| Lower Limit | ˗ 0.39263 | |
| Upper Limit | ˗ 0.14214 | |
| Z | ˗ 4.18444 | |
| 0.00003 |
IL-10/STAT3/SOCS3 axis and PPI interaction
Figure 7 shows the IL-10/STAT3 pathway and its role in suppressing inflammation. IL-10 activates STAT3, and the IL-10/STAT3 axis can have a powerful anti-inflammatory property65 and its role can be crucial in limiting undesirable immune reactions. It is possible that this axis could play a role in regulating inflammation in the context of CAD17. SOCS3 plays a role in the mechanism by which IL-10 modulates inflammation and acts as a feedback inhibitor of the JAK/STAT pathway. Further research would be needed to determine the exact role of the IL-10/STAT3/SOCS3 axis in CAD. The STRING PPI interaction contains 5 nodes with 10 edges with the highest confidence (0.900). The average node degree was 4 and the PPI enrichment p-value was 6.73e-5. In addition, the KEGG suggested a network analysis for the JAK-STAT signaling pathway based on 5 nodes entered into STRING (Figure 8).
Discussion
Coronary artery disease (CAD), a prevalent cardiovascular condition, is characterized by the buildup of plaque within the coronary artery walls, leading to reduced blood flow to the heart muscle66. Our main findings showed no difference in the blood levels of IL-10 between the CAD patients and the controls. The analyses reported that factors such as ethnicity, blood sample type (serum or plasma), sample size, and publication year could affect the pooled results.
Limited knowledge exists regarding the function of anti-inflammatory cytokines in CAD. IL-10, an anti-inflammatory cytokine that effectively inhibits immune responses, is produced by T and B cells, monocytes, and macrophages67. It suppresses pro-inflammatory cytokines and has a broad range of anti-inflammatory capabilities, including the inhibition of early pro-inflammatory transcription factors, which consequently reduces cytokine production68.
In vitro, IL-10 plays a crucial role in diminishing lesion growth and preventing the advancement of atherosclerosis69, 70, as well as in modulating the immune response to atherosclerosis, a major contributor to CAD71. However, in clinical settings, the role of IL-10 in patients with CAD has yielded inconsistent results25, 26, 33, despite numerous studies investigating the relationship between IL-10 and CAD25, 27, 40.
One study suggested that CAD patients of both sexes who smoked and consumed alcohol had lower levels of IL-1042, while another demonstrated that CAD patients with low serum iron had significantly higher levels of IL-10 compared to normal controls or CAD patients with normal/high serum iron36. The current meta-analysis suggested that factors such as ethnicity, blood sample, sample size, and publication year could significantly influence the pooled results. Therefore, CAD progression and development may be influenced by several factors, and future studies should examine the effect of each factor individually to identify the most significant influences. According to one study, there was no significant correlation between serum IL-10 levels in CAD patients and factors such as smoking, hypertension, diabetes, obesity, and dyslipidemia72. The findings suggest that certain risk factors for CAD, such as dietary habits (e.g., low intake of fruits and vegetables, high intake of saturated fats), smoking, physical inactivity, and obesity, may reduce IL-10 levels and increase inflammation in the body73, 74, 75, 76, thus contributing to the disease's development. Understanding the underlying mechanisms of these associations may assist in identifying new preventive and therapeutic strategies for CAD.
IL-10 has been shown to suppress the generation of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and IL-1β, which are key contributors to the formation of atherosclerotic plaques68, 77. Additionally, IL-10 has been found to promote the survival of endothelial cells that line the inner surfaces of blood vessels and prevent their apoptosis, thereby reducing the risk of plaque formation78, 79.
Various studies have indicated that increased IL-10 levels in the bloodstream could signal ongoing systemic inflammation in CAD patients27, 33, 40, while other studies noted that in human coronary disease, IL-10 is present and associated with diminished signs of inflammation71, 80. The available evidence suggests that IL-10 plays a protective role against CAD by modulating the inflammatory response and promoting endothelial cell survival. Nonetheless, additional studies are needed to fully comprehend the mechanisms underlying the relationship between IL-10 and CAD.
Research has established that CAD is associated with a continuous inflammatory response81. STAT3 has been identified as a crucial molecule in IL-10's operation, with its activation necessary for the cytokine's anti-inflammatory effects82, 83. Furthermore, evidence suggests that SOCS3 plays a role in how IL-10 modulates inflammation17, 84. Acting as a feedback inhibitor of the JAK/STAT pathway, SOCS3 is crucial in preventing STAT3 activation, cytokine signaling, and the expression of inflammatory genes in immune cells such as macrophages and microglia85. One study found that IL-10 increased SOCS3 expression in cultured cardiomyocytes17. With regards to protein-protein interactions between IL-10, STAT3, and SOCS3, evidence supports that both STAT3 and SOCS3 are involved in IL-10's regulation of inflammation. Additionally, the KEGG analysis reported several biomarkers.
The effects of statins on IL-10 levels in CAD patients versus controls have varied across studies39. One trial reported that statin therapy did not affect IL-10 mRNA expression in patients with CAD86, while another study confirmed this for serum IL-10 levels87. Our meta-analysis included a few studies that involved cases with statin therapy; thus, we were unable to analyze data related to the effect of statins on IL-10 levels comprehensively. Future research should investigate the impact of statins on IL-10 levels in CAD patients. The meta-regression identified the publication year as a confounding factor in the pooled result, indicating possible significant differences in the therapeutic approach to CAD patients over time.
The significant limitations of this meta-analysis include heterogeneity among the studies, limited case count in some analyses, and the lack of patient-level data; diverse criteria for study inclusion, such as varying definitions of CAD, which could introduce bias; different patient populations; and varying methodologies. Additionally, the study mentions factors such as statin therapy that could influence IL-10 levels in CAD patients, but these confounding variables were not fully explored in the analysis. The quality assessment of the included studies was conducted by a single author, which may introduce subjective bias. A more systematic and independent quality assessment process could enhance the reliability of the results. While no evidence of publication bias was found, it is possible that studies with significant results may have been more likely to be published, leading to an over representation of certain findings in the meta-analysis. However, the strengths of the meta-analysis include the absence of publication bias, the high quality of most included studies, and the consistency of the combined results.
Conclusions
According to this systematic review and meta-analysis, no significant difference was observed in blood IL-10 levels between CAD patients and controls, suggesting that IL-10 may not serve as a reliable biomarker for CAD. The analysis included a sufficient number of cases for robust comparison, highlighting the complex and multifactorial nature of CAD. Further research is needed to better understand the role of inflammation and specific inflammatory markers in the development of CAD. Future studies should include larger sample sizes and explore the interaction of IL-10 with other proteins to enhance our understanding of CAD's pathogenesis and identify potential therapeutic targets. This study underscores the importance of continued research efforts to improve our comprehension of the mechanisms and risk factors involved in CAD.
This meta-analysis is clinically significant as it informs clinicians and researchers that IL-10 levels may not be useful for diagnosing or predicting CAD. It highlights the complex and multifactorial nature of CAD, indicating that many factors, not just IL-10, contribute to the disease's development. This insight is vital for clinicians when diagnosing and treating CAD.
Abbreviations
CAD - Coronary Artery Disease, CI - Confidence Interval, CK - Creatine Kinase, CO - Control, DEGs - Differentially Expressed Genes, ECG - Electrocardiographic/Electrocardiography, HDs - Heart Diseases, IL-10 - Interleukin-10, JAK - Janus Kinase, KEGG - Kyoto Encyclopedia of Genes and Genomes, NR - Not Reported, NOS - Newcastle-Ottawa Scale, PECO - Population, Exposure, Comparator, Outcome, PPI - Protein-Protein Interactions, PRISMA - Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PROSPERO - International Prospective Register of Systematic Reviews, RIS - Required Information Size, SD - Standard Deviation, SE - Standard Error, SMD - Standardized Mean Difference, SOCS3 - Suppressor of Cytokine Signaling 3, STAT3 - Signal Transducer and Activator of Transcription 3, TNF-α - Tumor Necrosis Factor-Alpha, TSA - Trial Sequential Analysis
Acknowledgments
None.
Author’s contributions
Conceptualization, R.H.M.; methodology, M.S.; software, M.S.; validation, R.H.M., and N.S.; formal analysis, M.S.; investigation, M.R. and M.S.; resources, M.S.; data curation, M.S.; writing—original draft preparation, N.S.; writing—review and editing, M.R. and M.S.; visualization, R.H.M.; supervision, N.S.; project administration, R.H.M.; funding acquisition, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sidney
S.,
Go
A.S.,
Jaffe
M.G.,
Solomon
M.D.,
Ambrosy
A.P.,
Rana
J.S.,
Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiology.
2019;
4
(12)
:
1280-6
.
View Article PubMed Google Scholar -
Balakumar
P.,
Maung-U
K.,
Jagadeesh
G.,
Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacological Research.
2016;
113
:
600-9
.
View Article PubMed Google Scholar -
Prabhakaran
D.,
Jeemon
P.,
Sharma
M.,
Roth
G.A.,
Johnson
C.,
Harikrishnan
S.,
India State-Level Disease Burden Initiative CVD Collaborators
The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990-2016. The Lancet. Global Health.
2018;
6
(12)
:
e1339-51
.
View Article PubMed Google Scholar -
Qayyum
S.,
Rossington
J.A.,
Chelliah
R.,
John
J.,
Davidson
B.J.,
Oliver
R.M.,
Prospective cohort study of elderly patients with coronary artery disease: impact of frailty on quality of life and outcome. Open Heart.
2020;
7
(2)
:
e001314
.
View Article PubMed Google Scholar -
Jowell
A.R.,
Bhattacharya
R.,
Marnell
C.,
Wong
M.,
Haidermota
S.,
Trinder
M.,
Genetic and clinical factors underlying a self-reported family history of heart disease. European Journal of Preventive Cardiology.
2023;
30
(15)
:
1571-9
.
View Article PubMed Google Scholar -
Austin
M.A.,
Hutter
C.M.,
Zimmern
R.L.,
Humphries
S.E.,
Familial hypercholesterolemia and coronary heart disease: a HuGE association review. American Journal of Epidemiology.
2004;
160
(5)
:
421-9
.
View Article PubMed Google Scholar -
Fülöp
P.,
Harangi
M.,
Seres
I.,
Paragh
G.,
Paraoxonase-1 and adipokines: potential links between obesity and atherosclerosis. Chemico-Biological Interactions.
2016;
259
:
388-93
.
View Article PubMed Google Scholar -
Castillo
E.C.,
Vázquez-Garza
E.,
Yee-Trejo
D.,
García-Rivas
G.,
Torre-Amione
G.,
What is the role of the inflammation in the pathogenesis of heart failure?. Current Cardiology Reports.
2020;
22
(11)
:
139
.
View Article PubMed Google Scholar -
Goswami
S.K.,
Ranjan
P.,
Dutta
R.K.,
Verma
S.K.,
Management of inflammation in cardiovascular diseases. Pharmacological Research.
2021;
173
:
105912
.
View Article PubMed Google Scholar -
Zhang
L.,
Lou
D.,
He
D.,
Wang
Y.,
Wu
Y.,
Cao
X.,
Dysregulated circulating apoptosis-and autophagy-related lncRNAs as diagnostic markers in coronary artery disease. BioMed research international.
2021;
2021
:
5517786
.
View Article Google Scholar -
Silvestre-Roig
C.,
de Winther
M.P.,
Weber
C.,
Daemen
M.J.,
Lutgens
E.,
Soehnlein
O.,
Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circulation Research.
2014;
114
(1)
:
214-26
.
View Article PubMed Google Scholar -
Nguyen
M.T.,
Fernando
S.,
Schwarz
N.,
Tan
J.T.,
Bursill
C.A.,
Psaltis
P.J.,
Inflammation as a therapeutic target in atherosclerosis. Journal of Clinical Medicine.
2019;
8
(8)
:
1109
.
View Article PubMed Google Scholar -
Carrizales-Sepúlveda
E.F.,
Ordaz-Farías
A.,
Vera-Pineda
R.,
Flores-Ramírez
R.,
Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung and Circulation.
2018;
27
(11)
:
1327-34
.
View Article PubMed Google Scholar -
Mirzaei
H.,
Ferns
G.A.,
Avan
A.,
Mobarhan
M.G.,
Cytokines and microRNA in coronary artery disease. Advances in Clinical Chemistry.
2017;
82
:
47-70
.
View Article PubMed Google Scholar -
Zhang
Y.H.,
He
K.,
Shi
G.,
Effects of microRNA-499 on the inflammatory damage of endothelial cells during coronary artery disease via the targeting of PDCD4 through the NF-Kβ/TNF-α signaling pathway. Cellular Physiology and Biochemistry.
2017;
44
(1)
:
110-24
.
View Article PubMed Google Scholar -
Dokumacioglu
E.,
Duzcan
I.,
Iskender
H.,
Sahin
A.,
RhoA/ROCK-1 signaling pathway and oxidative stress in coronary artery disease patients. Revista Brasileira de Cirurgia Cardiovascular ; Orgao Oficial da Sociedade Brasileira de Cirurgia Cardiovascular.
2022;
37
(2)
:
212-8
.
View Article PubMed Google Scholar -
Cevey
Á.C.,
Penas
F.N.,
Alba Soto
C.D.,
Mirkin
G.A.,
Goren
N.B.,
IL-10/STAT3/SOCS3 Axis Is Involved in the Anti-inflammatory Effect of Benznidazole. Frontiers in Immunology.
2019;
10
:
1267
.
View Article PubMed Google Scholar -
Kumari
R.,
Kumar
S.,
Ahmad
M.K.,
Singh
R.,
Pradhan
A.,
Chandra
S.,
TNF-α/IL-10 ratio: an independent predictor for coronary artery disease in North Indian population. Diabetes & Metabolic Syndrome.
2018;
12
(3)
:
221-5
.
View Article PubMed Google Scholar -
Sabat
R.,
Grütz
G.,
Warszawska
K.,
Kirsch
S.,
Witte
E.,
Wolk
K.,
Biology of interleukin-10. Cytokine & Growth Factor Reviews.
2010;
21
(5)
:
331-44
.
View Article PubMed Google Scholar -
Rezaei
F.,
Doulatparast
D.,
Sadeghi
M.,
Common Polymorphisms of Interleukin-10 (-1082A/G, -592A/C, and -819C/T) in Oral Cancers: An Updated Meta-Analysis. Journal of Interferon & Cytokine Research.
2020;
40
(7)
:
357-69
.
View Article PubMed Google Scholar -
Jamshidy
L.,
Tadakamadla
S.K.,
Choubsaz
P.,
Sadeghi
M.,
Tadakamadla
J.,
Association of IL-10 and TNF-α Polymorphisms with Dental Peri-Implant Disease Risk: A Meta-Analysis, Meta-Regression, and Trial Sequential Analysis. International Journal of Environmental Research and Public Health.
2021;
18
(14)
:
7697
.
View Article PubMed Google Scholar -
Shakiba
E.,
Ramezani
M.,
Sadeghi
M.,
Evaluation of serum interleukin-10 levels in hepatocellular carcinoma patients: a systematic review and meta-analysis. Clinical and Experimental Hepatology.
2018;
4
(1)
:
35-40
.
View Article PubMed Google Scholar -
Zuo
S.,
Zheng
T.,
Li
H.,
Association between interleukin-10-819T/C polymorphism and risk of ischemic stroke: A meta-analysis. Medicine.
2020;
99
(20)
:
e19808
.
View Article PubMed Google Scholar -
Munjal
A.,
Khandia
R.,
Atherosclerosis: orchestrating cells and biomolecules involved in its activation and inhibition. Advances in Protein Chemistry and Structural Biology.
2020;
120
:
85-122
.
View Article PubMed Google Scholar -
Bergström
I.,
Lundberg
A.K.,
Jönsson
S.,
Särndahl
E.,
Ernerudh
J.,
Jonasson
L.,
Annexin A1 in blood mononuclear cells from patients with coronary artery disease: its association with inflammatory status and glucocorticoid sensitivity. PLoS One.
2017;
12
(3)
:
e0174177
.
View Article PubMed Google Scholar -
Boles
U.,
Johansson
A.,
Wiklund
U.,
Sharif
Z.,
David
S.,
McGrory
S.,
Cytokine disturbances in coronary artery ectasia do not support atherosclerosis pathogenesis. International Journal of Molecular Sciences.
2018;
19
(1)
:
260
.
View Article PubMed Google Scholar -
Kharaeva
Z.F.,
Khokonova
T.M.,
Kambachokova
Z.A.,
Barokova
E.B.,
Nakova
L.V.,
[Serum values of cytokines in patients with ischemic heart disease and arterial hypertension]. Klinicheskaia Laboratornaia Diagnostika.
2018;
63
(10)
:
626-9
.
PubMed Google Scholar -
Krause
C.D.,
Mei
E.,
Mirochnitchenko
O.,
Lavnikova
N.,
Xie
J.,
Jia
Y.,
Interactions among the components of the interleukin-10 receptor complex. Biochemical and Biophysical Research Communications.
2006;
340
(2)
:
377-85
.
View Article PubMed Google Scholar -
Wolk
K.,
Witte
E.,
Reineke
U.,
Witte
K.,
Friedrich
M.,
Sterry
W.,
Is there an interaction between interleukin-10 and interleukin-22?. Genes and Immunity.
2005;
6
(1)
:
8-18
.
View Article PubMed Google Scholar -
Niemand
C.,
Nimmesgern
A.,
Haan
S.,
Fischer
P.,
Schaper
F.,
Rossaint
R.,
Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. The Journal of Immunology : Official Journal of the American Association of Immunologists.
2003;
170
(6)
:
3263-72
.
View Article PubMed Google Scholar -
Parums
D.V.,
Review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Medical Science Monitor.
2021;
27
:
e934475-1
.
View Article PubMed Google Scholar -
Alshammary
A.F.,
Alharbi
K.K.,
Alshehri
N.J.,
Vennu
V.,
Ali Khan
I.,
Metabolic Syndrome and Coronary Artery Disease Risk: A Meta-Analysis of Observational Studies. International Journal of Environmental Research and Public Health.
2021;
18
(4)
:
1773
.
View Article PubMed Google Scholar -
Cheng
Y.C.,
Kuo
W.W.,
Wu
C.H.,
Shu
W.T.,
Kuo
C.H.,
Hwang
J.M.,
Iron status and cardiovascular risk factors in patients with haemodialysis versus patients with ischaemic heart disease. Nephrology (Carlton, Vic.).
2009;
14
(1)
:
65-9
.
View Article PubMed Google Scholar -
Jha
H.C.,
Srivastava
P.,
Sarkar
R.,
Prasad
J.,
Mittal
A.S.,
Association of plasma circulatory markers, Chlamydia pneumoniae, and high sensitive C-reactive protein in coronary artery disease patients of India. Mediators of inflammation.
2009;
2009
:
561532
.
View Article Google Scholar -
Jha
H.C.,
Divya
A.,
Prasad
J.,
Mittal
A.,
Plasma circulatory markers in male and female patients with coronary artery disease. Heart & Lung.
2010;
39
(4)
:
296-303
.
View Article PubMed Google Scholar -
Khan
D.A.,
Ansari
W.M.,
Khan
F.A.,
Pro/anti-inflammatory cytokines in the pathogenesis of premature coronary artery disease. Journal of Interferon & Cytokine Research.
2011;
31
(7)
:
561-7
.
View Article PubMed Google Scholar -
Tapp
L.D.,
Shantsila
E.,
Wrigley
B.J.,
Pamukcu
B.,
Lip
G.Y.,
The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. Journal of Thrombosis and Haemostasis.
2012;
10
(7)
:
1231-41
.
View Article PubMed Google Scholar -
Karu
I.,
Starkopf
J.,
Zilmer
K.,
Zilmer
M.,
Growth factors serum levels in coronary artery disease patients scheduled for bypass surgery: perioperative dynamics and comparisons with healthy volunteers. BioMed research international.
2013;
2013
:
985404
.
View Article Google Scholar -
Li
S.H.,
Chen
W.J.,
Yan
M.,
Shu
Y.W.,
Liao
Y.H.,
Expression of coinhibitory PD-L1 on CD4⁺CD25⁺FOXP3⁺ regulatory T cells is elevated in patients with acute coronary syndrome. Coronary Artery Disease.
2015;
26
(7)
:
598-603
.
View Article PubMed Google Scholar -
Mirhafez
S.R.,
Zarifian
A.,
Ebrahimi
M.,
Ali
R.F.,
Avan
A.,
Tajfard
M.,
Relationship between serum cytokine and growth factor concentrations and coronary artery disease. Clinical Biochemistry.
2015;
48
(9)
:
575-80
.
View Article PubMed Google Scholar -
Cheng
J.,
Chen
Y.,
Xu
B.,
Wu
J.,
He
F.,
Association of soluble fibrinogen-like protein 2 with the severity of coronary artery disease. Internal Medicine (Tokyo, Japan).
2016;
55
(17)
:
2343-50
.
View Article PubMed Google Scholar -
Mazzone
A.,
De Servi
S.,
Vezzoli
M.,
Fossati
G.,
Mazzucchelli
I.,
Gritti
D.,
Plasma levels of interleukin 2, 6, 10 and phenotypic characterization of circulating T lymphocytes in ischemic heart disease. Atherosclerosis.
1999;
145
(2)
:
369-74
.
View Article PubMed Google Scholar -
Tajfard
M.,
Latiff
L.A.,
Rahimi
H.R.,
Moohebati
M.,
Hasanzadeh
M.,
Emrani
A.S.,
Serum concentrations of MCP-1 and IL-6 in combination predict the presence of coronary artery disease and mortality in subjects undergoing coronary angiography. Molecular and Cellular Biochemistry.
2017;
435
(1-2)
:
37-45
.
View Article PubMed Google Scholar -
Xu
Y.,
Wang
Y.,
Zhi
J.,
Qi
L.,
Zhang
T.,
Li
X.,
Impact of matrix metalloproteinase 9 rs3918242 genetic variant on lipid-lowering efficacy of simvastatin therapy in Chinese patients with coronary heart disease. BMC Pharmacology & Toxicology.
2017;
18
(1)
:
28
.
View Article PubMed Google Scholar -
Ansari
W.M.,
Humphries
S.E.,
Naveed
A.K.,
Khan
O.J.,
Khan
D.A.,
Khattak
E.H.,
Effect of Coronary Artery Disease risk SNPs on serum cytokine levels and cytokine imbalance in Premature Coronary Artery Disease. Cytokine.
2019;
122
:
154060
.
View Article PubMed Google Scholar -
Shipulin
V.M.,
Chumakova
S.P.,
Pogonchenkova
D.A.,
Urazova
O.I.,
Vins
M.V.,
Pryakhin
A.S.,
Interleukin-10 and non-classical monocytes as biomarkers of ischemic cardiomyopathy. Circulation Pathology and Cardiac Surgery..
2020;
24
(1)
:
45-53
.
-
Nowrouzi-Sohrabi
P.,
Seghatoleslam
A.,
Kalani
M.,
Erfani
M.,
Hosseini Abgir
A.,
Hatami
S.,
Up-regulated lncRNA-PVT1 expression in peripheral blood mononuclear cells of patients with coronary artery disease is correlated with decreased interleukin-10 production. Molecular Biology Reports.
2022;
49
(5)
:
3453-9
.
View Article PubMed Google Scholar -
Mizia-Stec
K.,
Mandecki
T.,
Zahorska-Markiewicz
B.,
Janowska
J.,
Szulc
A.,
Jastrzebska-Maj
E.,
Is there a relationship between left ventricular systolic function and serum cytokines level in patients with coronary artery disease?. Medical Science Monitor.
2002;
8
(2)
:
87-92
.
PubMed Google Scholar -
Mizia-Stec
K.,
Zahorska-Markiewicz
B.,
Mandecki
T.,
Janowska
J.,
Szulc
A.,
Jastrzekbska-Maj
E.,
Hyperlipidaemias and serum cytokines in patients with coronary artery disease. Acta Cardiologica.
2003;
58
(1)
:
9-15
.
View Article PubMed Google Scholar -
Lee
S.D.,
Huang
C.Y.,
Shu
W.T.,
Chen
T.H.,
Lin
J.A.,
Hsu
H.H.,
Pro-inflammatory states and IGF-I level in ischemic heart disease with low or high serum iron. Clinica Chimica Acta.
2006;
370
(1-2)
:
50-6
.
View Article PubMed Google Scholar -
Nilsson
L.,
Jonasson
L.,
Nijm
J.,
Hamsten
A.,
Eriksson
P.,
Increased plasma concentration of matrix metalloproteinase-7 in patients with coronary artery disease. Clinical Chemistry.
2006;
52
(8)
:
1522-7
.
View Article PubMed Google Scholar -
Szodoray
P.,
Timar
O.,
Veres
K.,
Der
H.,
Szomjak
E.,
Lakos
G.,
TH1/TH2 imbalance, measured by circulating and intracytoplasmic inflammatory cytokines-immunological alterations in acute coronary syndrome and stable coronary artery disease. Scandinavian Journal of Immunology.
2006;
64
(3)
:
336-44
.
View Article PubMed Google Scholar -
Paulsson
J.M.,
Dadfar
E.,
Held
C.,
Jacobson
S.H.,
Lundahl
J.,
In vivo transmigrated monocytes from patients with stable coronary artery disease have a reduced expression of CD11b. Clinical and Experimental Immunology.
2008;
153
(2)
:
196-204
.
View Article PubMed Google Scholar -
Liang
K.,
Dong
S.R.,
Peng
H.,
Serum levels and clinical significance of IFN-γ and IL-10 in patients with coronary heart disease. European Review for Medical and Pharmacological Sciences.
2016;
20
(7)
:
1339-43
.
PubMed Google Scholar -
Wells
G.A.,
Shea
B.,
O'Connell
D.,
Peterson
J.,
Welch
V.,
Losos
M.,
The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000 Jul 3-5; Oxford, UK.
2000
.
-
DerSimonian
R.,
Laird
N.,
Meta-analysis in clinical trials revisited. Contemporary Clinical Trials.
2015;
45
:
139-45
.
View Article PubMed Google Scholar -
Mantel
N.,
Haenszel
W.,
Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute.
1959;
22
(4)
:
719-48
.
PubMed Google Scholar -
Egger
M.,
Davey Smith
G.,
Schneider
M.,
Minder
C.,
Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.).
1997;
315
(7109)
:
629-34
.
View Article PubMed Google Scholar -
Begg
C.B.,
Mazumdar
M.,
Operating characteristics of a rank correlation test for publication bias. Biometrics.
1994;
50
(4)
:
1088-101
.
View Article PubMed Google Scholar -
Galbraith
R.,
Graphical display of estimates having differing standard errors. Technometrics.
1988;
30
(3)
:
271-81
.
View Article Google Scholar -
Song
F.,
Exploring heterogeneity in meta-analysis: is the L'Abbé plot useful?. Journal of Clinical Epidemiology.
1999;
52
(8)
:
725-30
.
View Article PubMed Google Scholar -
L'Abbé
K.A.,
Detsky
A.S.,
O'Rourke
K.,
Meta-analysis in clinical research. Annals of Internal Medicine.
1987;
107
(2)
:
224-33
.
View Article PubMed Google Scholar -
Wetterslev
J.,
Jakobsen
J.C.,
Gluud
C.,
Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology.
2017;
17
(1)
:
39
.
View Article PubMed Google Scholar -
Szklarczyk
D.,
Franceschini
A.,
Wyder
S.,
Forslund
K.,
Heller
D.,
Huerta-Cepas
J.,
STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Research.
2015;
43
(Database issue)
:
447-52
.
View Article PubMed Google Scholar -
Suarez
A.A. Roca,
Renne
N. Van,
Baumert
T.F.,
Lupberger
J.,
Viral manipulation of STAT3: Evade, exploit, and injure. PLoS Pathogens.
2018;
14
(3)
:
e1006839
.
View Article PubMed Google Scholar -
Malakar
A.K.,
Choudhury
D.,
Halder
B.,
Paul
P.,
Uddin
A.,
Chakraborty
S.,
A review on coronary artery disease, its risk factors, and therapeutics. Journal of Cellular Physiology.
2019;
234
(10)
:
16812-23
.
View Article PubMed Google Scholar -
T. Boonpiyathad,
P. Satitsuksanoa,
M. Akdis,
C.A. Akdis,
Il-10 producing T and B cells in allergy. InSeminars in immunology.
2019;
44
:
101326
.
-
Kessler
B.,
Rinchai
D.,
Kewcharoenwong
C.,
Nithichanon
A.,
Biggart
R.,
Hawrylowicz
C.M.,
Interleukin 10 inhibits pro-inflammatory cytokine responses and killing of Burkholderia pseudomallei. Scientific Reports.
2017;
7
(1)
:
42791
.
View Article PubMed Google Scholar -
Bu
T.,
Li
Z.,
Hou
Y.,
Sun
W.,
Zhang
R.,
Zhao
L.,
Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics.
2021;
11
(20)
:
9988-10000
.
View Article PubMed Google Scholar -
Pinderski
L.J.,
Fischbein
M.P.,
Subbanagounder
G.,
Fishbein
M.C.,
Kubo
N.,
Cheroutre
H.,
Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circulation Research.
2002;
90
(10)
:
1064-71
.
View Article PubMed Google Scholar -
Fernández
R. Pérez,
Kaski
J.C.,
[Interleukin-10 and coronary disease]. Revista española de cardiología.
2002;
55
(7)
:
738-50
.
PubMed Google Scholar -
Sun
Y.,
Pei
W.,
Welte
T.,
Wu
Y.,
Ye
S.,
Yang
Y.,
Cytomegalovirus infection is associated with elevated interleukin-10 in coronary artery disease. Atherosclerosis.
2005;
179
(1)
:
133-7
.
View Article PubMed Google Scholar -
Hosseini
B.,
Berthon
B.S.,
Saedisomeolia
A.,
Starkey
M.R.,
Collison
A.,
Wark
P.A.,
Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. The American Journal of Clinical Nutrition.
2018;
108
(1)
:
136-55
.
View Article PubMed Google Scholar -
Jacobs
M.,
Verschraegen
S.,
Salhi
B.,
Anckaert
J.,
Mestdagh
P.,
Brusselle
G.G.,
IL-10 producing regulatory B cells are decreased in blood from smokers and COPD patients. Respiratory Research.
2022;
23
(1)
:
287
.
View Article PubMed Google Scholar -
Docherty
S.,
Harley
R.,
McAuley
J.J.,
Crowe
L.A.,
Pedret
C.,
Kirwan
P.D.,
The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Science, Medicine and Rehabilitation.
2022;
14
(1)
:
5
.
View Article PubMed Google Scholar -
Charles
B.A.,
Doumatey
A.,
Huang
H.,
Zhou
J.,
Chen
G.,
Shriner
D.,
The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. The Journal of Clinical Endocrinology and Metabolism.
2011;
96
(12)
:
2018-22
.
View Article PubMed Google Scholar -
Enayati
S.,
Seifirad
S.,
Amiri
P.,
Abolhalaj
M.,
Mohammad-Amoli
M.,
Interleukin-1 beta, interferon-gamma, and tumor necrosis factor-alpha gene expression in peripheral blood mononuclear cells of patients with coronary artery disease. ARYA Atherosclerosis.
2015;
11
(5)
:
267-74
.
PubMed Google Scholar -
Short
W.D.,
Steen
E.,
Kaul
A.,
Wang
X.,
Olutoye
O.O.,
Vangapandu
H.V.,
IL-10 promotes endothelial progenitor cell infiltration and wound healing via STAT3. The FASEB Journal.
2022;
36
(7)
:
e22298
.
View Article PubMed Google Scholar -
Wang
Y.,
Chen
Q.,
Zhang
Z.,
Jiang
F.,
Meng
X.,
Yan
H.,
Interleukin-10 overexpression improves the function of endothelial progenitor cells stimulated with TNF-α through the activation of the STAT3 signaling pathway. International Journal of Molecular Medicine.
2015;
35
(2)
:
471-7
.
View Article PubMed Google Scholar -
Xu
S.,
Zhang
J.,
Liu
J.,
Ye
J.,
Xu
Y.,
Wang
Z.,
The role of interleukin-10 family members in cardiovascular diseases. International Immunopharmacology.
2021;
94
:
107475
.
View Article PubMed Google Scholar -
Fioranelli
M.,
Bottaccioli
A.G.,
Bottaccioli
F.,
Bianchi
M.,
Rovesti
M.,
Roccia
M.G.,
Stress and inflammation in coronary artery disease: a review psychoneuroendocrineimmunology-based. Frontiers in Immunology.
2018;
9
:
2031
.
View Article PubMed Google Scholar -
Michée-Cospolite
M.,
Boudigou
M.,
Grasseau
A.,
Simon
Q.,
Mignen
O.,
Pers
J.O.,
Molecular mechanisms driving IL-10-producing B cells functions: STAT3 and c-MAF as underestimated central key regulators?. Frontiers in Immunology.
2022;
13
:
818814
.
View Article PubMed Google Scholar -
Hutchins
A.P.,
Diez
D.,
Miranda-Saavedra
D.,
The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Briefings in Functional Genomics.
2013;
12
(6)
:
489-98
.
View Article PubMed Google Scholar -
Duncan
S.A.,
Sahu
R.,
Dixit
S.,
Singh
S.R.,
Dennis
V.A.,
Suppressors of cytokine signaling (SOCS) 1 and SOCS3 proteins are mediators of interleukin-10 modulation of inflammatory responses induced by chlamydia muridarum and its major outer membrane protein (MOMP) in mouse J774 macrophages. Mediators of inflammation.
2020;
2020
:
7461742
.
View Article Google Scholar -
Qin
H.,
Yeh
W.I.,
De Sarno
P.,
Holdbrooks
A.T.,
Liu
Y.,
Muldowney
M.T.,
Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proceedings of the National Academy of Sciences of the United States of America.
2012;
109
(13)
:
5004-9
.
View Article PubMed Google Scholar -
Jackowska
P.,
Cha\lubiński
M.,
\Luczak
E.,
Wojdan
K.,
Gorzelak-Pabis
P.,
Olszewska-Banaszczyk
M.,
The influence of statin monotherapy and statin-ezetimibe combined therapy on FoxP3 and IL 10 mRNA expression in patients with coronary artery disease. Advances in Clinical and Experimental Medicine.
2019;
28
(9)
:
1243-8
.
View Article PubMed Google Scholar -
Pereira
M.M.,
Santos
T.P.,
Aras
R.,
Couto
R.D.,
Atta
M.L.,
Atta
A.M.,
Serum levels of cytokines and chemokines associated with cardiovascular disease in Brazilian patients treated with statins for dyslipidemia. International Immunopharmacology.
2014;
18
(1)
:
66-70
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 7 (2024)
Page No.: 6603-6621
Published on: 2024-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2980 times
- PDF downloaded - 802 times
- XML downloaded - 80 times
 Biomedpress
Biomedpress









