Abstract
Background: Colon cancer is one of the most common cancers in Vietnam and globally. The KRAS gene (Kirsten rat sarcoma) is an oncogene showing a high mutation rate in colon cancer, affecting 30% to 40% of patients. The mutated KRAS gene, which keeps the MAPK signaling pathway permanently active, is considered a negative factor in the survival of colon cancer patients. Recently, several studies have been conducted to evaluate the specific prognoses related to distinct KRAS mutations, but the results were controversial. Mutations in different KRAS codons may impact colon cancer treatment models and prognoses. Therefore, the impacts of codon-specific KRAS mutations on survival require further clarification. This study aims to determine the associations between codon-specific KRAS mutations and survival in Vietnamese patients at stages II - III.
Methods: A descriptive design was applied and included 158 colon cancer patients at stages II-III at Bach Mai Hospital from January 2016 to August 2020. Testing for KRAS mutations was performed with formalin-fixed, paraffin-embedded tissue, and KRAS mutations were detected with the KRAS XL StripAssay (ViennaLab, Austria).
Results: Among 158 patients, 71 (44.9%) exhibited mutated KRAS genes. The most frequent mutated KRAS variant was p.Gly12Asp (G12D) at 35.2%; the proportion of KRAS G13D (p.Gly13Asp) was 15.5%. The 3-year DFS of the wild-type KRAS group was 60.3%, while that of the mutated KRAS Exon 2 group was 55.2%. The worse prognosis for DFS was observed in patients with KRAS codon 12 mutations (3-year DFS: 52.9%). Among them, patients with codon-specific KRAS mutations named p.Gly12Asp (G12D) and p.Gly12Val (G12V) experienced better prognoses (59.1% and 63.6%, respectively). However, the differences were not statistically significant (P > 0.05).
Conclusion: KRAS p.Gly12Asp (G12D) was the most common type among mutated KRAS genes. Codon-specific KRAS mutation was not a prognostic factor for DFS and OS in colon cancer stages II-III. The results support the current clinical practice that determining specific codon KRAS gene status is not conventional testing for resected colon cancer stages II-III.
Introduction
Colon cancer (CC) is one of the most common cancers in Vietnam and globally1. Many years of biological feature discovery attempts have provided opportunities for personalized treatment, thereby supporting the reduction of mortality from CC. The KRAS gene (Kirsten rat sarcoma) is an oncogene known for its high mutation rate in CC, affecting 30% to 40% of patients2, 3. The MAPK signaling pathway plays a significant role in the proliferation of cancer cells. The mutated KRAS gene, which keeps the aforementioned pathway permanently active, is considered a negative factor for survival in CC patients. KRAS mutations in exon 2 at codons 12 and 13 are predominant. Minor rates of mutations at codons 59 and 61 in exon 3 have been reported, while mutations at codons 117 and 146 are rarely detected. Additionally, many variants of KRAS mutations have been identified. Among them, the most frequent KRAS types are G12D (p.Gly12Asp), G12V (p.Gly12Val), G12C (p.Gly12Cys), and G13D (p.Gly13Asp)4, 5, 6. KRAS G12D is the most common KRAS mutation detected in carcinomas7. Recently, several studies have evaluated the specific prognoses related to individual KRAS mutations, but the results have been controversial8, 9. Therefore, the impacts of codon-specific KRAS mutations on survival require further clarification.
CC cases show an increasing trend in Vietnam, and most patients are diagnosed at pathological stages II or III10. However, there are only a few studies on KRAS status in CC patients, and the associations between codon-specific KRAS mutations and survival have not been elucidated3, 11, 12. Individual treatment based on a patient’s genetic information plays a significant role in patient management and decision-making. Therefore, this study aims to determine the associations between codon-specific KRAS mutations and survival in Vietnamese patients at stages II-III.
Methods
Data in this study covered all patients (n = 158) with colon cancer diagnosed at pathological stages II - III who were admitted to Bach Mai Hospital (Hanoi, Vietnam) from January 2016 to August 2020. The inclusion criteria include pathological stages II-III colon cancer according to the 8th edition of the AJCC staging system, receiving radical treatment, testing for the KRAS status with determination of mutated sites, and patients’ documents being fully accessible. The exclusion criteria include the diagnosis of a second cancer and the inability to answer the research questions due to illness.
Study Design
A descriptive design was applied. The significant research objects were the correlations of single KRAS mutations with disease-free survival (DFS) and overall survival (OS). The observation period began on the date of receiving radical surgery.
Mutation Analysis
Testing for KRAS mutations was performed with formalin-fixed, paraffin-embedded (FFPE) tissue. Paraffin in FFPE tissue slides was eliminated by using an FFPE deparaffinization solution (MERCK, Germany). DNA was then extracted from tissue samples with the aid of a PureLink™ Genomic DNA Mini Kit (Invitrogen, USA). KRAS mutations were detected with the KRAS XL StripAssay (ViennaLab, Austria) in a five-step procedure that included amplification, hybridization, stringent wash, and color development. The strips were analyzed to determine the mutational variants of the KRAS gene at codons 12, 13, 59, 60, 61, 117, and 146.13, 14
Statistical Analyses
Statistical analyses were performed using SPSS 21.0 software. The Kaplan-Meier method was used to calculate the survival rate, and the log-rank test was applied to compare the survival rates of two groups. A P-value of less than 0.05 was considered statistically significant.
Ethics Declarations
The study was permitted by the Ethics Committee of Hanoi Medical University (approval number: NCS28/HMU-IRB). The patients consented to participate in the study.
| Characteristic | Total (n = 158) | Percentage (%) | |
| Age | |||
| < 40 ys | 17 | 10.8 | |
| 40 - 49 ys | 28 | 17.7 | |
| 50 – 59 ys | 42 | 26.6 | |
| 60 – 69 ys | 57 | 36.1 | |
| ≥ 70 ys | 14 | 8.9 | |
| Sex | |||
| Male | 86 | 54.4 | |
| Female | 72 | 45.6 | |
| Histological types | |||
| Adenocarcinoma | 137 | 86.7 | |
| Mucinous Adenocarcinoma | 18 | 11.4 | |
| Others* | 3 | 1.9 | |
| Tumor sites | |||
| Right | 76 | 48.1 | |
| Left | 82 | 51.9 | |
| Tumor invasion | |||
| pT3 | 67 | 42.4 | |
| pT4 | 91 | 57.5 | |
| Lymph node stage | |||
| pN0 | 75 | 47.5 | |
| pN1 | 59 | 37.3 | |
| pN2 | 24 | 15.2 | |
| pTNM stage | |||
| II | 75 | 47.5 | |
| III | 83 | 52.5 | |
| KRAS status | |||
| Mutated | 71 | 44.9 | |
| Wild - type | 87 | 55.1 | |
| * Signet ring carcinoma and Micropapillary carcinoma | |||
| Specified group | n | 3-year DFS | p-value* | 4-year OS | p-value* |
| Mutated KRAS Exon 2 (Codon 12, 13) | 63 | 55.2% | 0.592 | 69.5% | 0.933 |
| Mutated KRAS codon 12 | 47 | 52.9% | 0.448 | 69.7% | 0.937 |
| Mutated KRAS codon 13 | 16 | 61.9% | 0.844 | 70.7% | 0.708 |
| Mutated type of p.Gly>Asp (Codon 12, 13) | 40 | 59.2% | 0.989 | 69.1% | 0.873 |
| Mutated type of p.Gly12Asp (G12D) | 25 | 59.1% | 0.983 | 71.6% | 0.896 |
| Mutated type of p.Gly13Asp (G13D) | 15 | 59.3% | 0.989 | 69.9% | 0.897 |
| Mutated type of Gly12Val (G12V) | 11 | 63.6% | 0.881 | 72.7% | 0.962 |
| KRAS wild-type | 87 | 60.3% | 69.9% | ||
| * compared with KRAS wild-type | |||||
Results
Patients’ Characteristics
A total of 158 patients were recruited for this study. Among them, 86/158 (54.4%) were males, and those aged 60-69 accounted for the highest proportion at 36.1%. The predominant histological type is adenocarcinoma, constituting 86.7% (137/158) of all cases, while other types were identified in 21 cases (13.3% of patients). The T4 stage (including T4a and T4b) was confirmed in 91 patients (57.5%); Stage III tumors were identified at a higher rate than Stage II tumors (52.5% vs. 44.9%). The KRAS-mutated rate was 44.9% (71/158) compared with 55.1% (87/158) of the KRAS wild-type rate (Table 1).
Mutated Types of KRAS Gene in Colon Cancer Stage II–III
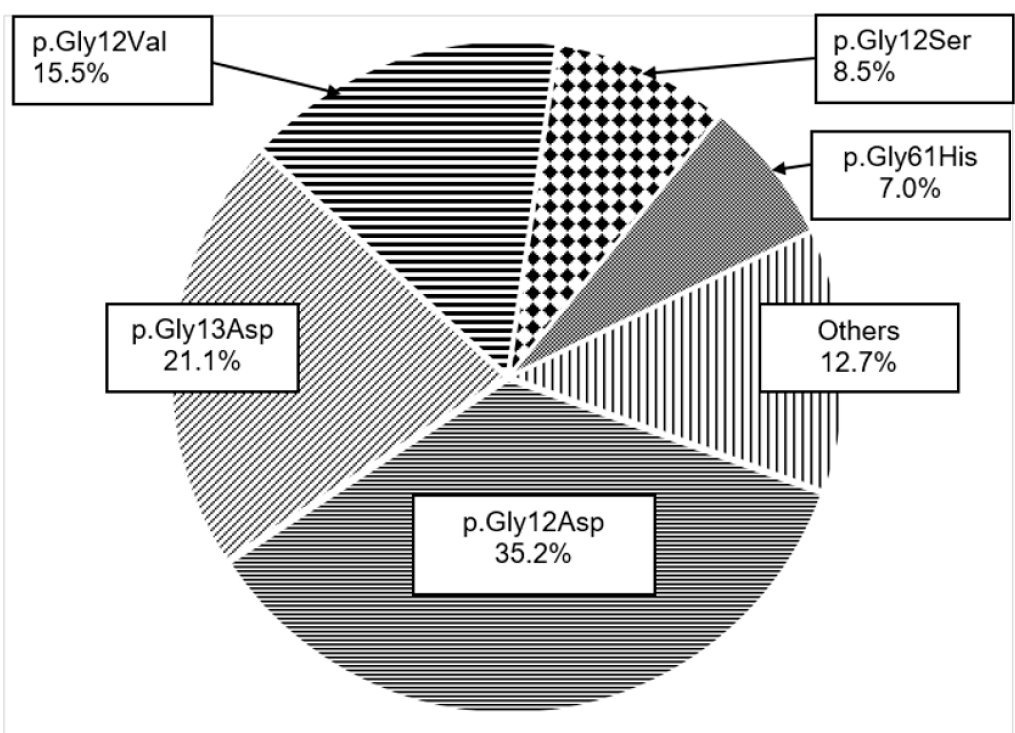
Among the 71 patients diagnosed with a mutated KRAS gene, the most frequent mutated KRAS variant was p.Gly12Asp (G12D) at 35.2%. The proportion of KRAS G13D (p.Gly13Asp) was 15.5%. All the mutated KRAS types of p.Gly>Val and p.Gly>Ser were identified at codon 12 (G12V and G12S, respectively), with rates of 15.5% and 8.5%. The other types, which accounted for 12.7% (9 out of 71 cases), include mutated KRAS variants: p.Ala146Val (A146V); p.Ala59Gly (A59G); p.Gly12Ala (G12A); p.Gly12Arg (G12R); p.Gly13Arg (G13R), p.Gly12Cys (G12C), and p.Lys117Asn (K117N) (Figure 1).
Association of Codon-Specific KRAS Mutations with Survival in Colon Cancer Stage II–III
The median follow-up duration was 40.0 months (min, 12 months; max, 78 months) (not shown in any table). Patients with KRAS mutations commonly experienced lower rates of 3-year DFS and 4-year OS, but the differences were minor and not statistically significant. The 3-year DFS of the wild-type KRAS group was 60.3%, while that of the mutated KRAS exon 2 group was 55.2%; however, the difference was not statistically significant (P = 0.592). Similarly, the 4-year OS of the mutated KRAS exon 2 and wild-type groups were 69.5% and 69.9%, respectively (P = 0.933). The worse prognosis for DFS was shown in patients with KRAS codon 12 mutations (3-year DFS; 52.9%). Among them, patients with codon-specific KRAS mutations p.Gly12Asp (G12D) and p.Gly12Val (G12V) had better prognoses (59.1% and 63.6%, respectively).
Discussion
The Raf/Ras/MAPK pathway is the downstream signaling pathway of EGFR; more than one-half of colorectal cancers are indicated with EGFR overexpression. Although overexpression of EGFR is not predictive of response to anti-EGFR therapy15, KRAS gene status plays a vital role in selecting candidates for anti-EGFR treatment in advanced colorectal cancer16. The impacts of KRAS gene status on prognosis and treatment choices in CC stage II-III are unstable; some studies indicate worse prognoses for mutated KRAS genes, while others do not17, 18. This uncertain judgment suggests that the different codons of the KRAS gene, and even KRAS mutated variants, may have different associations with tumor development and responsiveness to systemic therapies. Lee et al. indicated that patients with mutated KRAS G13D or G12D had worse prognoses for 3-year DFS compared with KRAS wild-type patients in CC stage II-III (76% vs. 92%, p = 0.008 and 33% vs. 92%, p = 0.002, respectively). DFS was equal in mutated KRAS G12D and wild-type (86% vs. 92%, p = 0.61)19.
Recently, the KRAS mutation rate in colorectal cancer has been reported in Viet Viet Nam but rarely in colon cancer. The rate of mutated KRAS gene was 41.0% in colorectal cancer at any stage. More than 85% of mutations were diagnosed at Exon 2 (codon 12 or codo3 13)$. According to the study, the KRAS mutation rate was 44.9% (71/158) in CC stage II-Table 1II ($). The point mutations of KRAS in Exons 2, 3, and 4 were determined, and KRAS mutations were mainly detected in Codon 12 or Codon 13. The KRAS variant of p.Gly12Asp (G12D) accounted for the highest ratio at 35.2%, followed by p.Gly13Asp (G13D; 21.1%); p.Gly12Val (G12V; 15.Figure 1%) ($). Several reports showed that the rate of mutated KRAS at G12D was higher than other point mutations. Hirose et al. reported that the more common KRAS mutations in 340 patients with metastatic colorectal cancers were KRAS G12D at 23.4% and KRAS G13D at 12.6%. Besides, KRAS G12V and G12C proportions were 21.2% and204.7%$. According to Koulouridi et al., KRAS G12D, G12V, and G13D were more frequently detected (33.1%, 21.2%, and 16.7%, respecti21ely)$.
Different KRAS gene mutations have diverse effects on the biochemical and structural properties of the KRAS protein. Hence, specific KRAS mutations influence treatment outcomes differently in CC7, 22. Results from a study in 200 CRCs with stage I-III indicated that the G12V and G12C mutations are related to worse DFS23. A larger retrospective analysis of five studies showed that KRAS G12C had the worst prognosis for overall survival (OS) and KRAS G13D had worse progression-free survival (PFS)24. However, in this study, no differences in survival were found in CC stage II-III according to the types of KRAS mutation. Although 3-year DFS and 4-year OS in KRAS p.Gly12Val (G12V) tend to be higher than KRAS wild-type (63.6% vs. 60.3%, p=0.881; 72.7% vs. 69.9%, p=0.962), the impact of the Gly to Val transitions at codon 12 on survival was not confirmed. The associations of the Gly to Asp transitions at KRAS gene codon 12 and 13 with survival were analyzed. The results indicated that variants of the KRAS gene were not a prognostic factor in CC stage II-III (Table 2).
The limitation of the research was the impact on survival, analyzed only by KRAS variants and not in association with other gene mutations such as BRAF, NRAS, and MMR.
Conclusions
This retrospective study indicated the rate of mutated KRAS variants and the insignificant impact of codon-specific KRAS mutations on survival outcomes in Vietnamese patients at stages II-III. KRAS p.Gly12Asp (G12D) and p.Gly13Asp (G13D) were more frequently confirmed in mutated KRAS patients, and this study has supplemented the clinical evidence to support the claim that codon-specific KRAS mutation is not a prognostic factor for survival in colon cancer stages II-III. The results contribute to a broader understanding of the prognostic value of KRAS mutations in early-stage colon cancer.
Abbreviations
AJCC - American Joint Committee on Cancer, BRAF - B-Raf Proto-Oncogene (Serine/Threonine Kinase), CRC - Colorectal Cancer, DFS - Disease-Free Survival, EGFR - Epidermal Growth Factor Receptor, FFPE - Formalin-Fixed, Paraffin-Embedded, KRAS - Kirsten Rat Sarcoma, MAPK - Mitogen-Activated Protein Kinase, MMR - Mismatch Repair, NRAS - Neuroblastoma RAS Viral Oncogene, OS - Overall Survival, PFS - Progression-Free Survival, SPSS - Statistical Package for the Social Sciences
Acknowledgments
None.
Author’s contributions
Hoang Minh Cuong, Vu Hong Thang was responsible for the conceptualization, data acquisition, formal analysis, and writing of the original draft. Hoang Minh Cuong, Vu Hong Thang, Tran Bao Ngoc, Nguyen Thuan Loi were responsible for data collection and investigation. Nguyen Thuan Loi was responsible for the critical review of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was permitted by the Ethics Committee of Hanoi Medical University (The approval number: NCS28/HMU-IRB).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sung
H.,
Ferlay
J.,
Siegel
R.L.,
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians.
2021;
71
(3)
:
209-49
.
View Article Google Scholar -
Guo
F.,
Gong
H.,
Zhao
H.,
Chen
J.,
Zhang
Y.,
Zhang
L.,
Mutation status and prognostic values of KRAS, NRAS, BRAF and PIK3CA in 353 Chinese colorectal cancer patients. Scientific Reports.
2018;
8
(1)
:
6076
.
View Article PubMed Google Scholar -
Ta
T.V.,
Nguyen
Q.N.,
Chu
H.H.,
Truong
V.L.,
Vuong
L.D.,
RAS/RAF mutations and their associations with epigenetic alterations for distinct pathways in Vietnamese colorectal cancer. Pathology, Research and Practice.
2020;
216
(4)
:
152898
.
View Article PubMed Google Scholar -
Hasbullah
H.H.,
Sulong
S.,
Jalil
N.A. Che,
Aziz
A.A. Abdul,
Musa
N.,
Musa
M.,
KRAS Mutational Profiles among Colorectal Cancer Patients in the East Coast of Peninsular Malaysia.. Diagnostics.
2023;
13
(5)
:
822
.
View Article Google Scholar -
Musselwhite
L.W.,
Trufan
S.J.,
Kadakia
K.C.,
Hwang
J.J.,
Salem
M.E.,
The prevalence of common KRAS variants and associated outcomes in patients with metastatic colorectal cancer (mCRC). Journal of Clinical Oncology.
2022;
4
(4 suppl)
:
173
.
View Article Google Scholar -
Aruquipa
M. Sunagua,
Peixoto
R. D'Alpino,
Jacome
A.,
Cesar
F.,
Lorandi
V.,
Dienstmann
R.,
Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer. Current Issues in Molecular Biology.
2024;
46
(2)
:
1374-82
.
View Article Google Scholar -
Zeissig
M.N.,
Ashwood
L.M.,
Kondrashova
O.,
Sutherland
K.D.,
Next batter up! Targeting cancers with KRAS-G12D mutations. Trends in Cancer.
2023;
9
(11)
:
955-67
.
View Article PubMed Google Scholar -
Chida
K.,
Kotani
D.,
Masuishi
T.,
Kawakami
T.,
Kawamoto
Y.,
Kato
K.,
The Prognostic Impact of KRAS G12C Mutation in Patients with Metastatic Colorectal Cancer: A Multicenter Retrospective Observational Study. The Oncologist.
2021;
26
(10)
:
845-53
.
View Article PubMed Google Scholar -
Scanu
A.M.,
De Miglio
M.R.,
Therapeutic Landscapes in Colorectal Carcinoma. Medicina (Kaunas, Lithuania).
2023;
59
(5)
:
821
.
View Article PubMed Google Scholar -
Tong
G.J.,
Zhang
G.Y.,
Liu
J.,
Zheng
Z.Z.,
Chen
Y.,
Niu
P.P.,
Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World Journal of Clinical Oncology.
2018;
9
(7)
:
148-61
.
View Article PubMed Google Scholar -
Nguyen
H.T.,
Le
D.T.,
Duong
Q.H.,
Tatipamula
V.B.,
Van Nguyen
B.,
High frequency of microsatellite instability and its substantial co-existence with KRAS and BRAF mutations in Vietnamese patients with colorectal cancer. Oncology Letters.
2021;
21
(1)
:
41
.
View Article PubMed Google Scholar -
Dan
N.V.,
Phuong
P.C.,
Thai
P.V.,
Nguyen
N.H.,
Loi
N.T.,
Cong
B.T.,
Relationship between PET/CT images and KRAS gene mutations in colorectal cancer in Vietnamese patients. European Review for Medical and Pharmacological Sciences.
2023;
27
(4)
:
1480-6
.
PubMed Google Scholar -
ViennaLab. K-ras StripAssay Procedure [Available from: https://www.viennalab.com/home/ifu/stripassay/199-5680-instructionsforuse-2020-01/file..
.
-
Abd El Kader
Y.,
Emera
G.,
Safwat
E.,
Kassem
H.A.,
Kassem
N.M.,
The KRAS StripAssay for detection of KRAS mutation in Egyptian patients with colorectal cancer (CRC): a pilot study. Journal of the Egyptian National Cancer Institute.
2013;
25
(1)
:
37-41
.
View Article PubMed Google Scholar -
Hurwitz
H.,
Fehrenbacher
L.,
Novotny
W.,
Cartwright
T.,
Hainsworth
J.,
Heim
W.,
Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England Journal of Medicine.
2004;
350
(23)
:
2335-42
.
View Article PubMed Google Scholar -
Karapetis
C.S.,
Khambata-Ford
S.,
Jonker
D.J.,
O'Callaghan
C.J.,
Tu
D.,
Tebbutt
N.C.,
K-ras mutations and benefit from cetuximab in advanced colorectal cancer. The New England Journal of Medicine.
2008;
359
(17)
:
1757-65
.
View Article PubMed Google Scholar -
Roth
A.D.,
Tejpar
S.,
Delorenzi
M.,
Yan
P.,
Fiocca
R.,
Klingbiel
D.,
Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. Journal of Clinical Oncology.
2010;
28
(3)
:
466-74
.
View Article PubMed Google Scholar -
Boutin
A.T.,
Liao
W.T.,
Wang
M.,
Hwang
S.S.,
Karpinets
T.V.,
Cheung
H.,
Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes & Development.
2017;
31
(4)
:
370-82
.
View Article PubMed Google Scholar -
Lee
D.W.,
Kim
K.J.,
Han
S.W.,
Lee
H.J.,
Rhee
Y.Y.,
Bae
J.M.,
KRAS mutation is associated with worse prognosis in stage III or high-risk stage II colon cancer patients treated with adjuvant FOLFOX. Annals of Surgical Oncology.
2015;
22
(1)
:
187-94
.
View Article PubMed Google Scholar -
Hirose
T.,
Matsuguma
K.,
Hirano
H.,
Okita
N.T.,
Shoji
H.,
Takashima
A.,
A retrospective analysis of the prognostic impact of KRAS G12D mutation in patients with RAS-mutated metastatic colorectal cancer. Journal of Clinical Oncology.
2024;
42
(3 Suppl)
:
105
.
View Article Google Scholar -
Koulouridi
A.,
Karagianni
M.,
Messaritakis
I.,
Sfakianaki
M.,
Voutsina
A.,
Trypaki
M.,
Prognostic Value of KRAS Mutations in Colorectal Cancer Patients. Cancers (Basel).
2022;
14
(14)
:
3320
.
View Article PubMed Google Scholar -
Cook
J.H.,
Melloni
G.E.,
Gulhan
D.C.,
Park
P.J.,
Haigis
K.M.,
The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nature Communications.
2021;
12
(1)
:
1808
.
View Article PubMed Google Scholar -
Hayama
T.,
Hashiguchi
Y.,
Okamoto
K.,
Okada
Y.,
Ono
K.,
Shimada
R.,
G12V and G12C mutations in the gene KRAS are associated with a poorer prognosis in primary colorectal cancer. International Journal of Colorectal Disease.
2019;
34
(8)
:
1491-6
.
View Article PubMed Google Scholar -
Modest
D.P.,
Ricard
I.,
Heinemann
V.,
Hegewisch-Becker
S.,
Schmiegel
W.,
Porschen
R.,
Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2016;
27
(9)
:
1746-53
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 9 (2024)
Page No.: 6723-6729
Published on: 2024-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2251 times
- PDF downloaded - 598 times
- XML downloaded - 73 times
 Biomedpress
Biomedpress



